Abstract
AIM: To evaluate a new immunohistological marker, annexin A1 (ANXA1), in cholangiocarcinoma (CCA) and hepatocellular carcinoma (HCC).
METHODS: Expression of ANXA1 protein was investigated in liver tissues from patients with CCA and HCC by immunohistochemistry. Its expression on differences stages of tumor development was investigated in hamster CCA tissues induced by Opisthorchis viverrini and N-nitrosodimethylamine. Moreover, mRNA expression of ANXA1 was assessed in CCA cell lines by quantitative real-time polymerase chain reaction and silencing of ANXA1 gene expression using small interfering RNA.
RESULTS: In human CCA tissue arrays, immunohistochemical analysis revealed that the positive expression of ANXA1 was 94.1% (64/68 cases) consisting of a high expression (66.2%, 45/68 cases) and a low expression (33.8%, 23/68 cases). However, expression of ANXA1 protein was negative in all histologic patterns for HCC (46/46 cases) and healthy individuals (6/6 cases). In hamster with opisthorchiasis-associated CCA, the expression of ANXA1 was observed in the cytoplasm of inflammatory cells, bile duct epithelia and tumor cells. Grading scores of ANXA1 expression were significantly increased with tumor progression. In addition, mRNA expression of ANXA1 significantly increased in all of the various CCA cell lines tested compared to an immortalized human cholangiocyte cell line (MMNK1). Suppressing the ANXA1 gene significantly reduced the matrix metalloproteinase (MMP) 2 and MMP9, and transforming growth factor-β genes, but increased nuclear factor-κB gene expression.
CONCLUSION: ANXA1 is highly expressed in CCA, but low in HCC, suggesting it may serve as a new immunohistochemical marker of CCA. ANXA1 may play a role in opisthorchiasis-associated cholangiocarcinogenesis.
Keywords: Cholangiocarcinoma, Opisthorchis viverrini, Hepatocellular carcinoma, Annexin A1, Biomarker
INTRODUCTION
Cholangiocarcinoma (CCA) is the leading cancer cause of death in northeastern Thailand. The incidence of CCA, a bile duct cancer, from the Khon Kaen Cancer Registry, Thailand, between 1985 and 2009 was 44.3 per 100000 in the males and 17.6 per 100000 in the females[1] which was the highest incidence rate in Southeast Asia[2,3], while its incidence is quite low in European countries[4]. The high incidence of CCA is found mainly in persons over 35 years of age and varies from 93.8 to 317.6 per 100000 person-years[5]. The incidence of mainly the intrahepatic type of CCA most typical in the northeastern part of Thailand is associated with the high prevalence of opisthorchiasis caused by Opisthorchis viverrini (O. viverrini) infection[5,6]. Because CCA is lacking specific symptoms and with no early diagnostic markers, the patients often present at late onset when the disease is in the advanced stage and the patients end up with a poor prognosis[7]. After surgery, no effective drug treatment is available[8] resulting in short survival outcomes[8,9].
The histologic distinction between CCA and hepatocellular carcinoma (HCC) is difficult due to heterogeneity and similarities in morphology[10-13]. Although several immunohistochemical markers such as cytokeratin (CK) 7, CK20, and hepatocyte paraffin 1 (HepPar1) are widely used to distinguish CCA and HCC, these markers can be expressed by both cancers[14,15]. Therefore, novel diagnostic markers for diagnostic differentiation of primary liver tumors are required.
Since the liver fluke associated with intrahepatic CCA is believed to have an immunopathological effect[6,16], a proteomics based approach was used to search for protein alterations during the host-parasite interaction in an in vitro study. A candidate molecule namely annexin A1 (ANXA1) was found which significantly upregulated persistently during the long-term host-parasite interaction. ANXA1 has diverse functions including the regulation of cell division, proliferation, apoptosis and cell growth. Its function participates in stimulation of epithelial cell motility which is crucial in the development of metastasis by disruption of cell morphology[17]. To date, however, the expression of ANXA1 in CCA and its relationship with clinicopathologic factors is unclear. Up-regulation[18] and down-regulation[19] expressions of ANXA1 have been found in sporadic CCA. Similar to in HCC, its expression is controversial, both up-regulation[20,21] and down-regulation[22] have been reported.
In the present study, the expression of ANXA1 in tumor tissues of intrahepatic CCA and HCC patients and its relationship with the clinicopathologic factors was investigated by immunohistochemical staining. In addition, opisthorchiasis-associated CCA in hamsters at the different stages of tumor development was assessed. The expression and regulation in CCA cell lines of ANXA1 was also determined in vitro using small interfering RNA (siRNA) to suppress the ANXA1 gene.
MATERIALS AND METHODS
Patient tissue samples
Tissue microarrays (TMAs) were constructed from archival paraffin embedded tissue samples of 68 intrahepatic CCA patients, 46 HCC patients and tissues from 6 normal healthy livers. These patients underwent liver resection at Srinagarind Hospital, Khon Kaen University, Thailand during 1999-2010. Diagnosis of both CCA and HCC patients were evaluated by clinical data, imaging analysis, tumor markers, and pathology. Immunohistochemical studies for pathological diagnosis included antibodies to CK7, cancer antigen or carbohydrate antigen 19-9 (CA19-9), HepPar1 and alpha-fetoprotein (AFP). The tumor tissues were verified based on the following criteria: as CCA when either CK7+ or HepPar1-, with or without CA19-9+, were found; or as HCC when either HepPar1+ or CK7-, with or without AFP+, were found. The study protocol was approved by The Human Research Ethics Committee, Khon Kaen University, Thailand (HE551407).
TMA and immunohistochemistry
TMAs of 68 O. viverrini-associated CCA cases and 46 HCC cases were generated manually from the paraffin-embedded tissues. In brief, four randomly selected regions from each paraffin block were identified on a hematoxylin-eosin (HE)-stained slide, after which the slide was aligned with the surface of the original paraffin block to locate the sampling areas. The designated areas in the paraffin block were punched with a 1-mm-diameter needle before each punched tissue was then manually transferred to a new recipient paraffin block to generate a TMA block. Five-micrometer-thick sections were cut from the TMA block, and applied on silane-coated slides (Sigma, St. Louis, MO, United States). After TMA construction, to confirm the presence of intact tumor tissue, a HE stained section of the TMA block was prepared and reviewed by two independent pathologists.
An immunohistochemical reaction was performed on 5 μm-thick sections of TMA on silane-coated slides (Sigma) by an immunoperoxidase method. Tissue samples were deparaffinized and dehydrated before the endogenous peroxidase activity was blocked by adding 3% H2O2 in phosphate buffer saline (PBS) for 30 min. After washing with PBS, pH 7.4, and blocking with 2% skim milk in PBS, pH 7.4, for 30 min, the samples were incubated with rabbit polyclonal anti-ANXA1 antibody (1:100, Santa Cruz, Heidelberg, Germany) diluted in 2% nonfat dried milk, followed by a horseradish peroxidase (HRP)-conjugated secondary antibody (1:400, GE healthcare, Piscataway, NJ, United States). The appearance of the brown color corresponding to the peroxidase activity was developed using 3,3-diaminobenzidine tetrahydrochloride as a chromogen and counterstained with Mayer’s hematoxylin. Negative controls were performed in a similar manner but omitting the primary antibody. The ANXA1 staining was scored based on signal intensity and positive area as follows: negative, < 10%; weak (+), 10%-25%; moderate (++), 26%-75%; and strong (+++), > 75%. Consensus evaluation from at least two of the three investigators was considered acceptable.
Animal tissue samples
The Animal Ethics Committee of Khon Kaen University, Thailand approved the study protocols for the animal experiments (AEKKU 17/2552). Thirty male Syrian golden hamsters (Mesocricetus auratus) aged between 4 and 6 wk were obtained from the Animal Unit, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand. Animals were divided into two groups; normal control group and infection with O. viverrini plus N-nitrosodimethylamine (NDMA) (O. viverrini + NDMA). In the treated group, hamsters induced by infection with O. viverrini metacercariae isolated from naturally infected cyprinid fish by pepsin (Wako, Japan) digestion and subsequently treated with 12.5 ppm NDMA at the same time point as described previously[23]. NDMA was given in drinking water for 2 mo, and withdrawn thereafter until the animals were sacrificed at 21 d, and then at 3 and 6 mo post-treatment (n = 5 for each sub-group). Animals were anaesthetized and killed with an overdose of diethyl ether. The liver was dissected and was placed in 10% buffered formalin and used to evaluate histopathological changes and immunohistochemical studies[23].
Cell lines and cell culture
Four human CCA cell lines, namely M156, M055, M213 and M214 were isolated from intrahepatic CCA patients from northeastern Thailand. CCA tissues were characterized as M156, M055 and M214 moderately differentiated CCA and M213 adenosquamous cell carcinoma. Those CCA cell lines were used to assess ANXA1 gene expression and compared to an immortalized human cholangiocyte cell line (MMNK1). In addition, the M214 CCA cell line was used to verify ANXA1-related molecule regulation. These CCA cell lines were kindly provided by Associate Professor Banchop Sripa. All cell culture materials (media, serum and antibiotics) were purchased from Gibco, Invitrogen (Auckland, New Zealand). The CCA cells were cultured in HAM’s F-12 medium supplemented with 10% heat-inactivated fetal bovine serum, 1% Penicillin-streptomycin, 1.176 g/L sodium bicarbonate and adjusted to pH 7.1 with 1 mol/L HCl. The cells were incubated at 37 °C under a humidified 5% CO2 atmosphere.
Western blot analysis
Protein was extracted from the CCA cell lines and the concentration was measured by the Bradford assay (Bio-Rad, Hercules, United States) according to the manufacturer’s instructions. Twenty micrograms of protein were separated on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred to a polyvinylidene difluoride membrane (Amersham Bioscience, Piscataway, NJ, United States) for 2 h at 60 V. The membrane was incubated overnight at 4 °C with rabbit polyclonal anti-ANXA1 antibody (1:1000, Santa Cruz, Heidelberg, Germany) diluted in 2% nonfat dried milk/phosphate buffered saline with Tween 20 (PBS-T). Subsequently, the membrane was incubated with an appropriate HRP-conjugated secondary antibody (1:3000, GE healthcare) diluted in 2% nonfat dried milk/PBS-T. The immunoreactive materials were developed by enhanced chemiluminescence using the ECL Western blotting Detection Reagent (GE Healthcare).
Quantitative real-time reverse transcription-polymerase chain reaction
Total RNA extraction was performed from various CCA cell lines (3 × 105 cells) of each experiment using the TRIzol reagent (Invitrogen, Carlsbad, CA, United States). An aliquot of total RNA was reverse transcribed into cDNA using reverse transcriptase (Invitrogen) following the manufacturer’s protocol. Polymerase chain reaction (PCR) was carried out in duplicate in a 20-μL volume using Faststart Universal SYBR Green Master (ROX, Roche Applied Science, Penzberg, Germany) using the following sets of primers: matrix metalloproteinase 2 (MMP2) (5′-TTGATGGCATCGCTCAGATC-3′ and 5′-CTGCGAAGAACACAGCCTTC-3′), MMP9 (5′-CATTGTCATCCAGTTTGGT G-3′ and 5′-ACCACAACTCGT CGTCGTC-3′), transforming growth factor (TGF)-β (5′-ACATCGACTTTCGCAAGGAC-3′ and 5′-TGGTTGTAG AGGGCAAGGAC-3′), nuclear factor-κB (NF-κB) (5′-GCTTTGCAAACCTGGGAATA-3′ and 5′-CAAGG TCAGAATGCACCAGA-3′), and GAPDH (5’-AGAAGACTGTGGATGGCCCC-3’ and 5’-TGACCTTGCCCACAGCCTT-3’). Reactions were performed in the ABI 7500 thermal cycler (Applied Biosystems, Foster City, CA, United States). All data were analyzed using 7500 system software with a cycle threshold (Ct) in the linear range of amplification and then processed by the 2‾ΔΔCt method.
Knockdown of ANXA1 using siRNA against the ANXA1 gene
The M214 CCA cells were transiently transfected with siRNA against the ANXA1 gene (Silencer® Select siRNA; siRNA ID s1380, Ambion, TX, United States) using the lipofectamine™ 2000 transfection reagent (Invitrogen) following the manufacturer’s protocol with some modifications. In brief, 5 μL of 5 μmol/L stock concentration siRNA or scrambled siRNA and 5 μL lipofectamine™ 2000 transfection reagent were separately diluted in 250 μL OPTI-MEM I medium (Invitrogen). The diluted siRNA solution was mixed with the diluted transfection reagent and incubated for 20 min at room temperature before being added to a six-well plate seeded with 1 × 105 CCA cells in 2 mL transfection medium. The level of ANXA1 mRNA was accessed at 48 and 72 h after transfection. The negative controls were performed by using Silencer® Negative Control siRNA No. 1 (Ambion), a non-targeted sequence.
Statistical analysis
The data were expressed as mean ± SD. To compare the data between groups, statistical significance was determined by Student’s t-test. The χ2 test was used to analyze the correlation between ANXA1 expression and the categorical variables regarding clinicopathological parameters. Survival analysis was done by Kaplan-Meier and log-rank tests. Statistical analyses were performed using SPSS version 15 (SPSS, Inc, Chicago, IL). A P value of less than 0.05 was considered statistically significant.
RESULTS
Expression of ANXA1 as a potential diagnostic marker for CCA but not HCC
ANXA1 expression in TMA obtained from 68 CCA and 46 HCC patients was examined using immunohistochemistry. Expression of ANXA1 was observed in the cytoplasm of epithelial bile duct tumor cells and some of inflammatory cells but was seen faintly or with no staining in tumor stroma and in hepatocytes (Figure 1). Sixty-four cases were positive (94.1%, 64/68) for ANXA1 expression. A high expression was coded in 21 cases for ++ and 24 cases for +++ or 66.2% (45/68 cases) and low expression where 4 cases were negative and 19 cases for + or 33.8% (23/68 cases) (Table l; Figure 1). The histological feature in the tubular type showed that high expression (78.8%, 26/33) was significantly higher than low expression (21.2%, 7/33) (P < 0.05) (Table 1).
Figure 1.
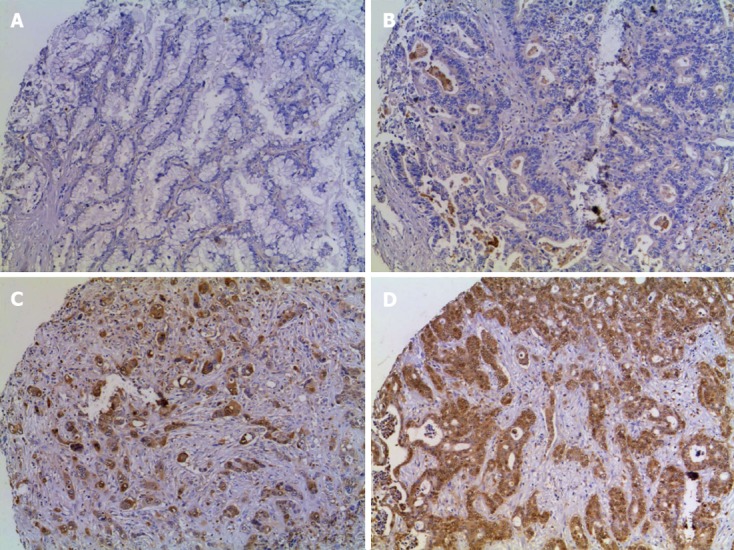
Immunostaining of annexin A1 protein expression in cholangiocarcinoma tissue microarrays. A-D: Tissue microarray of cholangiocarcinoma samples was stained with anti-annexin A1 antibody and counter stained with Mayer's hematoxylin and represented by A for negative, B for +, C for ++, and D for +++ when expression was < 10%, 10%-25%, 26%-75% and > 75%, respectively (magnification, × 200).
Table 1.
Clinical-pathological variables and the expression status of annexin A1 in cholangiocarcinoma tissues
| Variables |
Annexin A1 |
P value | ||
| Low | High | Total | ||
| Age (yr) | 0.328 | |||
| ≤ 56 | 11 | 16 | 27 | |
| > 56 | 12 | 29 | 41 | |
| Gender | 0.487 | |||
| Male | 15 | 33 | 48 | |
| Female | 8 | 12 | 20 | |
| Histopathologic feature | 0.033 | |||
| Tubular type | 7 | 26 | 33 | |
| Papillary type | 16 | 19 | 35 | |
| The intrahepatic location | 0.197 | |||
| Peripheral | 11 | 16 | 27 | |
| Hilar | 11 | 29 | 40 | |
| Gallbladder bed | 1 | 0 | 1 | |
| Tumor size (cm) | 0.889 | |||
| < 3 | 5 | 10 | 15 | |
| 3-6 | 10 | 17 | 27 | |
| > 6 | 8 | 18 | 26 | |
| Gross type | 0.244 | |||
| Mass forming | 10 | 28 | 38 | |
| Periductal infiltrating | 6 | 9 | 15 | |
| Intraductal | 7 | 7 | 14 | |
| Lymph node metastasis | 0.382 | |||
| Absent | 7 | 21 | 28 | |
| Present | 11 | 20 | 31 | |
When the sum of subset numbers does not match patient totals, data were missing or unavailable.
To determine ANXA1 expression distinguishing CCA from HCC, ANXA1 expression was determined in TMA of 46 HCC patients. Slight expression of ANXA1 was observed in the cytoplasm of some inflammatory cells, but was seen faintly or with no staining in tumor cells and hepatocytes. All histologic patterns of HCC samples 100% (46/46) were negative or of low expression (44 for negative and 2 for +) as shown in Figure 2. In addition, no staining or faintly stained immunoreactivity in normal liver tissues of healthy individuals (6/6) was also found. ANXA1 protein as an immunohistological marker could detect CCA and differential CCA from HCC in the primary liver cancer similar to CK7 and CA19-9 (Figure 3). ANXA1 immunohistochemistry showed high sensitivity (94%) and specificity (100%) and the positive prediction value was 100%.
Figure 2.
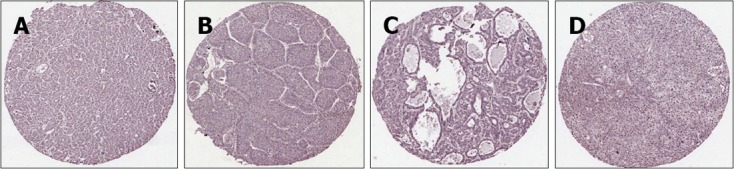
Immunostaining of annexin A1 in hepatocellular carcinoma tissue microarrays. Tissue microarray of hepatocellular carcinoma (HCC) samples was stained with anti-annexin A1 (ANXA1) antibody and counter stained with Mayer's Hematoxylin. ANXA1 shows low expression in all histologic patterns of HCC. A: Trabecular pattern; B: Broad trabecular pattern; C: Trabecular with pseudoacinar pattern; D: Solid growth pattern (magnification, × 40).
Figure 3.
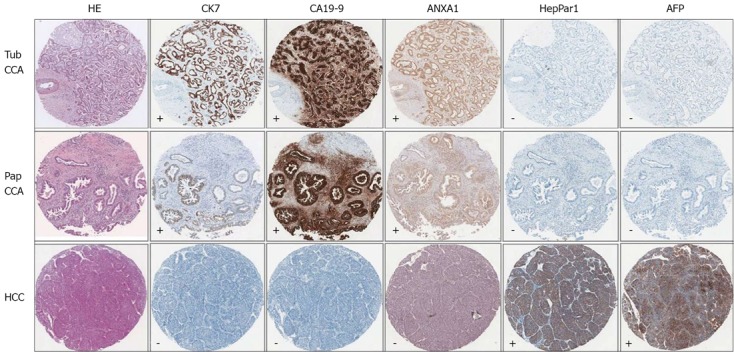
Illustration of the comparative immunohistochemical stains in 3 representative cases. The 3 representative cases include tubular type cholangiocarcinoma (CCA) (Tub CCA), papillary type CCA (Pap CCA) and hepatocellular carcinoma (HCC). Immunopositivity of cytokeratin 7 (CK7), annexin A1 (ANXA1), hepatocyte paraffin 1 (HepPar1) and alpha-fetoprotein (AFP) appeared as brown cytoplasmic staining of tumor cells, while positivity to carbohydrate antigen 19-9 (CA19-9) appeared as cytoplasmic and luminal staining of tumor (magnification, × 40). Noted that CCA are CK7+/CA19-9+/ANXA1+ and HCC is HepPar1+/AFP+/ANXA1-. -: Negative result; +: Positive result.
The correlation between ANXA1 expression and clinicopathological parameters was analyzed as shown in Table 1. There were no correlations between ANXA1 expression level and age, sex, tumor location, tumor size, gross type, lymph node metastasis (Table 1) and patients’ survival outcome (data not shown). Notably, high ANXA1 expression levels were positively correlated with the histological features of the tubular type (P = 0.03) but not the papillary type. All 26 cases of high expression in the tubular type had positive lymph nodes.
Expression of ANXA1 in hamsters CCA tissues
To evaluate the expression of ANAX1 in the different stages of CCA tissues in O. viverrini + NDMA-induced CCA in the hamster model, immunohistochemistry was used to evaluate the inflamed tissues at 21 d, when the tumors began at 3 mo, and tumor progression at 6 mo post-treatment. The percentage of CCA 60% (3/5) at 3 mo, and 100% (5/5) at 6 mo post-treatment were described previously[23]. Here, it was shown that the expression of ANXA1 was observed mainly in the cytoplasm of the epithelial bile ducts, some inflammatory cells (large cell, macrophage-like cell), and tumor cells which increased with time as bile duct proliferation and CCA development progressed (Figure 4). The level of its expression at 21 d was +, 3 mo was ++, and 6 mo was +++ for all five animals per group. In addition, low or a little expression was found in the normal livers with no changes in each time-sacrificed group.
Figure 4.
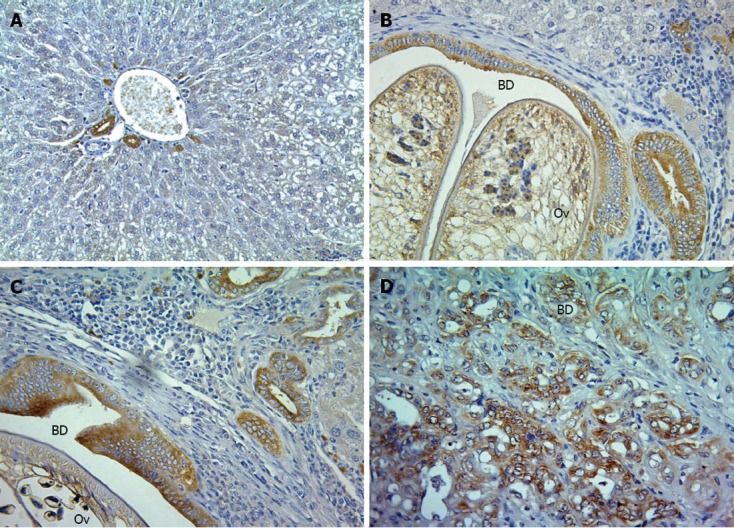
Immunostaining of annexin A1 protein expression in hamster tissues. A-D: Hamster tissues were stained with anti-annexin A1 antibody and counterstained with Mayer's hematoxylin represented by negative for A (normal at 6 mo post-treatment), grade + for B (21 d post-treatment), grade ++ for C (3 mo post-treatment) and grade +++ for D (6 mo post-treatment) (magnification, × 200). Ov: Opisthorchis viverrini; BD: Bile duct lumen.
Expression of ANXA1 and the effect of its gene inhibition in CCA cell lines
Since ANXA1 was positive in CCA tissue but not in HCC tissue, the expression was further confirmed in various intrahepatic human CCA cell lines compared to MMNK1. Real-time reverse transcription (RT)-PCR revealed that a significantly increased expression of the ANXA1 gene was observed in all four CCA cell lines including M156, M055, M213 and M214 compared to in MMNK1 (P < 0.01) (Figure 5).
Figure 5.
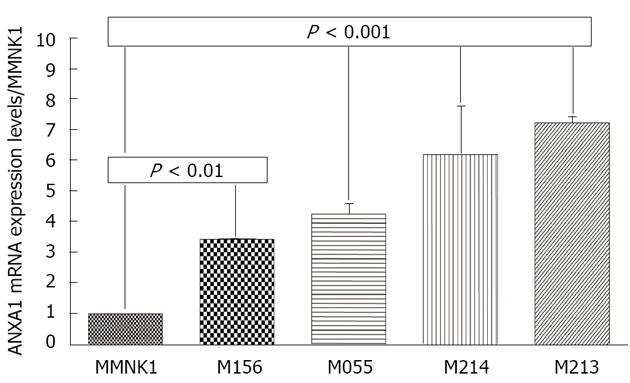
Expression of the annexin A1 gene in various cholangiocarcinoma cell lines. The mRNA expression level of annexin A1 (ANAX1) was evaluated by real-time reverse transcription-polymerase chain reaction in various cholangiocarcinoma (CCA) cell lines including M156, M055 and M214 moderately differentiated CCA, M213 adenosquamous cell carcinoma and compared to an immortalized human cholangiocyte cell line (MMNK1). Data are derived from duplicate independent experiments and presented as mean ± SD, P < 0.01, P < 0.001 vs MMNK1 is significantly different by the Student t-test.
In addition, ANXA1 was also investigated whether it was involved in MMPs in CCA metastasis by silencing ANXA1 using siRNA to target of ANXA1 gene expression in M214 CCA cells. Real-time RT-PCR and Western blotting analysis showed that the mRNA level of cells transfected with siRNA at 48 h was suppressed by 87.3% and the protein level was decreased by 80% compared to that of controls (Figure 6). In vitro proliferation of the ANXA1 silenced cells was also reduced (data not shown). Suppression of the ANXA1 gene significantly inhibited the mRNA expression of MMP2 by 50% and MMP9 by 45% (P < 0.05), and TGF-β by 15%, but induced NF-κB by 70% (Figure 7).
Figure 6.
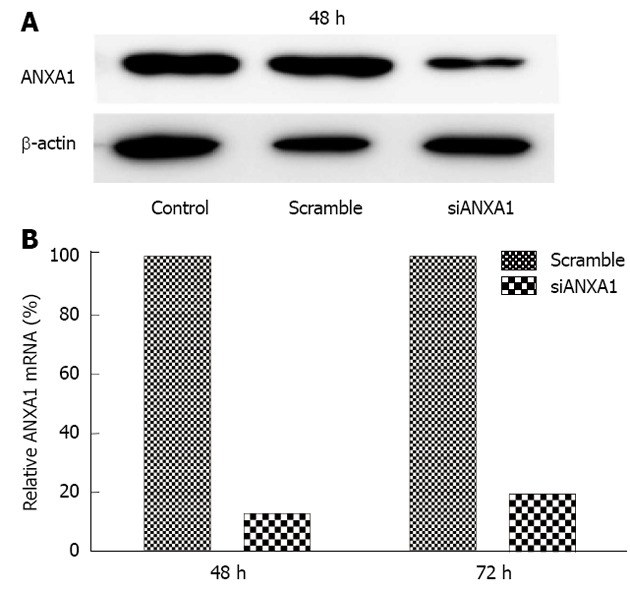
Effect of annexin A1 knockdown on the M214 cholangiocarcinoma cell line. A, B: Suppression of annexin A1 (ANXA1) expression at the translational level (at 48 h) and transcriptional level (at 48 and 72 h) in knockdown M214 cells was evaluated by Western blotting (A) and real-time reverse transcription-polymerase chain reaction (B).
Figure 7.
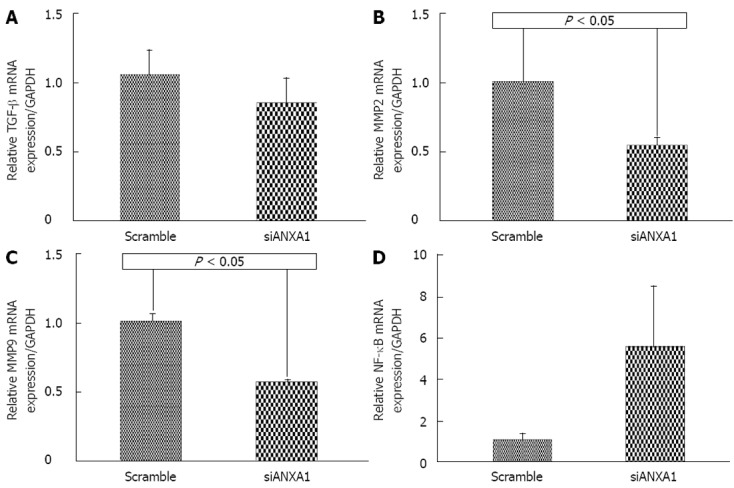
Effect of gene expression in the knockdown of the annexin A1 gene in the M214 cholangiocarcinoma cell line. A: Relative transforming growth factor-β (TGF-β) mRNA expression; B: Relative matrix metalloproteinase (MMP) 2 mRNA expression; C: Relative MMP9 mRNA expression; D: Relative nuclear factor (NF)-κB mRNA expression. Real-time reverse transcription-polymerase chain reaction was used to confirm the expression of TGF-β, MMP2, MMP9 and NF-κB in the knockdown of the annexin A1 (ANXA1) gene in the M214 cholangiocarcinoma cell line relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data are derived from duplicate independent experiments and presented as mean ± SD, and P < 0.05 vs scramble is significantly different by the Student t-test.
DISCUSSION
The most frequent malignancy among Thais from northeastern Thailand registered between 1985 and 2009 in liver cancers was 42% HCC and 58% CCA[1]. Distinguishing CCA from HCC can be problematic due to heterogeneous morphological. Here, ANAX1, a candidate marker obtained from proteomics was used to investigate CCA in humans and hamsters by immunohistochemistry and tested its regulation expression in an in vitro study. In human TMAs of intrahepatic CCA, expression of ANXA1 protein was highly expressed (94.1%, 64/68), similar to a previous study (83.3%, 10/12) in sporadic intrahepatic CCA[18]. An increased expression of the ANXA1 gene was verified in all of four CCA cell lines, supporting its over-expression in CCA tissue. In contrast, a decreased expression of ANXA1 is a common event in extrahepatic CCA and is significantly correlated with a poorer outcome in Chinese patients[19], indicating that its expression is varied according to tumor location. Moreover, down-regulation of ANXA1 expression was found in the HCC tissue array reported by Xue et al[22] but opposite from the previous report in transgenic mice with HCC[20] and in a human HCC cell line[21]. Therefore, expression of ANXA1 has different expression in primary liver cancer. With the current results and previous study as evidence, this indicates that ANXA1 is a new immunohistochemical marker to distinguish between CCA and HCC. Nevertheless, more studies in sporadic CCA are warranted.
ANXA1 is bound to cellular membranes in a Ca2+-dependent manner. It is a glucocorticoid-induced protein with multiple actions in the regulation of inflammatory cell activation, cellular processes and involved in carcinogenesis[24]. Recently, its expression has been proposed for the regeneration of skeletal muscle tissue[25] and in liver regeneration and transformation[20]. In hamster CCA, ANXA1 expression has been observed mainly in bile duct epithelia and increasing with bile duct proliferation and bile duct cancer progression in this current study. It may imply that ANXA1 may contribute to regenerate bile duct injury triggered by the fluke and NDMA-mediated CCA. Likewise, in mice after hepatectomy, regeneration of hepatocytes and bile ducts may lead to activate ANXA1 expression in the liver[20].
In addition, although its expression was not positively correlated with patients’ survival, and lymph node invasion, most cases of a high expression were found in the tubular type having positive lymph nodes, implying that it may be involved in tumor invasion[26] and specific for the tubular type but not in the papillary type. ANXA1 may regulate MMPs expression for CCA progression. The in vitro study revealed that ANXA1 in CCA cell lines of intrahepatic CCA patients was positively correlated with TGF-β and MMP2 and MMP9, but had a negative relationship with NF-κB. ANXA1 inhibits NF-κB, a key regulator of inflammation, the common pathophysiological mechanism of inflammatory bowel diseases[27]. These results indicated that ANXA1 functions as a positive regulator of TGF-β, MMP2 and MMP9 expression and invasion of cancer cells through specific activation of the NF-κB signaling pathway[28]. ANXA1 may regulate TGF-β signaling and promote metastasis[29] leading to up-regulation of MMP2 and MMP9[30] to degrade extracellular matrix (ECM) for tumor development and metastasis. The chronological expression of ANXA1 was shown to have different expression levels according to tumor development. An increased expression of ANXA1 with time is likely to be positively correlated with an increased accumulation of fibrosis and ECM, MMP9 expression[23] for maintenance of cytoskeleton and ECM integrity, and differentiation[25] for tumor onset[31] and tumor progression[32].
Recently, expression of ANXA1 was reported in inflamed tissue such as in ulcerative colitis[33] and in chronic granulomatous inflammation which might become activated at different stages of this chronic inflammatory response[34]. Moreover, expression of ANXA1 was correlated with the tumor staging in adenocarcinoma of the esophagus and the esophagogastric junction[35] and bladder cancer[32]. The results of the current study revealed that expression of the ANXA1 level in the inflamed tissues on 21 d was lower than when the tumor began at 3 mo and lower than in the tumor progression at 6 mo post-treatment in the hamster model, this finding may be supported by previous findings. In contrast, in benign breast tissue, myoepithelial cells showed stronger expression of ANXA1 than in tumorous tissue of breast cancer. A decreased expression of ANXA1 is correlated with breast cancer development and progression[36].
In conclusion, strong expression of ANXA1 was observed in CCA, but was low in HCC. In diagnosis of primary liver cancer, ANXA1 could be a new immunohistochemical marker for differential diagnosis of CCA from HCC. ANXA1 expression increased along with cholangiocarcinogenesis in hamsters induced by O. viverrini infection and NDMA administration. Its expression was positively correlated with TGF-β and MMPs but negatively correlated with the NF-κB signaling pathway, suggesting that ANXA1 is involved in inflammation-associated CCA, and a potential for therapeutic drug targeting.
ACKNOWLEDGMENTS
We thank Miss Orapan Kingchaiyaphum, Research Assistant, Faculty of Medicine, Khon Kaen University, Thailand, for her technical support. We thank the Publication Clinic, Research and Technology Transfer Affairs, Khon Kaen University, for their assistance. We also thank Professor Yukifumi Nawa, Research Affairs, Faculty of Medicine, Khon Kaen University, Thailand, for his suggestion and critical reading the manuscript.
COMMENTS
Background
Cholangiocarcinoma (CCA) is associated with late presentation, has high mortality rate, and poses challenges for diagnostic. The histological CCA is difficult distinguish from hepatocellular carcinoma (HCC). New diagnostic markers with better diagnostic differentiation are required. Annexin A1 (ANXA1) is a multipotent protein involved in several functions including regulation of cell differentiation, apoptosis, and carcinogenesis. ANXA1 involved in this process is frequently overexpressed in many types of cancers. Moreover, its expression in the same cancer is controversial.
Research frontiers
Although the expression of ANXA1 has been demonstrated in many types of cancers, its expression in CCA and HCC is controversial. Moreover, its expression at different stages of CCA development and its regulation in CCA has not been reported.
Innovations and breakthroughs
In this study, strong expression of ANXA1 was observed in CCA, but was low in HCC, which was similar to the available markers for CCA (cytokeratin 7 and carbohydrate antigen 19-9), implying its expression could present as a new marker for differential diagnosis of primary liver cancer. ANXA1 increased expression along with cholangiocarcinogenesis in hamsters induced by Opisthorchis viverrini infection and N-nitrosodimethylamine administration. Its expression was positively correlated with transforming growth factor-β (TGF-β) and matrix metalloproteinase (MMP) but negatively with the nuclear factor κB (NF-κB) signaling pathway. ANXA1 is involved in carcinogenesis of chronic inflammation related-CCA. ANXA1 is a promising biomarker and a potential for a therapeutic target in this aggressive cancer.
Applications
This study demonstrated that the ANXA1 protein was expressed in CCA, but had low expression in HCC and therefore it may provide a new diagnostic marker in CCA by the immunohistochemistry technique. By silencing of ANXA1 gene expression in the in vitro study the results demonstrate that ANXA1 represents a potential therapeutic target for the future intervention in CCA patients.
Terminology
Annexin A1 (also named macrocortin, renocortin, lipomodulin and lipocortin 1) is a 37 kDa protein member of an annexin superfamily which has a binding or annexing property to acidic phospholipid in a calcium dependent manner. It also has been shown to play a critical role in the regulation of inflammatory cell activation, cellular processes and involved in carcinogenesis.
Peer review
This is a good paper with sensible use of different experimental techniques. The authors examined the expression of ANXA1 in CCA and HCC tissues microarrays; ANXA1 protein had high sensitivity and specificity for immunohistochemistry diagnosis in CCA. Suppression of ANXA1 gene expression showed it inhibiting of TGF-β and MMPs but induction NF-κB expression. The results are interesting, represent a new immunohistochemistry analysis of CCA and ANXA1 is a promising therapeutic potential of drugs targeting in CCA.
Footnotes
Supported by The Commission on Higher Education, Thailand (Strategic Scholarships for Frontier Research Network for the PhD Program Thai Doctoral degree); Khon Kaen University Research Foundation, Invitation Research from Faculty of Medicine, No. I55113; The Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Thailand
P- Reviewers McKay SC, Ozmen O S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Kamsa-ard S, Wiangnon S, Suwanrungruang K, Promthet S, Khuntikeo N, Kamsa-ard S, Mahaweerawat S. Trends in liver cancer incidence between 1985 and 2009, Khon Kaen, Thailand: cholangiocarcinoma. Asian Pac J Cancer Prev. 2011;12:2209–2213. [PubMed] [Google Scholar]
- 2.Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, Wiangnon S, Sripa B, Hong ST. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–585. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, Mulvenna J, Laha T, Hotez PJ, Brindley PJ. Opisthorchiasis and Opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011;120 Suppl 1:S158–S168. doi: 10.1016/j.actatropica.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Sriamporn S, Pisani P, Pipitgool V, Suwanrungruang K, Kamsa-ard S, Parkin DM. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588–594. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 6.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–356. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8:189–200. doi: 10.1038/nrgastro.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seyama Y, Makuuchi M. Current surgical treatment for bile duct cancer. World J Gastroenterol. 2007;13:1505–1515. doi: 10.3748/wjg.v13.i10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khuntikeo N, Pugkhem A, Bhudhisawasdi V, Uttaravichien T. Major hepatic resection for hilar cholangiocarcinoma without preoperative biliary drainage. Asian Pac J Cancer Prev. 2008;9:83–85. [PubMed] [Google Scholar]
- 10.Nakajima T, Kondo Y. Well-differentiated cholangiocarcinoma: diagnostic significance of morphologic and immunohistochemical parameters. Am J Surg Pathol. 1989;13:569–573. [PubMed] [Google Scholar]
- 11.Jovanovic R, Jagirdar J, Thung SN, Paronetto F. Blood-group-related antigen Lewis(x) and Lewis(y) in the differential diagnosis of cholangiocarcinoma and hepatocellular carcinoma. Arch Pathol Lab Med. 1989;113:139–142. [PubMed] [Google Scholar]
- 12.Ganjei P, Nadji M, Albores-Saavedra J, Morales AR. Histologic markers in primary and metastatic tumors of the liver. Cancer. 1988;62:1994–1998. doi: 10.1002/1097-0142(19881101)62:9<1994::aid-cncr2820620920>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Sampatanukul P, Leong AS, Kosolbhand P, Tangkijvanich P. Proliferating ductules are a diagnostic discriminator for intrahepatic cholangiocarcinoma in FNA biopsies. Diagn Cytopathol. 2000;22:359–363. doi: 10.1002/(sici)1097-0339(200006)22:6<359::aid-dc6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Rullier A, Le Bail B, Fawaz R, Blanc JF, Saric J, Bioulac-Sage P. Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol. 2000;24:870–876. doi: 10.1097/00000478-200006000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Kakar S, Gown AM, Goodman ZD, Ferrell LD. Best practices in diagnostic immunohistochemistry: hepatocellular carcinoma versus metastatic neoplasms. Arch Pathol Lab Med. 2007;131:1648–1654. doi: 10.5858/2007-131-1648-BPIDIH. [DOI] [PubMed] [Google Scholar]
- 16.Yongvanit P, Pinlaor S, Bartsch H. Oxidative and nitrative DNA damage: key events in opisthorchiasis-induced carcinogenesis. Parasitol Int. 2012;61:130–135. doi: 10.1016/j.parint.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, Nusrat A. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem. 2006;281:19588–19599. doi: 10.1074/jbc.M513025200. [DOI] [PubMed] [Google Scholar]
- 18.Wang AG, Yoon SY, Oh JH, Jeon YJ, Kim M, Kim JM, Byun SS, Yang JO, Kim JH, Kim DG, et al. Identification of intrahepatic cholangiocarcinoma related genes by comparison with normal liver tissues using expressed sequence tags. Biochem Biophys Res Commun. 2006;345:1022–1032. doi: 10.1016/j.bbrc.2006.04.175. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Zhang H, Fang Z, Yu G. Annexin-1 downregulation is associated with clinical outcome in Chinese patients with hilar cholangiocarcinoma. Eur Surg Res. 2010;45:151–157. doi: 10.1159/000320237. [DOI] [PubMed] [Google Scholar]
- 20.de Coupade C, Gillet R, Bennoun M, Briand P, Russo-Marie F, Solito E. Annexin 1 expression and phosphorylation are upregulated during liver regeneration and transformation in antithrombin III SV40 T large antigen transgenic mice. Hepatology. 2000;31:371–380. doi: 10.1002/hep.510310217. [DOI] [PubMed] [Google Scholar]
- 21.Masaki T, Tokuda M, Ohnishi M, Watanabe S, Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M, et al. Enhanced expression of the protein kinase substrate annexin in human hepatocellular carcinoma. Hepatology. 1996;24:72–81. doi: 10.1053/jhep.1996.v24.pm0008707286. [DOI] [PubMed] [Google Scholar]
- 22.Xue LY, Teng LH, Zou SM, Ren LQ, Zheng S, Luo W, Bi R, Lü N. [Expression of annexin I in different histological types of carcinomas] Zhonghua Zhongliu Zazhi. 2007;29:444–448. [PubMed] [Google Scholar]
- 23.Prakobwong S, Yongvanit P, Hiraku Y, Pairojkul C, Sithithaworn P, Pinlaor P, Pinlaor S. Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int J Cancer. 2010;127:2576–2587. doi: 10.1002/ijc.25266. [DOI] [PubMed] [Google Scholar]
- 24.Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9:62–70. doi: 10.1038/nri2470. [DOI] [PubMed] [Google Scholar]
- 25.Bizzarro V, Petrella A, Parente L. Annexin A1: novel roles in skeletal muscle biology. J Cell Physiol. 2012;227:3007–3015. doi: 10.1002/jcp.24032. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Kumamoto K, Saito K, Okayama H, Hayase S, Kofunato Y, Miyamoto K, Nakamura I, Ohki S, Koyama Y, et al. Up-regulated Annexin A1 expression in gastrointestinal cancer is associated with cancer invasion and lymph node metastasis. Exp Ther Med. 2011;2:239–243. doi: 10.3892/etm.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang N, Zhu C, Zhou D, Nie T, Go MF, Richards RJ, Rigas B. MC-12, an annexin A1-based peptide, is effective in the treatment of experimental colitis. PLoS One. 2012;7:e41585. doi: 10.1371/journal.pone.0041585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang H, Ko J, Jang SW. The role of annexin A1 in expression of matrix metalloproteinase-9 and invasion of breast cancer cells. Biochem Biophys Res Commun. 2012;423:188–194. doi: 10.1016/j.bbrc.2012.05.114. [DOI] [PubMed] [Google Scholar]
- 29.de Graauw M, van Miltenburg MH, Schmidt MK, Pont C, Lalai R, Kartopawiro J, Pardali E, Le Dévédec SE, Smit VT, van der Wal A, et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci United States. 2010;107:6340–6345. doi: 10.1073/pnas.0913360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiercinska E, Naber HP, Pardali E, van der Pluijm G, van Dam H, ten Dijke P. The TGF-β/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Cancer Res Treat. 2011;128:657–666. doi: 10.1007/s10549-010-1147-x. [DOI] [PubMed] [Google Scholar]
- 31.Perretti M, Dalli J. Exploiting the Annexin A1 pathway for the development of novel anti-inflammatory therapeutics. Br J Pharmacol. 2009;158:936–946. doi: 10.1111/j.1476-5381.2009.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li CF, Shen KH, Huang LC, Huang HY, Wang YH, Wu TF. Annexin-I overexpression is associated with tumour progression and independently predicts inferior disease-specific and metastasis-free survival in urinary bladder urothelial carcinoma. Pathology. 2010;42:43–49. doi: 10.3109/00313020903434405. [DOI] [PubMed] [Google Scholar]
- 33.Vong L, Ferraz JG, Dufton N, Panaccione R, Beck PL, Sherman PM, Perretti M, Wallace JL. Up-regulation of Annexin-A1 and lipoxin A(4) in individuals with ulcerative colitis may promote mucosal homeostasis. PLoS One. 2012;7:e39244. doi: 10.1371/journal.pone.0039244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliani SM, Ciocca GA, Pimentel TA, Damazo AS, Gibbs L, Perretti M. Fluctuation of annexin-A1 positive mast cells in chronic granulomatous inflammation. Inflamm Res. 2008;57:450–456. doi: 10.1007/s00011-008-7222-7. [DOI] [PubMed] [Google Scholar]
- 35.Wang KL, Wu TT, Resetkova E, Wang H, Correa AM, Hofstetter WL, Swisher SG, Ajani JA, Rashid A, Hamilton SR, et al. Expression of annexin A1 in esophageal and esophagogastric junction adenocarcinomas: association with poor outcome. Clin Cancer Res. 2006;12:4598–4604. doi: 10.1158/1078-0432.CCR-06-0483. [DOI] [PubMed] [Google Scholar]
- 36.Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, Chia D, Seligson D, Chang HR, Goodglick L. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37:1583–1591. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]


