Abstract
AIM: To investigate whether a subcutaneous penrose drain would decrease the superficial surgical site infection (s-SSI) rate in elective colorectal surgery.
METHODS: This is a comparative study of the historical control type. Intervention consisted of the use of penrose drain in elective open colorectal surgical wounds. The outcome was an incidence of s-SSI. The patients were risk stratified according to the depth of subcutaneous tissue.
RESULTS: There were 131 patients (40 patients with high s-SSI risk) in the prior period (from July 2008 to June 2009, when no penrose drains were inserted) and 151 patients (75 patients with high s-SSI risk) in the latter period (from June 2010 to November 2011, when penrose drains were inserted). The overall s-SSI rate was 6.1% and 5.3% during the two periods (P = 0.770), and the s-SSI rate in the high s-SSI risk group was 15.0% and 8.0% (P = 0.242).
CONCLUSION: Although penrose drain was not observed to significantly reduce s-SSI, there tended to be a reduced risk of s-SSI in the high s-SSI risk group.
Keywords: Surgical site infections, Subcutaneous penrose drains, Colorectal surgery, Open surgery, Subcutaneous tissue
Core tip: In this article, the authors investigated whether a subcutaneous penrose drain would decrease the superficial surgical site infection rate in elective colorectal surgery. Although penrose drain were not observed to significantly reduce superficial surgical site infection, there tended to be a reduced risk of superficial surgical site infection in the high superficial surgical site infection risk group(depth of subcutaneous tissue was over 20 mm).
INTRODUCTION
Surgical site infections (SSI) are still a major problem in general surgery, because they are responsible for significant discomfort for patients and excess morbidity and mortality, which also translates into a financial burden on the health system[1]. Superficial SSI (s-SSI) account for about 60% of SSI, and the occurrence is associated with wound separation, ventral hernia, and so on[2].
SSI surveillance by an infection control team (ICT) started in September 2003 at this hospital. The incidence of s-SSI in colorectal surgery decreased from 12% to about 5% by the intervention of the ICT[3]. However, s-SSI still occurs at a low rate yet, and we therefore need further interventions to decrease the incidence of s-SSI. One consideration is to remove the blood and serous fluids from the wound by drains before fluids can get infected[4]. This concept is frequently implemented in clinics. However, a meta-analysis showed that prophylactic subcutaneous drainage to prevent wound complications is not efficient in gynecology[5]. On the other hand, there have so far been few reports on the efficacy of prophylactic subcutaneous drain for the prevention of s-SSI following digestive surgery. Recently one study described a systematic randomized evaluation in patients undergoing laparotomy in digestive surgery while clarifying whether subcutaneous closed suction drains affect wound infection, and the authors concluded that there were no indications for prophylactic subcutaneous suction drain[4]. Furthermore, there is no evidence about the use of prophylactic subcutaneous penrose drains (PD) which are likely to be used more widely than suction drains in digestive surgery due to the fact that they are cheaper. Moreover, there is no evidence about the effect of PD following elective colorectal surgery, in which the incidence of s-SSI is usually higher than other fields.
This study analyzed the efficacy of PD for the prevention of s-SSI in elective colorectal surgery.
MATERIALS AND METHODS
This study was a prospective cohort with historic controls in order to assess the use of PD. Patients undergoing elective open colorectal surgery were included in this study. Patients who underwent emergency surgery, laparoscopic surgery, and re-do operations were excluded. The study classified two periods, the prior period and the latter period. The prior period was from July 2008 to June 2009, in which no PD was inserted subcutaneously in patients that underwent elective colorectal surgery. The latter period was from June 2010 to November 2011, and PDs (an open drain, 8 mm; Fuji Systems Corporation, Japan) were inserted subcutaneously in patients that underwent elective colorectal surgery for prevention of s-SSI. The data of the prior period were collected retrospectively from the medical records, and PDs were prospectively inserted in cases that met the eligibility criteria during the latter period. Moreover, the patients from each period were divided into two groups, the low s-SSI risk group and the high s-SSI risk group. The two groups were based on whether the depth of subcutaneous tissue was over 20 mm, because Soper et al[6] reported that the depth of subcutaneous tissue is the most significant risk factor associated abdominal wound infection after hysterectomy. The depth of subcutaneous tissue was measured preoperatively at the level of the umbilicus based on abdominal computed tomography (Figure 1).
Figure 1.
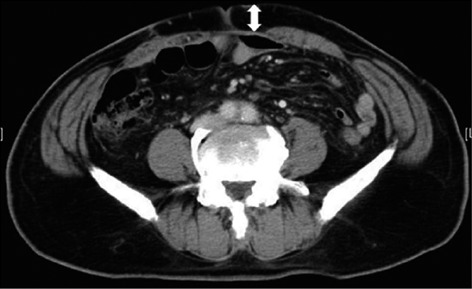
Depth of subcutaneous tissue at the level of the umbilicus.
Every patient received the same preparations, that is, sennoside and magnesium citrate were administered following fasting 1 d before surgery and followed by a glycerin enema in the morning of the day of surgery. No patient underwent chemical bowel preparation. The patients took showers 1 d before surgery, and underwent body hair removal just before the operation. Moreover, the surgical field was disinfected by the use of iodine and the patients received antibiotic prophylaxis with cefmetazole just before the initial skin incision, every 3 h during the operation, and twice per day on the first and second postoperative days.
The skin incision was performed with a scalpel; subcutaneous fat was dissected by electrocautery. Wound protection was achieved during the operation by a ring drape device. The surgical instruments were exchanged just before the peritoneal-muscle closure, and the wound was irrigated with 1000 mL of saline solution just before skin closure. The fascia/muscle layer was closed by interrupted VICRYL® sutures (Ethicon, Somerville, NJ, United States) and the skin was closed by stapling. There were no differences in the surgical procedures between the latter an prior period, except that a PD was inserted along the entire length of the subcutaneous tissue. The exit of the drain was separated from the incisions. The PD was removed on postoperative day three.
SSI cases were diagnosed within 30 postoperative day by ICT according to the Centers for Disease Control and Prevention (CDC) criteria: (1) purulent drainage with or without laboratory confirmation from the superficial incision; (2) organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision; (3) at least one of the following signs or symptoms of infection: Pain or tenderness, localized swelling, redness, or heat and superficial incision were deliberately opened by surgeon, unless the incision was culture-negative; and (4) diagnosis of s-SSI by the surgeon or ICT.
Statistical analysis
Numerical data are given as the mean ± SD, and they conformed to the normal distribution. Discrete data were tested for significance by means of the χ2 test or Fisher’s exact test. Continuous data were tested for significance with Student’s t-test. P < 0.05 was considered to be significant.
RESULTS
One hundred thirty-one patients underwent surgery during the prior period, and 151 patients during the latter period. The PD was usually removed on postoperative day 3, but the physician in charge removed it depending on properties and amount of drainage. The median times of removal of PD were postoperative day three (range 2-12). There were no severe complications associated with the insertion of the PD. The characteristics of patients during the two periods are shown in Table 1. There was no significant difference between the two periods with regard to the characteristics, such as age, sex, diabetes mellitus, smoking history, American Society of Anesthesiologists classification, body mass index, operation time, blood loss, and presence of stoma. However, the proportion of high s-SSI patients was different between the two periods (30.5% vs 49.7%, P = 0.001). The types of surgery of all of the patients and the high s-SSI group patients are shown in Table 2, and there was no significant difference between each group during the two periods (overall P = 0.440, high risk group P = 0.190). The characteristics of the high s-SSI risk patients in the two periods are shown in Table 3. No significant differences were noted with regard to characteristics between the two periods.
Table 1.
Demographic characteristics of the patients in the two periods
| Prior period (n = 131) | Latter period (n = 151) | P value | |
| Age (yr, mean ± SD) | 62.7 ± 10.4 | 63.0 ± 12.4 | 0.847 |
| Sex (male/female) | 68/63 | 91/60 | 0.158 |
| Diabetes mellitus (yes/no) | 17/114 | 20/131 | 0.947 |
| Smoking history (yes/no) | 24/107 | 32/119 | 0.547 |
| ASA classification (ASA score ≤ 2/3 ≤) | 124/7 | 146/5 | 0.399 |
| Body mass index (kg/m2) | 22.4 ± 3.3 | 22.5 ± 3.5 | 0.802 |
| Subcutaneous fat (mm, mean ± SD) | 18.2 ± 7.8 | 19.7 ± 7.1 | 0.081 |
| Site (colon/rectum) | 73/58 | 95/56 | 0.220 |
| Operation time (min, median) | 206 | 219 | 0.864 |
| Blood loss(mL, median) | 205 | 200 | 0.169 |
| Stoma (yes/no) | 32/99 | 31/120 | 0.433 |
| Patients with high risk1 | 40 | 75 | 0.001 |
| Patiens with PD | 0 | 151 |
High risk patients whose depth of subcutaneous tissue are over 20 mm. ASA: American Society of Anesthesiologists; PD: Penrose drains.
Table 2.
Types of surgery during the two periods
| Prior period (n = 131) | Latter period (n = 151) | |||
| Overall | High risk group | Overall | High risk group | |
| Resection of the colon | 73 | 18 | 95 | 46 |
| Resection of the rectum with the stoma | 31 | 10 | 28 | 16 |
| Resection of the rectum without the stoma | 27 | 12 | 28 | 13 |
| Total | 131 | 40 | 151 | 75 |
Table 3.
Demographic characteristics of the high risk patients
| Prior period (n = 40) | Later period (n = 75) | P value | |
| Age (yr, mean ± SD) | 61.8 ± 9.7 | 62.5 ± 11.0 | 0.743 |
| Sex (male/female) | 15/25 | 36/39 | 0.280 |
| Diabetes mellitus (yes/no) | 5/35 | 10/65 | 0.899 |
| Smoking history (yes/no) | 8/32 | 15/60 | > 0.999 |
| ASA classification (ASA score ≤ 2/3 ≤) | 40/0 | 75/0 | > 0.999 |
| Body mass index (kg/m2) | 24.2 | 24 | 0.767 |
| Site (colon/rectum) | 18/22 | 46/29 | 0.093 |
| Operation time (min, median) | 245 | 239 | 0.588 |
| Blood loss (mL, median) | 275 | 205 | 0.110 |
| Stoma (yes/no) | 9/31 | 18/57 | 0.857 |
ASA: American Society of Anesthesiologists.
The incidences of s-SSI in the two periods are shown in Table 4. The overall s-SSI rate was 6.1% (8/131) in the prior period, and 5.3% (8/151) in the latter period. The s-SSI rate in the high risk group during the two periods was 15.0% and 8.0% (P = 0.242). The s-SSI rate was reduced by half. However, there was no significant difference between two the periods. In contrast, the s-SSI rate of the low risk group during the two periods was 2.2% and 2.6% (P = 0.855). There was no significant difference between the two periods. Moreover, 6 s-SSI cases of the high risk group in the latter period are presented in Table 5. There were 4 culture-positive cases among in the latter period. Three of 4 cultures showed bacteria in the intestines, and only one culture was skin bacteria.
Table 4.
Incidence of superficial surgical site infections during the two periods n (%)
| Prior period (n = 40) | Later period (n = 75) | P value | |
| Patients with s-SSI (high s-SSI risk group) | 6/40 (15.0) | 6/75 (8.0) | 0.242 |
| Patients with s-SSI (low s-SSI risk group) | 2/91 (2.2) | 2/76 (2.6) | 0.855 |
| Patients with s-SSI (overall) | 8/131 (6.1) | 8/151 (5.3) | 0.770 |
s-SSI: Superficial surgical site infections.
Table 5.
Superficial surgical site infections cases in the later period
| Case | Age | Sex | ASA | Fat tissue (mm) | s-SSI risk | Operation | Daysof drainage | Culture |
| 1 | 72 | M | 2 | 23 | High | Resection of the rectum with the stoma | 4 | Not done |
| 4 | 71 | F | 2 | 24 | High | Resection of the colon | 4 | Not detected |
| 2 | 55 | M | 2 | 27 | High | Resection of the colon | 3 | Staphylococcus aureus (skin bacteria) |
| 3 | 73 | M | 2 | 27 | High | Resection of the colon | 3 | Enterococcus facium (intestine bacteria) |
| 5 | 56 | F | 2 | 22 | High | Resection of the rectum with the stoma | 3 | Pseudomonus aeruginosa (intestine bacteria) |
| 6 | 81 | F | 2 | 21 | High | Resection of the colon | 3 | Bacteroides fragilis (intestine bacteria) |
| 7 | 64 | M | 2 | 18 | Low | Resection of the colon | 4 | Enterococcus facium (intestine bacteria) |
| 8 | 62 | M | 2 | 16 | Low | Resection of the colon | 4 | Not detected |
ASA: American Society of Anesthesiologists; M: Male; F: Female; s-SSI: Superficial surgical site infections.
DISCUSSION
SSI is one of the most serious infectious complications of surgery. The occurrence is associated with a high incidence of reoperation, a long duration of hospitalization, and a large increase in the cost of any postoperative surgery complication. In addition, patient discomfort and the inconvenience of caring for a healing open wound at home make the prevention of this complication a high priority[6]. s-SSI has a high incidence among SSI, and it is generally thought that the incidence of s-SSI is related to amount of bacterium of the wound, formation of hematoma, pool of effusion, potential subcutaneous dead space, disturbance of the local circulation, and the amount of bacterium in the surgical organ[7].
A subcutaneous drain might reduce the amount of bacterium around the wound and remove residual effusion and blood from the wound that could serve as a medium for bacterial growth. This study selected a PD, which is an open drain, because of its convenience and inexpensiveness. Generally, a PD or closed suction drain is used as a subcutaneous drain. A closed drain is an active drain that employs the power of suction. The luminal obstruction of such drains increase with time, and drainage becomes poor 48 h after insertion[7]. On the other hand, long term insertion of a PD is associated with retrograde infection. Moro et al[8] pointed out that the insertion of an opened drain for more than 3 d increases the risk of SSI. In addition, Numata et al[9] reported that 25% of cultures of discharge from subcutaneous PDs that was inserted over 3 d postoperatively, were positive for skin bacteria. Therefore, the PD was removed on postoperative day three. Table 5 shows the s-SSI cases in the latter period. There were 5 culture positive cases among the 8 s-SSI cases in the latter period. Four cultures of the 5 cases showed bacteria in the intestines, and only one culture was skin bacteria. Moreover, the cost of PD is less expensive than that of a closed drain. Each type of drain has specific advantages and disadvantages.
Numata et al[7] reported that PD is an effective means for preventing s-SSI in high s-SSI risk patients following digestive tract surgery. However, they classified contaminated operations and dirty-infected operations, or clean-contaminated operations accompanied by at least 20 mm thick subcutaneous fat into the high s-SSI risk group, and they reported that PD was more efficient in contaminated surgery, such as a perforation of the colon. However, the cases in the current study were restricted to elective colorectal surgeries, and the amount of bacteria was found to be small. As a result, a potential risk of bias in the intervention population may have existed. Moreover, the current protocol exchanged the surgical instruments just before peritoneal-muscle closure, and performed wound irrigation with 1000 mL of saline solution just before skin closure. So, the decrease of s-SSI has a possibility of limit from the aspect of drainage in elective colorectal surgeries.
In regard to suture choice, we always closed the fascia/muscle layer with VICRYL® sutures in clean-contaminated surgery. However, multifilament sutures, such as VICRYL®, are more prone to develop SSI than monofilament wire, such as PDS®. On the other hand, one recent study reported that antibacterial-coated multifilament (VICRYL PLUS®) was more effective than monofilament (PDS-II®)[10]. We therefore need to examine the suture choice to prevent s-SSI from now on.
The current study failed to demonstrate the efficacy of PD. However, dead space in the subcutaneous layer is a risk factor of s-SSI, and Inotsume-Kojima et al[11] reported that a combination of subcuticular sutures and a drain for the skin closure reduces wound complications in obese females undergoing surgery using vertical incisions in gynecology. Furthermore, interventions associated with subcuticular sutures may be necessary in elective colorectal surgery.
In conclusion, a PD was inserted subcutaneously to reduce s-SSI following colorectal surgery. However, the results failed to demonstrate that PD reduced the incidence of s-SSI (6.1% vs 5.3%). Although the difference was not significant, there was a trend toward a reduced risk of s-SSI (15.0% vs 8.0%) in the high s-SSI risk group.
COMMENTS
Background
Surgical site infections (s-SSI) are still a major problem in general surgery, because they are responsible for significant discomfort for patients and excess morbidity and mortality, which also translates into a financial burden on the health system. The major parts of surgical site infections are superficial surgical site infections, and it account for about 60% of surgical site infection.
Research frontiers
The incidence of superficial s-SSI is related to amount of bacterium of the wound, formation of hematoma, pool of effusion, potential subcutaneous dead space, disturbance of the local circulation, and the amount of bacterium in the surgical organ. A subcutaneous drain might reduce the amount of bacterium around the wound and remove residual effusion and blood from the wound that could serve as a medium for bacterial growth. In this study, the authors investigate whether a subcutaneous penrose drain would decrease the superficial surgical site infection rate in elective colorectal surgery.
Innovations and breakthroughs
Some surgeons use subcutaneous drains to prevent surgical site infection. But there is no evidence of the efficacy of prophylactic subcutaneous drain for the prevention of superficial surgical site infection following digestive surgery, including colorectal surgery. This study analyzed the efficacy of penrose drains for the prevention of s-SSI in elective colorectal surgery.
Applications
The current study failed to demonstrate the efficacy of penrose drains. But dead space in the subcutaneous layer is a risk factor of s-SSI, and there are some suture choices and some subcutaneous drains. So, the authors therefore need to examine the combinations of suture and drain to prevent s-SSI.
Terminology
SSI is one of the most serious infectious complications of surgery. The occurrence is associated with a high incidence of reoperation, a long duration of hospitalization, and a large increase in the cost of any postoperative surgery complication.
Peer review
This study compares a prospective cohort with historic controls in order to assess the use of Penrose drains in median laparotomies in patients undergoing colorectal resection in order to reduce superficial surgical site infections. It is a retrospective case control study on a simple but important question.
Footnotes
Supported by Osaka Medical Center for Cancer and Cardiovascular Diseases
P- Reviewers Ragg JL, Siassi M, Teeuwen PHE S- Editor Song XX L- Editor A E- Editor Lu YJ
References
- 1.Romy S, Eisenring MC, Bettschart V, Petignat C, Francioli P, Troillet N. Laparoscope use and surgical site infections in digestive surgery. Ann Surg. 2008;247:627–632. doi: 10.1097/SLA.0b013e3181638609. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe A, Kohnoe S, Shimabukuro R, Yamanaka T, Iso Y, Baba H, Higashi H, Orita H, Emi Y, Takahashi I, et al. Risk factors associated with surgical site infection in upper and lower gastrointestinal surgery. Surg Today. 2008;38:404–412. doi: 10.1007/s00595-007-3637-y. [DOI] [PubMed] [Google Scholar]
- 3.Tanida T, Yamada T, Tanaka K, Tomimaru Y, Kishi K, Noura S, Ohue M, Yano M, Ishikawa O, Imaoka S. Trial of ICT intervention for the prevention of wound infection after colorectal surgery. JJpanSocSurgInfect. 2007;4:499–502. [Google Scholar]
- 4.Baier PK, Glück NC, Baumgartner U, Adam U, Fischer A, Hopt UT. Subcutaneous Redon drains do not reduce the incidence of surgical site infections after laparotomy. A randomized controlled trial on 200 patients. Int J Colorectal Dis. 2010;25:639–643. doi: 10.1007/s00384-010-0884-y. [DOI] [PubMed] [Google Scholar]
- 5.Hellums EK, Lin MG, Ramsey PS. Prophylactic subcutaneous drainage for prevention of wound complications after cesarean delivery--a metaanalysis. Am J Obstet Gynecol. 2007;197:229–235. doi: 10.1016/j.ajog.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Soper DE, Bump RC, Hurt WG. Wound infection after abdominal hysterectomy: effect of the depth of subcutaneous tissue. Am J Obstet Gynecol. 1995;173:465–469; discussion 465-469. doi: 10.1016/0002-9378(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 7.Numata M, Tanabe H, Numata K, Suzuki Y, Tani K, Shiraishi R, Ooshima T, Rino Y, Imada T, Masuda M. The Efficacy of subcutaneous Penrose Drains for the Prevention of Superficial Surgical Site Infections. Jpn J Gastroenterol Surg. 2010;43:221–228. [Google Scholar]
- 8.Moro ML, Carrieri MP, Tozzi AE, Lana S, Greco D. Risk factors for surgical wound infections in clean surgery: a multicenter study. Italian PRINOS Study Group. Ann Ital Chir. 1996;67:13–19. [PubMed] [Google Scholar]
- 9.Numata K, Tanabe H, Numata M. Asessment about discharge from inserted subcutaneous Penrose Drain in gastroenterological surgery. JJpnSocSurg Infect. 2011;8:291–294. [Google Scholar]
- 10.Justinger C, Slotta JE, Schilling MK. Incisional hernia after abdominal closure with slowly absorbable versus fast absorbable, antibacterial-coated sutures. Surgery. 2012;151:398–403. doi: 10.1016/j.surg.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Inotsume-Kojima Y, Uchida T, Abe M, Doi T, Kanayama N. A combination of subcuticular sutures and a drain for skin closure reduces wound complications in obese women undergoing surgery using vertical incisions. J Hosp Infect. 2011;77:162–165. doi: 10.1016/j.jhin.2010.07.016. [DOI] [PubMed] [Google Scholar]


