Abstract
AIM: To elucidate the relationship between clinical presentation and outcome.
METHODS: A single institution retrospective chart review of patients admitted with the diagnosis of colon cancer. We used univariate and a multivariate analysis to identify symptoms association with mortality. An odds ratio based clinical score was created to evaluate the contribution of the quality of symptoms to outcome. Primary measure of outcome was survival.
RESULTS: During the study period, 236 patients met the inclusion criteria. Overall survival was 60.6%, mean follow-up 3.0 years. A bivariate analysis showed that increasing number of symptoms is not associated with mortality. However, a symptom-specific analysis performed using a logistic regression model controlling for age, stage and the duration of complaints revealed that the presence of melena was independently associated with mortality [P = 0.04, odds ratio (OR) 7.4], while rectal bleeding was associated with survival (P = 0.004, OR 3.9). Applying the proposed clinical score to an receiver operating characteristic curve showed that score > 1 had a strong association with mortality. The same logistic regression model was applied. The results showed that a score > 1 was an independent predictor of mortality (P < 0.001) and associated with node-positive disease (P = 0.008).
CONCLUSION: The quality of symptoms rather than quantity is correlated with outcome among patients with colon cancer. The proposed clinical scoring system may correctly predict the patient’s outcome.
Keywords: Colon cancer, Symptoms, Mortality
Core tip: Clinical presentation and its association with outcome among patients with colon cancer, although poorly studied in the past, may have an important role in predicting the outcomes among this important cohort of patients. Clinical scoring as proved in other areas in the past may have an important role in outcome prediction.
INTRODUCTION
Colon cancer is the third most common tumor worldwide and continues to cause significant mortality[1]. To date, pathological staging is the single most important independent predictor of outcome[2-6]. Other factors such as vascular invasion[2,7-9], residual tumor[10-13], serum carcino-embryogenic antigen levels[7,14-19], tumor grade[2,5,7], histologic type[5,20], perineural invasion[10-11] and radial margins are also linearly associated with outcome. Tumor DNA content, K-ras mutations, microvessel density and proliferative activity (Ki-67) are also associated with the prognosis of colon cancer. The clinical presentation of patients diagnosed with colon cancer and its association with outcome has not been studied extensively. The current study was performed to elucidate this association.
MATERIALS AND METHODS
We retrospectively reviewed the electronic charts of all patients admitted to the department of surgery at the Rambam Health Care Campus (RHCC) in Haifa, Israel, from January 2000 through December 2009. Patients with the diagnosis of colon cancer were identified and further scrutinized. Electronic charts were reviewed for age, symptoms at presentation, the duration of complaints, location of the tumor and post-operative TNM staging, according to Union for International Cancer Control. Follow-up and survival were also recorded. Symptoms were collected as described by the admitting physician in the patient’s electronic charts (preset menu).
The primary end point of the study was to seek an association between clinical presentation and outcome. The secondary end point was to examine whether various symptoms have different impacts on outcome and whether the quality and combination of complaints may predict the outcome. The primary measure of outcome was mortality.
Only patients who had undergone their index operation at RHCC were included. Patients presenting with sigmoid and rectal cancer were excluded. Mortality was adjusted for peri-operative mortality and patients who died within 30 d from the index operation where eliminated from the secondary analysis.
A logistic regression model controlling for age, stage and the duration of complaints was used to analyze the clinical symptoms and their association with mortality. The odds ratio and the event probability (mortality rate) predicted by this model were used to create a scoring system that incorporates the relative association of each symptom with mortality and/or patient survival. Points were given for each symptom according to a standardized legend (Table 1). The sum of all points for each patient was used to create a new variable with the corresponding score.
Table 1.
Estimates and scores used for creation of the clinical score
| Symptom | Odds ratio | Points |
| Pain | 1.2 | 1 |
| Diarrhea | 1.2 | 1 |
| Constipation | 0.91 | -1 |
| Vomiting | 1.3 | 2 |
| Weight loss | 1.1 | 1 |
| Rectal bleeding | 0.65 | -3 |
| Melena | 2.5 | 5 |
| Change in BH | 1.1 | 1 |
| Legend | ||
| 0.5-0.7 | -3 | |
| 0.7-0.9 | -2 | |
| 0.9-1.0 | -1 | |
| 1.0-1.2 | 1 | |
| 1.2-1.4 | 2 | |
| 1.4-1.6 | 3 | |
| 1.6-1.8 | 4 | |
| >1.8 | 5 |
BH: Bowel habits.
The Institutional Review Board of RHCC approved the study and waived the requirement for informed consent on the basis of preserving participants’ anonymity.
Statistical analysis
Continuous parametric variables were analyzed using Student’s t-test. The Mann-Whitney U test was used to analyze non-parametric variables. The chi-square test was applied to analyze the association between frequencies. A stepwise logistic regression model and a likelihood ratio test were applied to identify positive predictors of mortality among symptoms. The odds ratio and the event probability predicted by that model (mortality) were used to create a clinical scoring system. A receiver operating characteristic (ROC) curve was used to identify the score above which mortality was most likely. The results were applied to the same stepwise logistic regression model and likelihood ratio test, controlling again for age, stage and the duration of complaints. JMP Pro for Mac (Version 9.0.0) was used to analyze the data. P < 0.05 (2-sided) was considered to indicate statistical significance.
RESULTS
Over a period of ten years, 236 patients met the inclusion criteria for the study. The mean age was 71.5 ± 14.3 years; 124 (52.5%) patients were male, and 140 (59.3%) suffered from left-sided colon cancer. The mean duration of complaints prior to diagnosis was 1.8 mo. The median follow-up time was 36 mo (IQR 24-60 mo). The overall mortality was 39.4%. Adjusted mortality (overall-peri-operative mortality) was 33.9%. The most common symptom, abdominal pain, was present in 51.3% of patients, followed by a change in bowel habits in 41.5% and weight loss in 32.6%. Table 2 depicts the distribution of patient symptoms among the different TNM stages (Union for International Cancer Control) and shows significant differences in the manifestation of symptoms with an increased incidence of abdominal pain (P = 0.01), weight loss (P = 0.04) and a change in bowel habits (P = 0.03) within the node-positive stages.
Table 2.
Distribution of symptoms among patients, divided according to the Union for International Cancer Control TNM staging system n (%)
| Stage | Abdominal pain | Diarrhea | Constipation | Vomit | Weight loss | Rectal bleeding | Melena | Change in BH1 |
| 1 | 10 (30.3) | 3 (9.0) | 11 (33.3) | 1 (3.0) | 11 (33.3) | 8 (24.2) | 1 (3.0) | 11 (33.3) |
| 2a | 44 (62.8) | 11 (15.7) | 20 (28.6) | 11 (15.7) | 22 (31.4) | 11 (15.7) | 2 (2.8) | 34 (48.6) |
| 2b | 2 (66.6) | 0 (0.0) | 0 (0.0) | 1 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 3a | 5 (45.4) | 1 (9.0) | 2 (18.2) | 1 (9.0) | 3 (27.3) | 0 (0.0) | 0 (0.0) | 1 (9.0) |
| 3b | 15 (44.1) | 3 (8.8) | 8 (23.5) | 3 (2.9) | 6 (17.6) | 5 (14.7) | 1 (2.9) | 10 (29.4) |
| 3c | 10 (37.0) | 1 (3.7) | 10 (37.0) | 3 (11.1) | 7 (25.9) | 10 (37) | 0 (0.0) | 15 (55.5) |
| 4 | 35 (60.3) | 7 (12.1) | 18 (31.3) | 7 (12.1) | 28 (48.3) | 13 (22.4) | 3 (5.2) | 27 (46.5) |
| Total | 121 (51.3) | 26 (11) | 69 (29.2) | 27 (11.4) | 77 (32.6) | 47 (19.9) | 7 (2.9) | 98 (41.5) |
| P value | 0.01 | 0.78 | 0.79 | 0.35 | 0.04 | 0.14 | 0.95 | 0.03 |
Staging system according to the Union for International Cancer Control. BH: Bowel habits.
When comparing the distribution of symptoms with respect to tumor location, rectal bleeding and changes in bowel habits occurred in significantly higher rates in patients with tumors of the left colon (P = 0.002 and 0.006, respectively) (Table 3).
Table 3.
Comparison of symptoms with respect to tumor location n (%)
| Symptom | Left colon n = 139 | Right colon n = 97 | P value |
| Pain | 73 (52.5) | 48 (49.5) | 0.7 |
| Diarrhea | 12 (8.6) | 14 (14.4) | 0.2 |
| Constipation | 47 (33.8) | 22 (22.7) | 0.08 |
| Vomiting | 16 (11.5) | 11 (11.3) | 1 |
| Weight loss | 48 (34.5) | 29 (29.9) | 0.48 |
| Rectal bleeding | 37 (26.6) | 10 (10.3) | 0.002 |
| Melena | 5 (3.6) | 2 (2.1) | 0.71 |
| Change in BH | 68 (48.9) | 30 (30.9) | 0.006 |
Fisher’s exact test. BH: Bowel habits.
A univariate analysis comparing the symptoms among survivors and non-survivors showed that abdominal pain (P = 0.05) was the only symptom that was significantly associated with mortality; however, rectal bleeding was slightly associated with survival (0.06) (Table 4). A stepwise logistic regression model controlling for age, stage and the duration of complaints was applied. Our analysis showed that the presence of melena was associated with mortality (P = 0.04), whereas rectal bleeding was associated with survival (0.004), both independently of the controlled factors. Age (P = 0.005), stage (P < 0.001) and the duration of complaints (P = 0.03) were found to be independent predictors of mortality, as expected. We have applied the same logistic regression model on the adjusted mortality, melena was no longer associated with the adjusted mortality and rectal bleeding was (P = 0.01). Interestingly age was no longer an independent predictor, while duration of complaints (P = 0.04) and pathological stage (P < 0.001) remained strongly significant.
Table 4.
Crude univariate analysis comparing the presentation of symptoms among survivors and non-survivors n (%)
| Symptom | Survivors n = 142 | Non-survivors n = 93 | Univariate P value | Multivariate P value | OR (95%CI) |
| Pain | 66 (46.2) | 55 (59.1) | 0.05 | 0.38 | 1.4 (0.7-2.8) |
| Diarrhea | 13 (9.1) | 13 (14) | 0.24 | 0.51 | 1.5 (0.4-5.6) |
| Constipation | 41 (28.7) | 28 (30.1) | 0.81 | 0.91 | 0.94 (0.3-2.7) |
| Vomiting | 12 (8.4) | 15 (16.1) | 0.07 | 0.24 | 1.9 (0.6-5.6) |
| Weight loss | 43 (30) | 34 (36.6) | 0.32 | 0.63 | 0.8 (0.4-1.7) |
| Rectal bleeding | 34 (23.8) | 13 (14) | 0.06 | 0.004 | 0.25 (0.1-0.7) |
| Melena | 2 (1.4) | 5 (5.4) | 0.1 | 0.04 | 7.4 (1.1-72.7) |
| Change in BH | 56 (39.2) | 42 (45.2) | 0.36 | 0.96 | 1 (0.4-2.9) |
A stepwise logistic regression model utilizing a likelihood ratio test was applied controlling for, age, stage and the duration of complaints. BH: Bowel habits.
A quantitative analysis of numeric combinations of symptoms indicated that the number of symptoms present had no positive or negative association with mortality. Patients who presented with any numeric combination shared the same outcome on univariate or multivariate analysis when controlled for age, stage and the duration of complaints.
To perform a symptom-specific analysis with regard to mortality, we created a clinical scoring system according to the odds ratios and probability of an event (mortality) predicted by the logistic regression model and the likelihood ratio test applied previously. Each symptom had points assigned according to the legend depicted in Table 1. We summarized the total points assigned to each patient into one variable by creating the new scoring system. The new score showed a normal distribution among patients and a graded association with mortality.
The new clinical scoring system is an independent predictor of mortality (P < 0.001) when age (P = 0.02), stage (P < 0.001) and the duration of complaints (P = 0.05) are controlled. When applied to an ROC curve, scores higher than 1 had the highest likelihood of mortality with area under the curve (AUC) of 65%. In a multivariate analysis, scores higher than 1 proved to be a significant predictor of mortality (P = 0.001) independently of age (P = 0.003), stage (P < 0.001) and the duration of complaints (P = 0.05) (Table 5).
Table 5.
Clinical score predicts mortality independently of age, stage and the duration of complaints
| Mortality | OR (95%CI) | |
| Clinical score > 1 | 0.001 | 3.1 (1.5-6.1) |
| Age | 0.003 | 0.96 (0.94-0.99) |
| Stage | < 0.001 | 35 (5.3-703.7) |
| Duration of complaints | 0.05 | 0.6 (0.26-1.0) |
Stepwise logistic regression analysis with the application of a likelihood ratio test. OR: Odds ratio.
We have applied the described scoring system on the adjusted mortality, clinical score higher than 1 remained a significant independent predictor of mortality (P = 0.01) independently of duration of complaints (P = 0.04), stage (P < 0.001). Age was no longer an independent predictor of outcome.
A survival analysis was performed using the Kaplan Meier method and the survival curves of both groups (clinical scores < 1 and > 1) are depicted in Figure 1. The mean survival of patients with scores lower than 1 at 48 mo was 78%, whereas patients with scores higher than 1 presented with 43% survival at 48 mo. The curves shown in Figure 1 also indicate the significant differences between the groups in terms of survival, the log-rank test result was P < 0.001.
Figure 1.
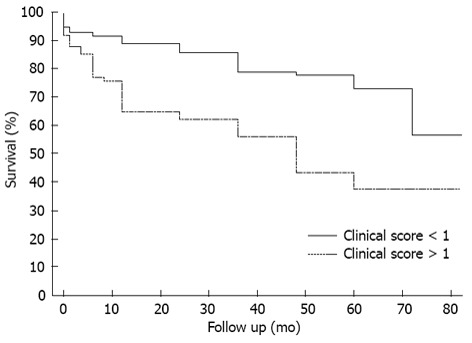
Kaplan-Meier survival curve comparing the survival of patients with clinical scores higher and lower than one.
The clinical score was also associated with the TNM stage when patients with scores higher than 1 presented with later TNM stages (P = 0.008). However, the score was not associated with age, gender, location of the tumor or the duration of complaints.
DISCUSSION
Although colon cancer is the third most common cancer worldwide[1], studies on the clinical presentation and its association with outcome are limited. Previous studies have shown that rectal bleeding is associated with early TNM stages of disease. Changes in bowel habits and abdominal pain are associated with advanced TNM stages[21-24]. Stapley et al[25] reported rectal bleeding to be associated with reduced mortality. The current study investigated the correlation between the number and the quality of symptoms and the outcome among patients with colon cancer, and created a clinical score that may be able to predict outcome based on the clinical presentation prior to diagnosis.
The characteristics of this cohort of 236 patients are comparable to those of other reported cohorts with respect to gender distribution, mean age and tumor location[1,26]. The rates of symptoms reported and the distribution of stages are also comparable to those reported in the English literature.
A univariate analysis of our sample indicated that patients in the late TNM stages presented with significantly more abdominal pain (P = 0.01), more weight loss (P = 0.04) and more drastic changes in bowel habits (0.03). Rectal bleeding was previously reported to be associated with early TNM stages[21], although this trend was not observed among the patients in our sample.
The comparison of symptoms with respect to laterality showed that patients with left-sided colonic tumors manifested significantly more rectal bleeding (P = 0.002) and more drastic changes in bowel habits (P = 0.006) than patients with right-sided tumors (Table 2).
Univariate analysis indicated that the number of symptoms regardless of their quality was not associated with mortality or with TNM staging. A multivariate analysis controlling for factors such as age, stage and the duration of complaints did not yield significant differences for any possible numeric combination. These results, although poorly reported in the past, are similar to those reported by Stapley et al [25].
Univariate analysis, of the quality of all symptoms showed that except for abdominal pain (0.05) no other symptom was associated with mortality. However, a multivariate analysis controlling for age, stage and the duration of complaints showed that melena was strongly associated with mortality (P = 0.04, OR 7.4), and rectal bleeding was strongly associated with survival (P = 0.004, OR 0.25). Applying the same univariate analysis on the adjusted mortality did not change the results and abdominal pain remained significantly associated with mortality (P = 0.05), while on a multivariate analysis rectal bleeding was associated mortality and no longer with survival (P = 0.02, OR 2.5)
Melena is typically not a symptom of colon cancer and was reported in only 2.9% (n = 7) of patients. Interestingly, the presentation of melena was not associated with tumor location, age, stage, duration of complaints or any of the other reported symptoms. The association of rectal bleeding with survival was previously reported[26], and the current study was able to validate this observation for overall mortality but the adjusted mortality did not replicate the same results.
We believe that although melena and rectal bleeding were the only symptoms associated with positive or negative outcomes, the clinical presentation prior to diagnosis may play a role in predicting outcomes among patients with colon cancer. In view of this belief, we developed a clinical scoring system based on the estimates of event probability yielded by the logistic regression model. We assigned points to each symptom according to its relative contribution to outcome, and the sum of these points for each patient allowed us to create a variable that incorporates all symptoms to quantify the relative event probability contributed by the combination of symptoms presented in each patient.
The new clinical score had a normal distribution among patients and a graded association with mortality. To validate the new score, we performed a univariate analysis that revealed a significant association with mortality (P < 0.001). A multivariate analysis controlling for age, stage and the duration of complaints showed that the score is an independent predictor of mortality (P < 0.001). We placed the various patient scores on a ROC curve and found that scores higher than one were associated with the highest event probability. By applying the same multivariate analysis, we confirmed that when controlled for age, stage and the duration of complaints, a score higher than one is an independent predictor of outcome (P = 0.001, OR 3.1) (Table 5).
The Kaplan-Meier survival curve (Figure 1) also demonstrates the negative impact of a score higher than one on survival.
The main limitation of this study is its retrospective nature; it is possible that the patients neglected to report symptoms and that the physicians neglected to document reported symptoms. However, the agreement of the reported symptoms and their distribution with what was previously reported in the literature encourages us to believe that the data accurately represent the symptoms present. The study is novel in its approach to clinical presentation and the predictive model proposed.
In conclusion, clinical presentation and its association with outcome among patients with colon cancer, although poorly studied in the past, may have an important role in predicting the outcomes among this important cohort of patients. The proposed clinical scoring system seems to be able to reliably predict outcome. Further large-scale, population-based studies should be applied to validate the proposed clinical scoring system.
COMMENTS
Background
Although colon cancer is third most common cancer world wide and research regarding it prognosis and outcome is held universally. The clinical presentation of patients diagnosed with colon cancer and its association with outcome has not been studies extensively. The current study was initiated to elucidate on this association.
Research frontiers
Very limited studies were published regarding the reported association between clinical presentation and outcome and prospective evaluation should be considered.
Innovations and breakthroughs
Clinical presentation is of utmost importance and its association with outcome should be further investigated prospectively. Preoperative clinical scoring may provide the clinicians with a toold.
Applications
The current clinical score may predict outcome preoperatively in patients with colon cancer and may imply as to how severe the disease is.
Peer review
This is an interesting work searching for correlation between the number and the quality of symptoms and the outcome among patients with colon cancer. The study has also clinical utility since authors explain how a clinical score can be created to predict outcome based on the clinical presentation prior to diagnosis.
Footnotes
P- Reviewer Cadena MP S- Editor Wen LL L- Editor A E- Editor Lu YJ
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, Colquhoun K. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985;72:698–702. doi: 10.1002/bjs.1800720909. [DOI] [PubMed] [Google Scholar]
- 3.Tominaga T, Sakabe T, Koyama Y, Hamano K, Yasutomi M, Takahashi T, Kodaira S, Kato T, Ogawa N. Prognostic factors for patients with colon or rectal carcinoma treated with resection only. Five-year follow-up report. Cancer. 1996;78:403–408. doi: 10.1002/(SICI)1097-0142(19960801)78:3<403::AID-CNCR4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd NA, Baxter KJ, Love SB. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997;112:1096–1102. doi: 10.1016/s0016-5085(97)70119-7. [DOI] [PubMed] [Google Scholar]
- 5.Newland RC, Dent OF, Lyttle MN, Chapuis PH, Bokey EL. Pathologic determinants of survival associated with colorectal cancer with lymph node metastases. A multivariate analysis of 579 patients. Cancer. 1994;73:2076–2082. doi: 10.1002/1097-0142(19940415)73:8<2076::aid-cncr2820730811>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.AJCC (American Joint Committee on Cancer) Cancer Staging Manual. 7th edition. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. New York: Springer; 2010. p. 143. [Google Scholar]
- 7.Wiggers T, Arends JW, Volovics A. Regression analysis of prognostic factors in colorectal cancer after curative resections. Dis Colon Rectum. 1988;31:33–41. doi: 10.1007/BF02552567. [DOI] [PubMed] [Google Scholar]
- 8.Mulcahy HE, Skelly MM, Husain A, O’Donoghue DP. Long-term outcome following curative surgery for malignant large bowel obstruction. Br J Surg. 1996;83:46–50. doi: 10.1002/bjs.1800830114. [DOI] [PubMed] [Google Scholar]
- 9.Betge J, Pollheimer MJ, Lindtner RA, Kornprat P, Schlemmer A, Rehak P, Vieth M, Hoefler G, Langner C. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer. 2012;118:628–638. doi: 10.1002/cncr.26310. [DOI] [PubMed] [Google Scholar]
- 10.Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 11.Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 12.Willett CG, Goldberg S, Shellito PC, Grossbard M, Clark J, Fung C, Proulx G, Daly M, Kaufman DS. Does postoperative irradiation play a role in the adjuvant therapy of stage T4 colon cancer? Cancer J Sci Am. 1999;5:242–247. [PubMed] [Google Scholar]
- 13.Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511–2516. doi: 10.1002/cncr.10492. [DOI] [PubMed] [Google Scholar]
- 14.Wolmark N, Fisher B, Wieand HS, Henry RS, Lerner H, Legault-Poisson S, Deckers PJ, Dimitrov N, Gordon PH, Jochimsen P. The prognostic significance of preoperative carcinoembryonic antigen levels in colorectal cancer. Results from NSABP (National Surgical Adjuvant Breast and Bowel Project) clinical trials. Ann Surg. 1984;199:375–382. doi: 10.1097/00000658-198404000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meling GI, Rognum TO, Clausen OP, Børmer O, Lunde OC, Schlichting E, Grüner OP, Hognestad J, Trondsen E, Havig O. Serum carcinoembryonic antigen in relation to survival, DNA ploidy pattern, and recurrent disease in 406 colorectal carcinoma patients. Scand J Gastroenterol. 1992;27:1061–1068. doi: 10.3109/00365529209028139. [DOI] [PubMed] [Google Scholar]
- 16.Lindmark G, Bergström R, Påhlman L, Glimelius B. The association of preoperative serum tumour markers with Dukes’ stage and survival in colorectal cancer. Br J Cancer. 1995;71:1090–1094. doi: 10.1038/bjc.1995.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison LE, Guillem JG, Paty P, Cohen AM. Preoperative carcinoembryonic antigen predicts outcomes in node-negative colon cancer patients: a multivariate analysis of 572 patients. J Am Coll Surg. 1997;185:55–59. doi: 10.1016/s1072-7515(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 18.Park IJ, Choi GS, Lim KH, Kang BM, Jun SH. Serum carcinoembryonic antigen monitoring after curative resection for colorectal cancer: clinical significance of the preoperative level. Ann Surg Oncol. 2009;16:3087–3093. doi: 10.1245/s10434-009-0625-z. [DOI] [PubMed] [Google Scholar]
- 19.Thirunavukarasu P, Sukumar S, Sathaiah M, Mahan M, Pragatheeshwar KD, Pingpank JF, Zeh H, Bartels CJ, Lee KK, Bartlett DL. C-stage in colon cancer: implications of carcinoembryonic antigen biomarker in staging, prognosis, and management. J Natl Cancer Inst. 2011;103:689–697. doi: 10.1093/jnci/djr078. [DOI] [PubMed] [Google Scholar]
- 20.Secco GB, Fardelli R, Campora E, Lapertosa G, Gentile R, Zoli S, Prior C. Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology. 1994;51:30–34. doi: 10.1159/000227306. [DOI] [PubMed] [Google Scholar]
- 21.Alexiusdottir KK, Möller PH, Snaebjornsson P, Jonasson L, Olafsdottir EJ, Björnsson ES, Tryggvadottir L, Jonasson JG. Association of symptoms of colon cancer patients with tumor location and TNM tumor stage. Scand J Gastroenterol. 2012;47:795–801. doi: 10.3109/00365521.2012.672589. [DOI] [PubMed] [Google Scholar]
- 22.Majumdar SR, Fletcher RH, Evans AT. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol. 1999;94:3039–3045. doi: 10.1111/j.1572-0241.1999.01454.x. [DOI] [PubMed] [Google Scholar]
- 23.Speights VO, Johnson MW, Stoltenberg PH, Rappaport ES, Helbert B, Riggs M. Colorectal cancer: current trends in initial clinical manifestations. South Med J. 1991;84:575–578. [PubMed] [Google Scholar]
- 24.Cappell MS, Goldberg ES. The relationship between the clinical presentation and spread of colon cancer in 315 consecutive patients. A significant trend of earlier cancer detection from 1982 through 1988 at a university hospital. J Clin Gastroenterol. 1992;14:227–235. doi: 10.1097/00004836-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Stapley S, Peters TJ, Sharp D, Hamilton W. The mortality of colorectal cancer in relation to the initial symptom at presentation to primary care and to the duration of symptoms: a cohort study using medical records. Br J Cancer. 2006;95:1321–1325. doi: 10.1038/sj.bjc.6603439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]


