Abstract
AIM: To investigate the expression and prognostic value of carbonic anhydrase II (CA II) and Ki-67 in gastrointestinal stromal tumors (GISTs).
METHODS: One hundred and thirteen GIST patients admitted to Chinese People’s Liberation Army General Hospital from January 2004 to December 2010 were retrospectively followed up, and immunohistochemistry was used to detect CA II, Ki-67 and CD117 expression in tumor samples. The survival rates of the patients were analyzed using the Kaplan-Meier method. Log-rank test, χ2 test and Cox proportional hazards model were used to determine the relationships between CA II, Ki-67 and CD117 expression and prognostic value in GISTs.
RESULTS: The survival rates at 1, 3 and 5 years were 90.0%, 82.0% and 72.0% in all patients. However, in patients with positive CA II or Ki-67, the survival rates were 92.0%, 83.0% and 77.0% or 83.0%, 66.6% and 53.0%, respectively. Compared with the negative groups, the survival rates in the positive groups were significantly lower (CA II log-rank P = 0.000; Ki-67 log-rank P = 0.004). Multivariate Cox analysis revealed that CA II, CD117 and Ki-67 were considerable immune factors in prognosis of GIST patients (CA II P = 0.043; CD117 P = 0.042; Ki-67 P = 0.007). Besides, tumor diameter, mitotic rate, tumor site, depth of invasion, complete resection, intraoperative rupture, and adjuvant therapy were important prognosis predictive factors. Our study indicated that CA II had strong expression in GISTs and the prognosis of GISTs with high CA II expression was better than that of GISTs with low or no expression, suggesting that CA II is both a diagnostic and prognostic biomarker for GIST.
CONCLUSION: CA II and Ki-67 are significant prognostic factors for GISTs. CA II associated with neovascular endothelia could serve as a potential target for cancer therapy.
Keywords: Gastrointestinal stromal tumors, Carbonic anhydrase, CD117, Ki-67, Prognostic factor
Core tip: Gastrointestinal stromal tumors (GISTs) are mesenchymal tumors with a wide spectrum of clinical behavior. This is the first study showing the prognostic significance of carbonic anhydrase II (CA II) and Ki-67. The 1-, 3- and 5-year survival rates were 90.0%, 82.0% and 72.0%. However, in patients with positive CA II or Ki-67, the survival rates were 92.0%, 83.0% and 77.0% or 83.0%, 66.6% and 53.0%, respectively. Our study indicates that CA II has strong expression in GISTs and prognosis with high CA II expression is better than that with low or no expression, suggesting that CA II is both a diagnostic and prognostic biomarker for GIST.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are mesenchymal tumors that arise from the gastrointestinal tract. They show differentiation towards the interstitial cells of Cajal and account for < 1% of all gastrointestinal neoplasms[1]. GISTs positively express discovered on GIST-1 (98%) and CD117 (95%) immune globulin. The estimated incidence of GISTs is 10-20 per million people annually worldwide. The majority of GISTs arise in the stomach (60%), small bowel (30%), esophagus and rectum (10%)[2] and the remainder outside the gastrointestinal tract, comprising a wide spectrum from a curable disorder to highly malignant disease. As far as the molecular markers are concerned, previous studies have revealed that p53, CD147, monocarboxylate transporter 1 (MCT1), DEAD (Asp-Glu-Ala-Asp) box polypeptide 39 (DDX39) and natural killer cell p30 (NKp30) are related to the prognosis of GISTs[3-7]. However, considering that these markers are different from tumor size, mitotic rate or tumor site, and due to their weak correlation, they are often mentioned in recurrence risk of GIST or prediction of patient prognosis.
Carbonic anhydrases (CAs) are a group of zinc-containing metalloenzymes that catalyze the reversible hydration of carbon dioxide, CO2 + H2O <=> HCO3- + H+ and participate in various physiological processes, including respiration, gluconeogenesis, bone resorption, renal acidification, and formation of cerebrospinal fluid and gastric acid[8]. To date, 15 isoforms of human (h) α-CA, 15 enzymatically active α CAs have been identified and characterized in mammals, including five cytoplasmic (CA I, CA II, CA III, CA VII and CA XIII), two mitochondrial (CA VA and CA VB), one secreted (CA VI), four membrane-associated (CA IV, CA IX, CA XII, and CA XIV) forms and three CA-related proteins (CA-RP VIII, CA-RP X and CA-RP XI). These functionally active CA isozymes, having been identified in mammals[9-11], differ in their tissue distribution and enzymatic activity. Furthermore, in the present study, GIST cells demonstrated strong expression of CA-RPs VIII and XI. Overexpressed CA-RP XI is possibly substituted for CA-RP VIII in the cytoplasm and enhances the proliferation and invasion of GIST cells[12]. These enzymes are commonly expressed in malignant tumor cells in which they promote tumor growth by contributing to intracellular alkalization and extracellular acidification[13]. However, because of its wide distribution, high catalytic efficiency, and an important physiological role, CA II has become one of the hot research topics. CA II expression in the cytoplasm is a single polypeptide chain of molecular weight 29 kDa and its gene is located on chromosome 8, 8q22, 760 bp. It is present in most tissues with high enzyme activity, including gastric cancer, liver and bile duct cancer, colon cancer, renal cell carcinoma, melanoma, brain astrocyte tumors, pancreatitis cells in mice[14], and cardiomyocyte hypertrophy[15]. In a recent study, high CA II expression was associated with a better disease-specific survival rate than low or no expression in GIST[16]. Although CA II has been reported to represent potential diagnostic and therapeutic targets in the above cancers, there have been fewer reports discussing the predictive value of prognosis in GIST patients in Asia from a clinicopathological aspect.
Ki-67 is a nuclear marker that is closely related to tumor cell proliferation. It has been found to have a positive correlation with prognosis of various malignant tumors including GIST. One recent study has suggested that Ki-67 is a strong prognostic indicator even though it is less valuable than mitotic rate in GIST[17]. A study by Nakamura et al[18] supported the hypothesis that Ki-67 and risk grade are useful for predicting the aggressive biological behavior of GIST.
The aim of our study was to reveal the relationship between the above two molecular markers (CA II and Ki-67) and prognosis of GIST. As a diagnostic index, CD117 is located in the tumor cell membrane and cytoplasm[19] and has a positive rate as high as 95% in GISTs. The predictive value of CD117 in prognosis was also explored.
MATERIALS AND METHODS
Study population and follow-up
We retrospectively followed up GIST patients who were admitted and operated upon in Chinese People’s Liberation Army General Hospital between January 2004 and December 2010. Clinical follow-up was completed in February 2011. Inclusion criteria were: (1) age ≥ 18 years; (2) GISTs diagnosed by the histopathological and immunohistochemical methods; and (3) not receiving any previous treatment. Exclusion criteria were: (1) female patients with pregnancy or lactation; (2) patients developing other malignancies during the past 5 years; and (3) patients with other serious diseases.
Pathological examination of tumor samples
Paraffin wax sections (5 μm thick) of GIST specimens were dewaxed in xylene and transferred to alcohol. Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in methanol and the sections were subjected to heat-induced antigen retrieval using a microwave oven. Sections were incubated overnight at 4 °C with polyclonal antibodies for CA II, CD117 and Ki-67 (CD117, rabbit anti-human polyclonal antibody, 1:100, Abcam, Cambridge, United Kingdom; Ki67, rabbit polyclonal antibody to proliferation marker, 1:1000, Abcam; CA II, rabbit anti-human polyclonal antibody, 1:100, Abcam). Polyperoxidase-anti-mouse/rabbit immunoglobulin G was applied to the sections for 30 min at 37 °C, followed by detection with 3,3’-diaminobenzidine (Bioss, Beijing, China). The reactions were developed with hematoxylin and were mounted with glue[20,21]. The immunohistochemical reactions were visualized under high-power magnification (× 400) using an Olympus BH2 microscope (Tokyo, Japan). Positive expression for CA II, Ki-67 and CD117 was defined by the percentage of positively stained cells (1 positive; 0 negative). The following scoring assessments for CA II, Ki-67 and CD117 were used. Score 0 was assigned for ≤ 10%, and 1 for > 10% staining positive cells.
Ethics
Approval for the use of clinical material for research was obtained from the hospital ethics committee, along with patient consent.
Statistical analysis
SPSS version 17.0 (SPSS Inc. Chicago, IL, United States) was used for statistical analysis. Analysis was performed assuming a nonparametric distribution using the χ2 test. Actuarial survival rates were evaluated by Kaplan-Meier analysis and log-rank test. Multivariate survival analysis was performed by Cox proportional hazards model. All tests were two-tailed and statistical significance was set at P < 0.05.
RESULTS
Clinical characteristics
A total of 113 GIST patients (61 male, 52 female) with a median age of 60 years were included. Twenty-five patients died from GIST. Median follow-up time was 35.5 mo (1-90 mo).
Expression of CA II, Ki-67 and CD117 in tumor samples
Immunohistochemistry showed that the positive rate for CA II, CD117 and Ki-67 was 87.6% (99/113), 85.8% (97/113) and 65.5% (74/113) in all patients, respectively. CA II protein was strongly expressed in the cytoplasm of GIST cells (Figure 1B). Ki-67 protein was expressed in the nuclei of GIST cells (Figure 1D). In the control group, CA II was negatively expressed in GIST cells (Figure 1C), and only partially expressed in neural astrocytoma, schwannoma, leiomyoma of the stomach, and malignant solitary fibrous tumors (Figure 1E-H). The histopathological type (spindle cell, epithelioid or mixed type) was noted and mitoses were counted using a × 40 objective for 50 high-power fields, as recommended.
Figure 1.
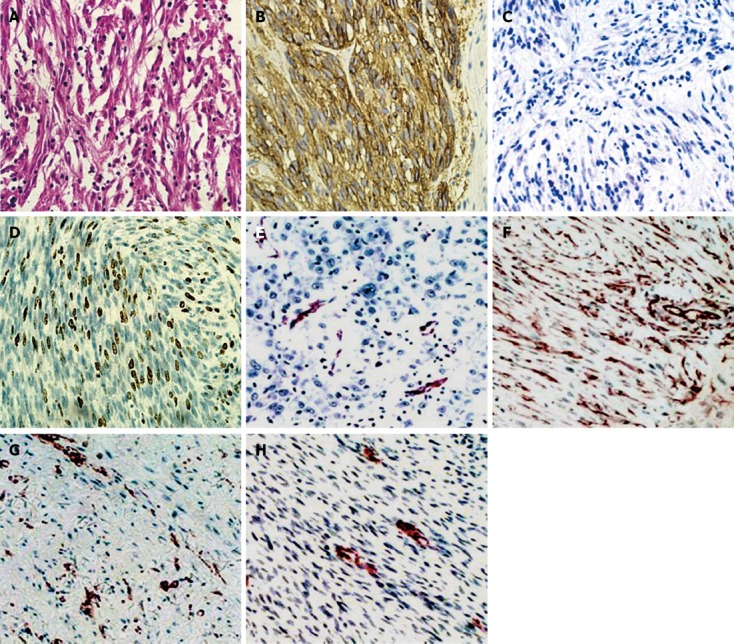
The scale is 5 μm using a × 400 objective as recommended. Carbonic anhydrase II (CA II) and Ki-67 proteins expressed in gastrointestinal stromal tumors (GISTs) and other malignant tumors. A: Hematoxylin and eosin in GISTs; B: CA II positive protein in the GISTs; C: CA II and Ki-67 negative protein in GISTs; D: Ki-67 positive protein in the GISTs; E: CAII positive protein in neural astrocytoma; F: CA II positive protein in schwannoma; G: CA II positive protein in leiomyoma of the stomach; H: CA II positive protein in malignant solitary fibrous tumor.
Relationship between expression of CA II, Ki-67 and CD117 and clinicopathological characteristics of GISTs
The survival analysis for all GIST patients showed that the 1-, 3- and 5-year survival rates were 90.0%, 82.0% and 72.0%. The recurrence rate was 10.6% with a recurrence time of 6-20 mo. The highest survival rate was found in those patients who received complete tumor resection and took imatinib (400 mg/d) postoperatively. However, in those patients who did not undergo complete tumor resection and were not treated with imatinib postoperatively, the survival rate was the lowest. However, in patients with positive CA II or Ki-67 expression, the survival rates were 92.0%, 83.0% and 77.0% or 83.0%, 66.6% and 53.0%, respectively. Considering molecular markers, the survival rates in the CA II-negative group or CD117-negative group were significantly lower than in the positive groups (CA II, log-rank P = 0.000; CD117, log-rank P = 0.000). However, it was higher in the Ki-67-positive group compared to the Ki-67-negative group (log-rank P = 0.004) (Figure 2).
Figure 2.
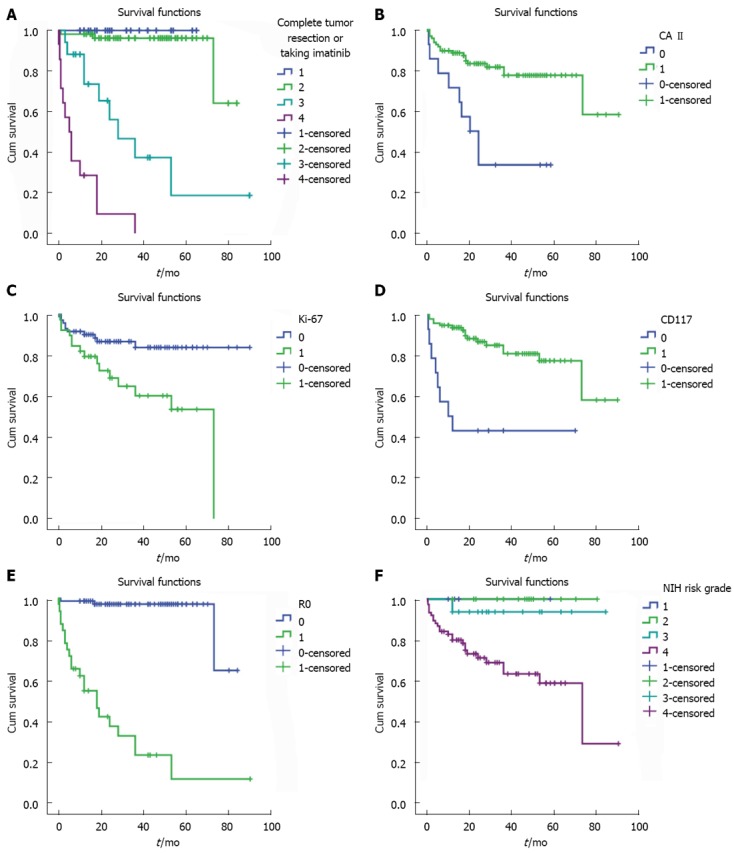
Analysis of survival rates (Kaplan-Meier) and comparison of survival rates in gastrointestinal stromal tumors groups (log-rank test). A: The highest survival rate was found in those patients that received complete tumor resection and postoperatively took imatinib (400 mg/d), while in those patients not receiving complete tumor resection and postoperative imatinib, the survival rate was lowest (log-rank P = 0.000). 1: Resected completely with imatinib administrated postoperatively, 2: Resected completely without imatinib administrated postoperatively, 3: Resected incompletely with imatinib administrated postoperatively, 4: Resected incompletely without imatinib administrated postoperatively; B: The survival rates in carbonic anhydrase (CA) II-positive group were significantly higher than those in negative groups (CA II, log-rank P = 0.000); C: The survival rates in Ki-67-positive group were higher than those in negative groups (Ki-67, log-rank P = 0.004); D: The survival rates in CD117-positive group were significantly higher than those in negative groups (CD117, log-rank P = 0.000); E: The higher survival rates were found in those patients that received complete tumor resection (R0) (R0, log-rank P = 0.000); F: The higher survival rates were found in those patients with National Institutes of Health (NIH) high risk (NIH risk, log-rank P = 0.006).
According to the National Institutes of Health (NIH) risk grade[22] in GIST, there were five cases of extremely low risk, 15 of low risk, 16 of medium risk, and 77 of high risk. Comparing these parameters (tumor diameter, tumor site, mitotic rate, NIH risk, and depth of invasion), the differences were significant between the Ki-67-positive and -negative group, while for CD117 marker, there was no difference. For CA II, significant differences were found between positive and negative groups only when they were compared by tumor diameter, mitotic rate and NIH risk. In the CA II-positive group, high NIH risk accounted for 66.6% (66/99) of the cases (Figure 3). As for the other pathological characteristics, CD34, SMA and desmin protein positive rates were 79.4%, 46.8% and 5%, respectively.
Figure 3.
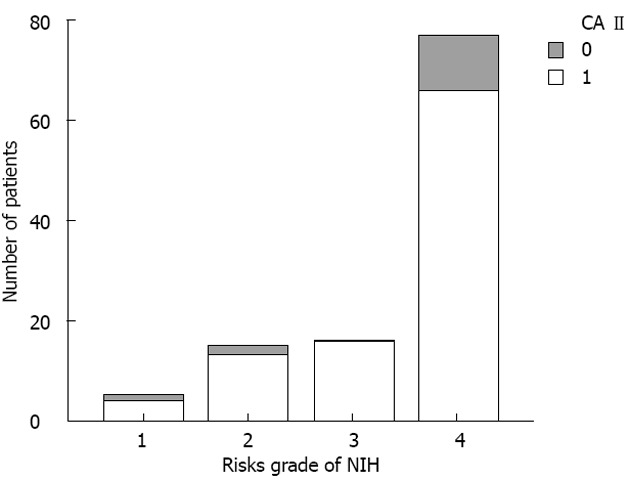
Carbonic anhydrase II positive cases in gastrointestinal stromal tumors according to National Institutes of Health risk grade. There were five cases with extremely low risk, 15 with low risk, 16 with medium risk, and 77 with high risk. Carbonic anhydrase (CA) II expressed in 113 gastrointestinal stromal tumors: “0” represents the negative cases, and “1” the positive cases. NIH: National Institutes of Health.
When comparing tumor site, mitotic rate, NIH risk, and depth of invasion, the differences were significant between Ki-67-positive and -negative groups (P < 0.05), whereas for CD117, significant differences were found for age, tumor site and depth of invasion (P < 0.05). For CA II, significant differences were not found between the positive and negative groups (P > 0.05) (Table 1).
Table 1.
Pathological parameters of gastrointestinal stromal tumors in CD117, carbonic anhydrase II and Ki-67 proteins
| Variable | Total |
CD117 |
P value |
CA II |
P value |
Ki-67 |
P value | |||
| + | - | + | - | + | - | |||||
| Sex | ||||||||||
| Male | 61 | 56 | 5 | 0.317 | 53 | 8 | 0.515 | 25 | 36 | 0.171 |
| Female | 52 | 41 | 11 | 46 | 6 | 14 | 38 | |||
| Age (yr) | ||||||||||
| ≤ 60 | 61 | 48 | 13 | 0.036 | 54 | 7 | 0.485 | 22 | 39 | 0.859 |
| > 60 | 52 | 49 | 3 | 45 | 7 | 17 | 35 | |||
| Diameter | ||||||||||
| ≤ 5 cm | 30 | 27 | 3 | 0.647 | 25 | 5 | 0.297 | 8 | 22 | 0.406 |
| > 5 cm | 83 | 70 | 13 | 74 | 9 | 31 | 52 | |||
| Site | ||||||||||
| Stomach | 45 | 40 | 5 | 41 | 4 | 13 | 32 | |||
| Small bowel | 35 | 34 | 1 | 0.004 | 31 | 4 | 0.459 | 8 | 27 | 0.014 |
| Others | 33 | 23 | 10 | 27 | 6 | 18 | 15 | |||
| Mitotic rate | ||||||||||
| ≤ 5 MF/50 HPFs | 52 | 45 | 7 | 0.941 | 46 | 6 | 0.515 | 11 | 41 | 0.009 |
| > 5 MF/50 HPFs | 61 | 52 | 9 | 53 | 8 | 28 | 33 | |||
| NIH risk | ||||||||||
| Very low | 5 | 4 | 1 | 4 | 1 | 2 | 3 | |||
| Low | 15 | 13 | 2 | 0.980 | 13 | 2 | 0.424 | 2 | 13 | 0.031 |
| Medium | 16 | 14 | 2 | 16 | 0 | 2 | 14 | |||
| High | 77 | 66 | 11 | 66 | 11 | 33 | 44 | |||
| Depth of invasion | ||||||||||
| Mucosa | 24 | 21 | 3 | 21 | 3 | 6 | 18 | |||
| Muscular | 41 | 33 | 8 | 38 | 3 | 10 | 31 | |||
| Serous | 30 | 30 | 0 | 0.033 | 27 | 3 | 0.168 | 13 | 17 | 0.013 |
| Adjacent tissue | 18 | 13 | 5 | 13 | 5 | 10 | 8 | |||
This table shows that Being compared by tumor site, mitotic rate, National Institutes of Health (NIH) risk, and depth of invasion of tumor cells, the differences were significant between the Ki-67-positive and -negative groups, while for CD117, significant differences were found for age, tumor site and depth of invasion of tumor cells. For carbonic anhydrase (CA) II, no significant differences were found between positive and negative groups. MF: Mitotic figures; HPFs: High-power fields.
Multivariate Cox model analysis suggested that CA II, Ki-67 and CD117, along with tumor site, tumor diameter, mitotic rate, depth of invasion, complete resection, intraoperative rupture, and adjuvant therapy were important prognosis predictive factors (P < 0.05). However, age, sex, mucosal erosion, biopsy and CD34 were not important prognosis predictive factors (Table 2).
Table 2.
Multivariate survival analysis (Cox proportional hazards model) in gastrointestinal stromal tumors
| Variable | B | Wald | df | P value | HR |
95%CI for HR |
|
| Lower | Upper | ||||||
| Age | 0.023 | 1.696 | 1 | 0.193 | 1.024 | 0.988 | 1.060 |
| Sex | -0.239 | 0.342 | 1 | 0.559 | 0.788 | 0.354 | 1.754 |
| Mucosal erosion or not | 1.485 | 2.115 | 1 | 0.146 | 4.416 | 0.597 | 32.684 |
| Biopsy or not | -0.404 | 0.901 | 1 | 0.340 | 0.668 | 0.291 | 1.531 |
| CD34 | -0.071 | 0.086 | 1 | 0.769 | 0.932 | 0.582 | 1.493 |
| CA II | -0.319 | 4.113 | 1 | 0.043 | 0.727 | 0.543 | 0.989 |
| CD117 | -0.609 | 4.114 | 1 | 0.042 | 0.544 | 0.303 | 0.978 |
| Ki-67 | 1.103 | 7.282 | 1 | 0.007 | 3.014 | 1.352 | 6.717 |
| Diameter | 1.645 | 4.974 | 1 | 0.026 | 5.182 | 1.216 | 22.085 |
| Mitotic rate | 0.972 | 11.081 | 1 | 0.001 | 2.644 | 1.491 | 4.686 |
| Site | 1.334 | 15.581 | 1 | 0.000 | 3.796 | 1.955 | 7.371 |
| Depth of invasion | 0.559 | 7.445 | 1 | 0.006 | 1.748 | 1.170 | 2.612 |
| Complete resection or not | 2.807 | 24.674 | 1 | 0.000 | 16.555 | 5.470 | 50.104 |
| Intraoperative rupture | 1.937 | 17.997 | 1 | 0.000 | 0.144 | 0.059 | 0.353 |
| Adjuvant therapy | 1.757 | 35.579 | 1 | 0.000 | 5.796 | 3.254 | 10.325 |
This table shows that carbonic anhydrase (CA) II, CD117 and Ki-67 expression, tumor diameter, mitotic rate, tumor site, depth of invasion, complete resection, intraoperative rupture, and adjuvant therapy were important prognosis predictive factors. HR: Hazard ratios.
DISCUSSION
It has been proved that tumor size, mitotic index, tumor location, and intraoperative tumor rupture are related to prognosis and recurrence of GIST[23-25]. For instance, although p53, CD147, MCT1, DDX39 and NKp30 are related to prognosis of GIST, they have never been mentioned as prognostic predictors due to their weak correlation.
Multivariate analysis showed that CA II provided additional information on patient survival as compared to age, sex, NIH risk classification and mutational status. Based upon the comprehensive recognition that CA II-positive tumor cells have oxidative activity, it is safe to suggest that CA II plays an important role in occurrence and development of GIST. By contrast, various studies have included only the membrane-bound isoforms, CA IX and XII, which are overexpressed in several types of cancers[26-29]. There has been only scattered evidence that CA II is expressed to some extent in malignant cells such as leukemic blast cells, and brain, colorectal and pancreatic cancers[30-32]. A more recent study has indicated that CA II expression is induced in neovascular endothelial cells of malignant melanoma and in esophageal, renal and lung cancers. It has been suggested that CA II associated with the neovascular endothelia could serve as a potential target for cancer therapy. It has also been proposed that the presence of CA II in the endothelium could contribute to generation of autoantibodies that could, in turn, be a desired outcome in immunotherapy of cancer. Combined with our present results, a new therapeutic approach targeting CA II, as well as CA II to predict prognosis of GIST, might be promising. However, more studies are necessary.
Ki-67 protein exists in actively proliferating cells (G1, S and G2 phase), which is a proliferation-related nuclear marker of tumor cell[33]. Some studies have shown that Ki-67 expression is closely related to aggressive biological behavior of tumor cells in GISTs[18] and represents a good prognostic predictor for GIST[34]. However, the significance of Ki-67 in predicting prognosis is still in dispute.
Wong et al[17] have found that Ki-67 was less reliable than mitotic count, even though it was useful in assessing the proliferation rate of tumor cells in GIST. We believe that the prognostic predictive value of Ki-67 in GIST might have been evaluated more objectively in a large survival study, with the various prognostic factors being taken into account. This was one of the aims of our present study. We found that the 1-, 3- and 5-year survival rates of patients with Ki-67-positive GIST were lower than in the Li-67-negative group. Our survival analysis further indicated that the Ki-67 expression was also an important prognostic predictor for GIST. The Wald indexes of Ki-67, diameter, mitotic rate and tumors site were all > 1 (7.282, 4.974, 11.081 and 15.581, respectively), which indicated that the Ki-67 was another useful molecular marker in predicting the prognosis of GISTs.
As for molecular markers, the negative expression of CD117 is believed to be associated with early postoperative recurrence of GIST[35]. This was confirmed once again by our study. At the same time, our study indicated that positive expression of Ki-67 or negative expression of CA II and CD117 was a cue for poor prognosis in GIST. However, there was a limitation to our study, namely, its small sample size. Our results could promote the clinical application of these two markers and provide clues to a novel therapeutic target for GIST in the future.
In conclusion, our study showed CA II expression in GIST. The prognosis of GIST with high CA II expression was better than that of GIST with low or no expression, suggesting that CA II is both a diagnostic and prognostic biomarker. Further validation studies with other CA antibodies should be undertaken to characterize CA II expression in a larger cohort of patients with GIST and other mesenchymal tumors of the gastrointestinal tract.
COMMENTS
Background
Gastrointestinal stromal tumors (GISTs) are mesenchymal tumors that arise from the gastrointestinal tract with a wide spectrum of clinical behavior. Thus, it is of great importance to define prognostic factors that could indicate survival rates. Carbonic anhydrase (CA) II is expressed in many malignant tumors, and higher expression is associated with a better disease-specific survival rate. Ki-67 is related to cell proliferation in various tumors and has a markedly positive correlation with prognosis.
Research frontiers
According to National Institutes of Health risk classification, some prognostic factors are well documented as prognostic factors of further tumor behavior, such as mitotic count and tumor size, along with tumor primary localization. However, the value of immunohistochemistry index in GISTs has not been clearly indicated. Furthermore, there have been a limited number of studies investigating the relationship between the prognosis of GISTs and CA II and Ki-67.
Innovations and breakthroughs
This is believed to be the first study showing so clearly the significance of CA II and Ki-67 as prognostic factors. The 1-, 3- and 5-year survival rates were 90.0%, 82.0% and 72.0% in all patients. However, in patients with positive CA II or Ki-67, the survival rates were 92.0%, 83.0% and 77.0% or 83.0%, 66.6% and 53.0%, respectively. This study indicated that CA II has strong expression in GISTs and the prognosis of GISTs with high CA II expression was better than that of GISTs with low or no expression, suggesting that CA II is both a diagnostic and a prognostic biomarker for GIST.
Applications
CA II and Ki-67 were useful for predicting the aggressive biological behavior of GISTs. CA II associated with neovascular endothelia could serve as a potential target for cancer therapy.
Terminology
CAs are a group of zinc-containing metalloenzymes that catalyze the reversible hydration of CO2 and participate in various physiological processes, including respiration, gluconeogenesis, bone resorption, renal acidification, and formation of cerebrospinal fluid and gastric acid. Ki-67 is a protein that is encoded by the MKI-67 gene in humans. Ki-67 antigen is associated, and probably necessary, for cellular proliferation, and is associated with rRNA transcription.
Peer review
This study revealed that CA II is highly expressed in GIST cell lines and 87.6% of GISTs selectively. Until now, there have been few reports of CA II expression in GISTs. The result of this study showed that high CA II expression was associated with a better disease-specific survival rate than low or no expression, therefore, it might be a useful biomarker in diagnosis and prognosis of GISTs. This is a retrospective study and the results should be helpful for clinical practice and a potential therapeutic target.
Footnotes
P- Reviewers Hsiao KCW, Fu DL, Sazci A S- Editor Huang XZ L- Editor A E- Editor Xiong L
References
- 1.Gupta P, Tewari M, Shukla HS. Gastrointestinal stromal tumor. Surg Oncol. 2008;17:129–138. doi: 10.1016/j.suronc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Katz SC, DeMatteo RP. Gastrointestinal stromal tumors and leiomyosarcomas. J Surg Oncol. 2008;97:350–359. doi: 10.1002/jso.20970. [DOI] [PubMed] [Google Scholar]
- 3.Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. 2011;17:700–707. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 4.Kikuta K, Kubota D, Saito T, Orita H, Yoshida A, Tsuda H, Suehara Y, Katai H, Shimada Y, Toyama Y, et al. Clinical proteomics identified ATP-dependent RNA helicase DDX39 as a novel biomarker to predict poor prognosis of patients with gastrointestinal stromal tumor. J Proteomics. 2012;75:1089–1098. doi: 10.1016/j.jprot.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 5.González-Cámpora R, Delgado MD, Amate AH, Gallardo SP, León MS, Beltrán AL. Old and new immunohistochemical markers for the diagnosis of gastrointestinal stromal tumors. Anal Quant Cytol Histol. 2011;33:1–11. [PubMed] [Google Scholar]
- 6.Zong L, Chen P, Xu Y. Correlation between P53 expression and malignant risk of gastrointestinal stromal tumors: evidence from 9 studies. Eur J Surg Oncol. 2012;38:189–195. doi: 10.1016/j.ejso.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira AT, Pinheiro C, Longatto-Filho A, Brito MJ, Martinho O, Matos D, Carvalho AL, Vazquez VL, Silva TB, Scapulatempo C, et al. Co-expression of monocarboxylate transporter 1 (MCT1) and its chaperone (CD147) is associated with low survival in patients with gastrointestinal stromal tumors (GISTs) J Bioenerg Biomembr. 2012;44:171–178. doi: 10.1007/s10863-012-9408-5. [DOI] [PubMed] [Google Scholar]
- 8.Esbaugh AJ, Tufts BL. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir Physiol Neurobiol. 2006;154:185–198. doi: 10.1016/j.resp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 10.Hewett-Emmett D, Tashian RE. Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol. 1996;5:50–77. doi: 10.1006/mpev.1996.0006. [DOI] [PubMed] [Google Scholar]
- 11.Hewett-Emmett D. Evolution and distribution of the carbonic anhydrase gene families. EXS. 2000;90:29–76. doi: 10.1007/978-3-0348-8446-4_3. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto K, Nishimori I, Takeuchi T, Kohsaki T, Okamoto N, Taguchi T, Yunoki S, Watanabe R, Ohtsuki Y, Onishi S. Overexpression of carbonic anhydrase-related protein XI promotes proliferation and invasion of gastrointestinal stromal tumors. Virchows Arch. 2005;447:66–73. doi: 10.1007/s00428-005-1225-3. [DOI] [PubMed] [Google Scholar]
- 13.Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 14.Uchida K, Okazaki K, Nishi T, Uose S, Nakase H, Ohana M, Matsushima Y, Omori K, Chiba T. Experimental immune-mediated pancreatitis in neonatally thymectomized mice immunized with carbonic anhydrase II and lactoferrin. Lab Invest. 2002;82:411–424. doi: 10.1038/labinvest.3780435. [DOI] [PubMed] [Google Scholar]
- 15.Brown BF, Quon A, Dyck JR, Casey JR. Carbonic anhydrase II promotes cardiomyocyte hypertrophy. Can J Physiol Pharmacol. 2012;90:1599–1610. doi: 10.1139/y2012-142. [DOI] [PubMed] [Google Scholar]
- 16.Parkkila S, Lasota J, Fletcher JA, Ou WB, Kivelä AJ, Nuorva K, Parkkila AK, Ollikainen J, Sly WS, Waheed A, et al. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod Pathol. 2010;23:743–750. doi: 10.1038/modpathol.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong NA, Young R, Malcomson RD, Nayar AG, Jamieson LA, Save VE, Carey FA, Brewster DH, Han C, Al-Nafussi A. Prognostic indicators for gastrointestinal stromal tumours: a clinicopathological and immunohistochemical study of 108 resected cases of the stomach. Histopathology. 2003;43:118–126. doi: 10.1046/j.1365-2559.2003.01665.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura N, Yamamoto H, Yao T, Oda Y, Nishiyama K, Imamura M, Yamada T, Nawata H, Tsuneyoshi M. Prognostic significance of expressions of cell-cycle regulatory proteins in gastrointestinal stromal tumor and the relevance of the risk grade. Hum Pathol. 2005;36:828–837. doi: 10.1016/j.humpath.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 20.Elias J. Immunhistopathology: a practical Approach to Diagonsis. Chocago, United States: ASCP Press; 1990. [Google Scholar]
- 21.Taylor CR. Immunoperoxidase techniques: practical and theoretical aspects. Arch Pathol Lab Med. 1978;102:113–121. [PubMed] [Google Scholar]
- 22.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–1419. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Dematteo RP. Personalized therapy: prognostic factors in gastrointestinal stromal tumor (GIST) J Gastrointest Surg. 2012;16:1645–1647. doi: 10.1007/s11605-012-1944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PW, Demetri GD, Blanke CD, von Mehren M, Brennan MF, McCall L, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012;215:53–59; discussion 59-60. doi: 10.1016/j.jamcollsurg.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attili SV, Ananda B, Mandapal T, Anjaneyulu V, Sinha S, Reddy OC. Factors influencing progression-free survival in gastrointestinal stromal tumors with special reference to pathologic features, cytogenetics, and radiologic response. Gastrointest Cancer Res. 2011;4:173–177. [PMC free article] [PubMed] [Google Scholar]
- 26.Parkkila S, Rajaniemi H, Parkkila AK, Kivela J, Waheed A, Pastorekova S, Pastorek J, Sly WS. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proc Natl Acad Sci USA. 2000;97:2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem. 2004;19:199–229. doi: 10.1080/14756360410001689540. [DOI] [PubMed] [Google Scholar]
- 28.Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64:6160–6165. doi: 10.1158/0008-5472.CAN-03-2224. [DOI] [PubMed] [Google Scholar]
- 29.Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol. 2001;158:905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leppilampi M, Koistinen P, Savolainen ER, Hannuksela J, Parkkila AK, Niemelä O, Pastoreková S, Pastorek J, Waheed A, Sly WS, et al. The expression of carbonic anhydrase II in hematological malignancies. Clin Cancer Res. 2002;8:2240–2245. [PubMed] [Google Scholar]
- 31.Parkkila AK, Herva R, Parkkila S, Rajaniemi H. Immunohistochemical demonstration of human carbonic anhydrase isoenzyme II in brain tumours. Histochem J. 1995;27:974–982. [PubMed] [Google Scholar]
- 32.Hosoda H, Okawa-Takatsuji M, Shinmura W, Hasimoto N, Ozaki Y, Ikeda Y. Potential for differential diagnosis of autoimmune pancreatitis and pancreatic cancer using carbonic anhydrase II antibody. Pancreas. 2008;37:e1–e7. doi: 10.1097/MPA.0b013e318162cb3a. [DOI] [PubMed] [Google Scholar]
- 33.Hutchins JR, Toyoda Y, Hegemann B, Poser I, Hériché JK, Sykora MM, Augsburg M, Hudecz O, Buschhorn BA, Bulkescher J, et al. Systematic analysis of human protein complexes identifies chromosome segregation proteins. Science. 2010;328:593–599. doi: 10.1126/science.1181348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Artigiani Neto R, Logullo AF, Stávale JN, Lourenço LG. Ki-67 expression score correlates to survival rate in gastrointestinal stromal tumors (GIST) Acta Cir Bras. 2012;27:315–321. doi: 10.1590/s0102-86502012000500007. [DOI] [PubMed] [Google Scholar]
- 35.Lamba G, Ambrale S, Lee B, Gupta R, Rafiyath SM, Liu D. Recent advances and novel agents for gastrointestinal stromal tumor (GIST) J Hematol Oncol. 2012;5:21. doi: 10.1186/1756-8722-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]


