Abstract
AIM: To evaluate the safety, efficacy and tolerability of Nigella sativa (N. sativa) in patients with hepatitis C not eligible for interferon (IFN)-α.
METHODS: Thirty patients with hepatitis C virus (HCV) infection, who were not eligible for IFN/ribavirin therapy, were included in the present study. Inclusion criteria included: patients with HCV with or without cirrhosis, who had a contraindication to IFN-α therapy, or had refused or had a financial constraint to IFN-α therapy. Exclusion criteria included: patients on IFN-α therapy, infection with hepatitis B or hepatitis I virus, hepatocellular carcinoma, other malignancies, major severe illness, or treatment non-compliance. Various parameters, including clinical parameters, complete blood count, liver function, renal function, plasma glucose, total antioxidant capacity (TAC), and polymerase chain reaction, were all assessed at baseline and at the end of the study. Clinical assessment included: hepato and/or splenomegaly, jaundice, palmar erythema, flapping tremors, spider naevi, lower-limb edema, and ascites. N. sativa was administered for three successive months at a dose of (450 mg three times daily). Clinical response and incidence of adverse drug reactions were assessed initially, periodically, and at the end of the study.
RESULTS: N. sativa administration significantly improved HCV viral load (380808.7 ± 610937 vs 147028.2 ± 475225.6, P = 0.001) and TAC (1.35 ± 0.5 vs 1.612 ± 0.56, P = 0.001). After N. sativa administration, the following laboratory parameters improved: total protein (7.1 ± 0.7 vs 7.5 ± 0.8, P = 0.001), albumin (3.5 ± 0.87 vs 3.69 ± 0.91, P = 0.008), red blood cell count (4.13 ± 0.9 vs 4.3 ± 0.9, P = 0.001), and platelet count (167.7 ± 91.2 vs 198.5 ± 103, P = 0.004). Fasting blood glucose (104.03 ± 43.42 vs 92.1 ± 31.34, P = 0.001) and postprandial blood glucose (143.67 ± 72.56 vs 112.1 ± 42.9, P = 0.001) were significantly decreased in both diabetic and non-diabetic HCV patients. Patients with lower-limb edema decreased significantly from baseline compared with after treatment [16 (53.30%) vs 7 (23.30%), P = 0.004]. Adverse drug reactions were unremarkable except for a few cases of epigastric pain and hypoglycemia that did not affect patient compliance.
CONCLUSION: N. sativa administration in patients with HCV was tolerable, safe, decreased viral load, and improved oxidative stress, clinical condition and glycemic control in diabetic patients.
Keywords: Hepatitis C virus, Nigella sativa, Oxidative stress, Viral load
INTRODUCTION
Egypt has the highest prevalence of hepatitis C virus (HCV) worldwide (15%) and the highest prevalence of HCV-4 (67%) with a predominance of subtype 4a (55%)[1-4].
The natural history of HCV infection and disease progression are influenced by several factors such as age at infection onset, sex, duration of infection, co-infection with hepatitis B virus (HBV), level of HCV viremia and its genotype[5].
HCV is an important etiological factor for the development of hepatocellular carcinoma (HCC) and 23% of HCV patients develop HCC[6]. It has been shown that there is an alarming increase in the incidence of HCC in HCV patients in Egypt[7].
Presently, the only approved therapy for HCV is pegylated interferon-α (PEG-IFN-α) and ribavirin treatment, and their success is heavily influenced by patient adherence, which correlates directly with tolerance to their side effects[8]. Moreover, financial constraints for the combined therapy in many patients often contribute to therapy non-adherence, potentially lowering its success rates[9].
Oxidative-stress-related molecules may act as mediators modulating cellular events responsible for progression to liver fibrosis[10,11]. It has been shown that increased production of reactive oxygen species, in part catalyzed by iron overload, is involved in HCV-related liver damage through a pathway that involves DNA oxidative injury[12].
Silymarin is one of the alternative therapies that has been previously tested for the management of HCV patients who are not candidates for PEG-IFN; however, it has not shown any appreciable effects on viral load[13].
Nigella sativa (N. sativa) is used as a food condiment in the Middle East, and its seeds/oil have been shown to possess anti-inflammatory, antiviral and antineoplastic activity in various in vitro and in vivo studies[14]. The antioxidant effects of N. sativa have been shown in the essential oil obtained from six different extracts of its seeds, as well as from a commercial fixed oil[15]. The crude N. sativa oil and its fractions have shown potent in vitro radical scavenging activity[16].
The effect of N. sativa has been evaluated in animal studies. There are many reports of its biological activities including: immunopotentiation, antitumor, anti-inflammatory, analgesic, antihypertensive, antidiabetic, respiratory stimulation, antibacterial, antifungal, anticestode and antinematode effects[17-19].
A striking reduction of murine cytomegalovirus (CMV) virus titer in both spleen and liver was found in mice treated with N. sativa seed oil compared with control mice[20]. Moreover, oral feeding with N. sativa extract suppressed chemically induced hepatic tumors in rats[21]. N. sativa treatment has been shown to ameliorate disturbed hematological parameters in diabetic rabbits through modulation of lipid peroxide red blood cell (RBC) membrane content, leading to an increase in RBC count[22].
To date, no studies have addressed the use of N. sativa in HCV patients and its potential benefits; hence, we sought to evaluate the efficacy, safety, and tolerability of N. sativa supplementation as an alternative therapy in the management of HCV patients who are non-candidates for IFN-α therapy.
MATERIALS AND METHODS
This was a prospective, single-armed, self-controlled pilot study, conducted at the Tropical Medicine Department, El-Demerdash Hospital, Ain Shams University, Cairo, Egypt.
Patients
All HCV patients presenting to the department were assessed for eligibility. Inclusion criteria included all patients diagnosed with HCV with or without cirrhosis who either had a contraindication to IFN-α therapy[23], or had refused or had a financial constraint to IFN-α therapy. Exclusion criteria included: patients on IFN-α therapy; infection with HBV or hepatitis I virus; HCC or other malignancies; major severe illness such as renal failure, congestive heart failure, respiratory failure or autoimmune disease; or non-compliance to treatment. Informed consent was obtained from all patients, and the institutional ethical committee approved the study protocol, which conformed with the ethical guidelines of the 1975 Declaration of Helsinki.
Methods
Hepatitis markers were assessed for all patients at enrollment, including: hepatitis B core immunoglobulin G, hepatitis B surface antigen, and HCV antibody. All eligible patients were subjected to the following at enrollment and after 3 mo therapy: (1) Full clinical assessment with an emphasis on hepato- and/or splenomegaly, jaundice, palmar erythema, flapping tremors, spider naevi, lower-limb edema, and ascites; (2) Abdominal ultrasonography; (3) Laboratory investigations including complete blood count, liver functions [aspartate aminotransferase (AST), alanine aminotransferase (ALT), total proteins, albumin, total and direct bilirubin, prothrombin time and international standard ratio (INR)], renal function (serum creatinine, blood urea nitrogen), serum α-fetoprotein, polymerase chain reaction (PCR) for HCV (lower detection limit, < 50 copies) and total antioxidant capacity (TAC); (4) The antioxidants assessed in the estimation of TAC included enzymes such as superoxide dismutase, catalase, glutathione peroxidase; macromolecules such as albumin, ceruloplasmin, ferritin; small molecules, including ascorbic acid, α-tocopherol, β-carotene, reduced glutathione, uric acid, and bilirubin; (5) The assay principle depended on the determination of the antioxidative capacity by the reaction of antioxidants in the sample with a defined amount of exogenously provide H2O2. The antioxidants in the sample eliminated a certain amount of the provided H2O2. The residual H2O2 was determined colorimetrically by an enzymatic reaction that involved the conversion of 3,5,dichloro-2-hydroxy benzensulfonate to a colored product; (6) TAC was analyzed using a TAC kit from Bio-diagnostic and measured spectrophotometrically using KENZA (Biolabo) analyzer; and (7) Real-time PCR was performed on COBAS TaqMan 48 PCR analyzer, using Roche COBAS Ampliprep Taqman Kit.
Drug administration
After performing the baseline evaluation, all patients received one capsule of N. sativa seed oil (450 mg) available as soft gelatin capsules (Baraka; Pharco Pharmaceuticals) three times daily after meals continuously for 3 mo. Patients were followed up every 2 wk throughout the study period for assessing treatment adherence, tolerability and incidence of adverse reactions.
Statistical analysis
Statistical analysis was performed using SPSS version 17 software. Numerical data were summarized using means and standard deviations or medians and ranges. Categorical data were summarized as percentages. Differences between numerical variables over two time measurements were tested using paired t test or medians test for non-normally distributed data. Repeated measures analysis of variance was used to test differences between three-time numerically normally distributed variables and Friedman test was used for non-normally distributed variables. McNemar’s test was used to compare categorical data overtime. All P values were two-sided, and P < 0.05 was considered significant. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Thirty patients (16 male, 14 female) with a mean age of 47 ± 10.2 years fulfilled the inclusion criteria and were enrolled in the study. Four of those patients (13.33%) had diabetes and 26 (86.67%) did not. Fifteen patients (30%) had chronic liver disease, five (16.7%) had compensated cirrhosis, and 10 (33.3%) had decompensated cirrhosis.
Patients’ clinical assessment data before and after treatment are presented in Table 1. After treatment, there was a significant decrease in the percentage of patients with lower-limb edema, while there was no change in the percentage of patients with jaundice, palmar erythema, spider naevi or ascites. Laboratory parameters before and after treatment are presented in Table 2.
Table 1.
Clinical assessment data at baseline and after treatment n (%)
| Characteristic | Baseline | After treatment | P value |
| Hepato and/or splenomegaly | 19 (63.30) | 19 (63.30) | |
| Jaundice | 8 (26.70) | 5 (16.70) | 0.25 |
| Palmar erythema | 10 (33.30) | 8 (26.70) | 0.5 |
| Spider naevi | 8 (26.70) | 4 (13.30) | 0.125 |
| Lower limb edema | 16 (53.30) | 7 (23.30) | 0.004 |
| Clinically detected ascites | 13 (43.30) | 8 (26.70) | 0.063 |
After treatment: 3 mo Nigella sativa treatment. McNemar’s test was used to compare categorical data overtime.
Table 2.
Laboratory data assessment at baseline and after treatment
| Parameter | Base line | After 3 mo treatment | P value |
| Hemoglobin (g%) | 11.8 ± 2.1 | 12.2 ± 2.2 | 0.1 |
| RBCs (× 106/μL) | 4.13 ± 0.9 | 4.3 ± 0.9 | 0.001 |
| WBCs (× 103/μL) | 6.4 ± 2.1 | 5.6 ± 2.2 | 0.013 |
| Platelets (× 103/μL) | 167.7 ± 91.2 | 198.5 ± 103 | 0.004 |
| Hematocrit (%) | 35.5 ± 6.3 | 37.3 ± 6.3 | 0.056 |
| ALT (IU/L) | 35.0 ± 15.7 | 41 ± 24.4 | 0.255 |
| AST (IU/L) | 40.9 ± 30.4 | 46.8 ± 32.2 | 0.307 |
| Total protein (g/dL) | 7.1 ± 0.7 | 7.5 ± 0.8 | 0.001 |
| Albumin (g/dL) | 3.5 ± 0.9 | 3.69 ± 0.9 | 0.008 |
| Direct bilirubin (mg/dL) | 0.5 ± 0.8 | 0.57 ± 1.5 | 0.745 |
| Total bilirubin (mg/dL) | 1.46 ± 1.5 | 1.36 ± 1.3 | 0.428 |
| Prothrombin time (s) | 14.1 ± 2.7 | 13.8 ± 2.2 | 0.562 |
| INR | 1.18 ± 0.2 | 1.2 ± 0.2 | 0.974 |
| BUN (mg/dL) | 13.5 ± 6.2 | 14.1 ± 5 | 0.540 |
| Creatinin (mg/dL) | 0.99 ± 0.4 | 0.88 ± 0.2 | 0.102 |
| Serum AFP (IU/mL) | 5.07 ± 1.8 | 4.67 ± 2.3 | 0.194 |
| Sodium (mmole/L) | 135.5 ± 6.1 | 133.5 ± 6 | 0.064 |
| Potassium (mmole/L) | 4.1 ± 0.5 | 4 ± 0.5 | 0.350 |
| TAC (mmol/L) | 1.35 ± 0.5 | 1.61 ± 0.6 | 0.001 |
| Fasting blood sugar (mg/dL) | 104.03 ± 43.4 | 92.1 ± 31.3 | 0.001 |
| Post prandial blood sugar (mg/dL) | 143.67 ± 72.6 | 112.1 ± 42.9 | 0.001 |
| PCR (copies) | 380808.7 ± 610937 | 147028.2 ± 475225.6 | 0.001 |
Paired t-test for all parameters, median test (equivalent to Wilcoxon matched pairs test) for polymerase chain reaction (PCR) levels. RBCs: Red blood cells; WBCs: White blood cells; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio; BUN: Blood urea nitrogen; AFP: α-fetoprotein; TAC: Total antioxidant capacity.
Liver functions tests
After 3 mo of N. sativa treatment, the mean HCV RNA levels (PCR) (147028.2 ± 475225.6) significantly decreased relative to their baseline levels (380808.7± 610937, P = 0.001) (Figure 1A). Table 3 presents the PCR responses after 3 mo treatment in patients with chronic liver disease and compensated and decompensated cirrhosis. Figure 2 presents individual patients’ HCV RNA (PCR) values before and after treatment. Table 4 presents the Child-Pugh score and PCR response at baseline and after 3 mo in patients with compensated and decompensated cirrhosis. All cirrhotic patients (compensated and decompensated) showed no change or an improvement in their Child-Pugh score, patients presented with variable Child-Pugh score, yet the proportions’ numbers were small for a valid statistical test. There was a significant increase in total protein and albumin levels after treatment. However, there was no significant change in liver enzymes (AST and ALT), bilirubin, or INR. Renal function did not show a significant change from baseline. TAC showed a significant increase after treatment (1.612 ± 0.56) relative to the baseline values (1.35 ± 0.05, P = 0.001, Figure 1B). Hematological functions varied significantly after 3 mo of N. sativa treatment. There was a significant increase in RBCs (P = 0.001) and platelets (P = 0.004) and a significant decrease (P = 0.013) in white blood cells.
Figure 1.
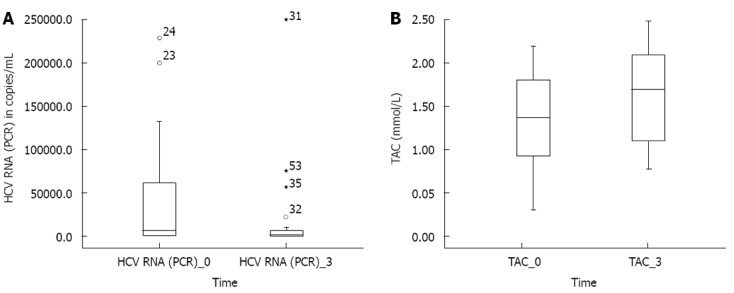
Box plot for hepatitis C virus RNA (polymerase chain reaction) levels (A), total antioxidant capacity (B) before and after treatment. A: Median test (equivalent to Wilcoxon matched pairs test), P < 0.001. Hepatitis C virus (HCV) RNA [(polymerase chain reaction (PCR)]_0: PCR values of patients before treatment; HCV RNA (PCR)_3: PCR values of patients after 3 mo treatment; B: Paired t test, P < 0.001. Total antioxidant capacity (TAC)_0: TAC levels of patients before treatment; TAC_3: TAC levels of patients after 3 mo treatment.
Table 3.
Polymerase chain reaction response after treatment n (%)
| Total responders | 5 (16.67) |
| Chronic liver disease | 3 |
| Compensated cirrhosis | 1 |
| Decompensated cirrhosis | 1 |
| Total partial responders | 15 (50) |
| Chronic liver disease | 5 |
| Compensated cirrhosis | 4 |
| Decreased 1 log | 1 |
| Decreased 2 log | 3 |
| Decompensated cirrhosis | 61 |
| Total non-responders | 10 (33.33) |
| Chronic liver disease | 7 |
| Compensated cirrhosis | |
| Decompensated cirrhosis | 3 |
Patients decreased polymerase chain reaction (PCR) but in same log. Non-responders: Patients did not show a decrease or showed an increase in PCR after 3 mo treatment with Nigella sativa (N. sativa); Responders: Patients became seronegative after 3 mo treatment with N. sativa; Partial responders: Patients showed a decrease in PCR but were still seropositive after 3 mo treatment with N. sativa.
Figure 2.
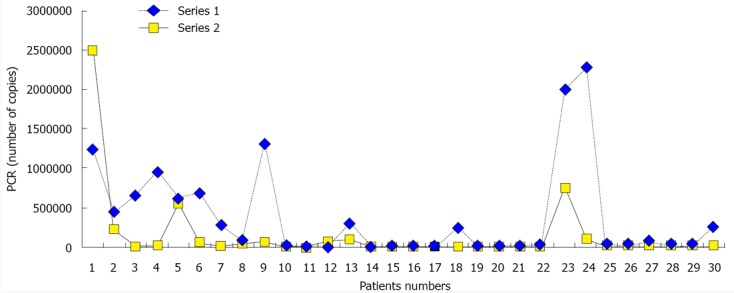
Line plot for polymerase chain reaction levels in individual patients at baseline and after 3 mo of treatment. Series 1: Polymerase chain reaction (PCR) values in all patients at baseline; Series 2: PCR values in all patients after 3 mo of treatment.
Table 4.
Child-Pugh score at baseline and after 3 mo in patients with compensated and decompensated cirrhosis
| Patients | Child-Pugh score at baseline | Child-Pugh score after 3 mo of treatment | HCV RNA (PCR) response |
| 1 | B | B | Partial responder |
| 2 | C | B | Partial responder |
| 3 | A | A | Partial responder |
| 4 | B | B | Partial responder |
| 5 | A | A | Partial responder |
| 6 | C | C | Partial responder |
| 7 | C | B | Non- responder |
| 8 | C | B | Partial responder |
| 9 | A | A | Responder |
| 10 | B | B | Non-responder |
| 11 | C | B | Partial responder |
| 12 | B | A | Responder |
| 13 | C | B | Non-responder |
| 14 | A | A | Partial responder |
| 15 | A | A | Partial responder |
HCV: Hepatitis C virus; PCR: Polymerase chain reaction.
Blood glucose
There was a significant decrease in both fasting and postprandial blood glucose after treatment (P = 0.001).
Incidence of side effects and drug interactions
The reported side effects throughout the study period were gastritis in one patient (3.33%) and hypoglycemia in five (16.76 %); of whom two had insulin-dependent diabetes, and the other three had advanced liver cirrhosis with possible glycogen depletion. Both side effects were treated and did not hinder completion of therapy. The only reported drug interaction was hypoglycemia due to concurrent use of insulin and N. sativa, which aggravated its hypoglycemic effects.
DISCUSSION
The main findings of our study were that administration of N. sativa significantly decreased HCV viral load, increased total antioxidant activity and total protein and albumin levels, lowered blood glucose levels, and improved lower-limb edema.
The anti-inflammatory, antiviral and antineoplastic activities of N. sativa have been previously documented in various in vitro and in vivo studies[14]. In the current study, N. sativa administration resulted in a significant decrease in viral load, with 16.67% of patients becoming seronegative, and 50% showing a significant decrease in the quantitative viral count. Among these, 66.7% had cirrhosis and 33.3% had chronic liver disease, implying antiviral activity. Patients with compensated and decompensated cirrhosis, either improved or maintained their baseline clinical condition and viral load, and none of them deteriorated, which signified the potential beneficial effects of N. sativa administration, as reflected by improvement in HCV RNA responses and clinical condition reflected in Child-Pugh class. Although the subcategory of cirrhosis patients was not large enough to detect significance, we recommend that larger studies should be conducted in patients with cirrhosis to confirm the potential beneficial effects offered by N. sativa, which might improve patients’ overall outcome. To the best of our knowledge, this is the first human study to evaluate the effects of N. sativa on viral load in patients with HCV infection. Our findings of improved viral load could be explained by the results of a previous study of murine CMV[20], which showed a significant increase in macrophages and CD4+ T cells, with a significant decrease in viral titer and increased serum IFN-γ levels in animals treated with N. sativa[24].
Oxidative-stress-related molecules have been shown to modulate cellular events responsible for the progression of liver fibrosis[10,11]. Moreover, HCV-related fibrosis, cirrhosis and liver failure have been found to be the result of an adaptive immune response to HCV-infected cells[25], which is mediated by induction of endoplasmic reticulum and oxidative stress and downregulation of antiapoptotic proteins nuclear factor-κB and Bcl-xl in infected hepatocytes[26].
In our study, N. sativa administration significantly increased TAC in HCV patients, implying the potential protective effect of N. sativa by halting the oxidative stress that contributes to disease progression. Furthermore, it is tempting to propose that increasing antioxidant capacity, with its cytoprotective role, contributed to decreasing the viral load.
The antioxidant effects of N. sativa have been previously elaborated in animal models of liver ischemia, in which it improved the antioxidant capacity and reduced oxidative stress[27]. Moreover, N. sativa increased hepatic glutathione and reduced elevated hepatic serum enzymes in carbon-tetrachloride-treated mice, ameliorating its hepatotoxic potential[15,28].
Some patients with acute and chronic liver disease develop diabetes mellitus[29,30]. HCV infection may also contribute to the development of diabetes, which has been observed in 21% of HCV-infected patients[31], and glucose intolerance has been seen in patients with HCV infection, compared with controls with liver diseases[32-35].
Insulin resistance is one of the pathological features in patients with HCV infection that may be associated with life-threatening complications, making HCV-associated insulin resistance a therapeutic target at any stage of HCV infection[36].
Our study showed that N. sativa treatment significantly decreased blood glucose levels in HCV patients, implying that it might offer a potential modulatory effect on HCV-induced glucose intolerance. This effect was beneficial in the control of diabetes in HCV patients because it allowed us to lower the insulin requirement. Similar results have been previously shown in a study of patients with diabetes, in whom administration of N. sativa (2 g/d) caused significant reductions in fasting blood glucose and 2-h postprandial blood glucose and hemoglobin A1c, and improved insulin resistance[37].
HCV infection itself can induce autoimmune hemolytic anemia, leukopenia, and thrombocytopenia, even in the absence of IFN-α treatment[38-42]. Hematopoietic growth factors modulating these complications have shown a beneficial role in HCV patients[43].
N. sativa therapy in our study significantly improved RBC and platelet counts in HCV patients, indicating a potential amelioration/prevention of HCV-induced hematological disorders. Hence, N. sativa may positively affect clinical outcome in HCV patients.
The ability of N. sativa to improve hematological indices has also been reported in animal studies in which it increased both the packed cell volume and hemoglobin in treated rats[18], as well as increased RBC count in diabetic rabbits[44]. The increased RBC count was attributed to lowering of the membrane lipid peroxide level, leading to decreased susceptibility to hemolysis.
Serum albumin is the most abundant plasma protein[45] and is essential for maintaining oncotic pressure of the vascular system[46]. Chronic HCV patients may suffer a decrease in serum albumin level[47], and improvement in hypoalbuminemia has been shown to improve prognosis[48] and quality of life[49]. Concentrations of < 30 g/L were associated with an 85% chance of liver-related complications at 5 years and a 3-year mortality of 70%[50], and was predictive of morbidity and mortality in patients with liver cirrhosis[51,52].
In the current study, N. sativa administration significantly increased serum albumin levels and significantly reduced lower-limb edema, indicating an improvement in clinical condition. Prior animal studies have shown similar effects in rats[53] and broiler chickens[54] in a dose-dependent manner[55].
N. sativa is used in Arab folk medicine as a diuretic plant[56], the mechanism that can also contribute to its efficacy in decreasing lower limb edema, and its resolution in many patients.
In our study, the number of patients with ascites decreased after treatment with N. sativa, although the change was not significant; nevertheless, the change in ascites severity could not be totally denied, because the degree of ascites was not assessed sonographically. We hence recommend assessment of ascites incidence and severity in future studies to confirm these results.
The safety and tolerability of N. sativa have been previously documented in various clinical trials[57-60]. However, to date, clinical studies addressing N. sativa efficacy, safety and tolerability in HCV patients are lacking. Our study has shown that N. sativa was tolerable in all patients, and the only side effects reported were one patient with epigastric pain that was controlled with antacids, and five patients with hypoglycemia, two of whom had diabetes and were receiving concomitant insulin and the hypoglycemia did not recur after decreasing the insulin dose. Of note, the dose of N. sativa used in the current study was (1.35 g/d), which was slightly lower than in the other studies - 2 g/d used by Bamosa et al[37] - because this dose was available in the Egyptian market and was close to the doses previously used. Although N. sativa in such patients had significantly positive effects on many parameters, perhaps higher doses or longer durations of therapy may accentuate such appreciable effects. Further studies are needed to confirm such findings.
It can therefore be concluded that N. sativa administration can have a potential beneficial effect on HCV disease progression and outcome through its prominent antiviral, antioxidant and immunomodulatory effects and can minimize HCV-related hematological complications.
Our study had some limitations. This was the first clinical study to be performed in HCV patients and larger studies are required to confirm the results of the current study. We did not assess all patients for the amount of ascites after therapy sonographically, because such a favorable effect of N. sativa was not anticipated. Hence, in view of significant improvement of serum albumin, this effect of N. sativa on the amount of ascites needs further study. Liver biopsy was not performed, because the patients were either not eligible or refused the procedure.
In conclusion, N. sativa administration in HCV patients is safe and tolerable and results in a significant improvement in viral load, oxidative stress and laboratory markers. Moreover, the clinical improvement and better glycemic control in patients with diabetes indicate a potential role for N. sativa in improving the clinical outcome of HCV patients. We recommend larger controlled multicenter randomized studies for longer periods for evaluation of the potential beneficial role of N. sativa in HCV patients with and without concurrent IFN therapy.
ACKNOWLEDGMENTS
We wish to express our gratitude and appreciation to Dr. Inas A Elattar, Professor and Head of Department of Cancer Epidemiology and Biostatistics, at the National Cancer Institute, Cairo, Egypt for her statistical evaluation and review of this study.
COMMENTS
Background
Hepatitis C virus (HCV) is an important etiological factor for the development of hepatocellular carcinoma. Pegylated interferon-α (PEG-IFN-α) and ribavirin treatment are the only currently approved therapy for HCV with variable response rate, and a success that is heavily influenced by patients’ response rate, adherence to treatment, and tolerance to side effects. Moreover, the financial constraints for the combined therapy in many patients often contribute to their non-adherence to therapy, potentially lowering its success rates. Nigella sativa (N. sativa), a food condiment used in the Middle East, has shown anti-inflammatory, antiviral, antioxidant and anticancer activities in various in vitro and in vivo studies. To date, no studies have addressed the use of N. sativa in HCV patients and its potential benefits.
Research frontiers
N. sativa is a natural food supplement, and has shown beneficial antioxidant, antiviral, anticancer and immunopotentiating properties in various in vitro and in vivo studies, but HCV studies are lacking. In exploring the potential role of N. sativa in improving HCV patients’ clinical outcome, the research hot spot is its beneficial effects on reducing viral load, improving antioxidant capacity, alleviating hematological parameters, and improving blood glucose control, especially in diabetes. All of which could have a potential beneficial effect on HCV patients’ responses and amelioration of HCV-related complications.
Innovations and breakthroughs
No prior clinical trials in HCV patients have evaluated the use of N. sativa and its potential beneficial effects. No studies have addressed any alternative treatments for IFN non-eligible patients or those who refuse or cannot tolerate IFN therapy. N. sativa offers hope for a safe tolerable alternative to those patients who cannot tolerate IFN or have a contraindication to its use. Moreover, N. sativa has a potential benefit in improving clinical outcome. It showed a preliminary improvement in viral load and antioxidant levels that could provide a potential cure for HCV infection. N. sativa also improved the hematological profile and total protein and albumin levels, which contribute to HCV-induced complications. Moreover, N. sativa decreased blood glucose levels, and hence decreased insulin requirement in patients with diabetes.
Applications
The study results suggest that N. sativa is a potentially beneficial, safe and tolerable alternative in IFN non-eligible HCV patients. It can improve clinical outcome, ameliorate HCV-induced hematological and diabetic complications, and improve lower-limb edema.
Terminology
Viral load, also known as viral burden or viral titer, is a measure of the severity of a viral infection, and can be calculated by estimating the amount of virus in an involved body fluid, for example, RNA copies/mL blood plasma. Human serum albumin is the most abundant protein in human blood plasma. It is produced in the liver and constitutes about half of the blood serum protein. It transports hormones, fatty acids, and other compounds, buffers pH, and maintains osmotic pressure, among other functions. Total antioxidant capacity measures collectively the amount of antioxidant components of the body that reflects the body’s capacity to combat oxidative stress.
Peer review
This was an interesting study in which the authors treated HCV patients with N. sativa, a food condiment used in the Middle East.
Footnotes
P- Reviewers Montalto G, Anand BS S- Editor Song XX L- Editor Kerr C E- Editor Xiong L
References
- 1.Nguyen MH, Keeffe EB. Prevalence and treatment of hepatitis C virus genotypes 4, 5, and 6. Clin Gastroenterol Hepatol. 2005;3:S97–S101. doi: 10.1016/s1542-3565(05)00711-1. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Aziz F, Habib M, Mohamed MK, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Thomas D, Fix AD, Strickland GT, et al. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology. 2000;32:111–115. doi: 10.1053/jhep.2000.8438. [DOI] [PubMed] [Google Scholar]
- 3.Elkady A, Tanaka Y, Kurbanov F, Sugauchi F, Sugiyama M, Khan A, Sayed D, Moustafa G, Abdel-Hameed AR, Mizokami M. Genetic variability of hepatitis C virus in South Egypt and its possible clinical implication. J Med Virol. 2009;81:1015–1023. doi: 10.1002/jmv.21492. [DOI] [PubMed] [Google Scholar]
- 4.Khattab MA, Ferenci P, Hadziyannis SJ, Colombo M, Manns MP, Almasio PL, Esteban R, Abdo AA, Harrison SA, Ibrahim N, et al. Management of hepatitis C virus genotype 4: recommendations of an international expert panel. J Hepatol. 2011;54:1250–1262. doi: 10.1016/j.jhep.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47–52. doi: 10.7150/ijms.3.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pisani P, Parkin DM, Muñoz N, Ferlay J. Cancer and infection: estimates of the attributable fraction in 1990. Cancer Epidemiol Biomarkers Prev. 1997;6:387–400. [PubMed] [Google Scholar]
- 7.Anwar WA, Khaled HM, Amra HA, El-Nezami H, Loffredo CA. Changing pattern of hepatocellular carcinoma (HCC) and its risk factors in Egypt: possibilities for prevention. Mutat Res. 2008;659:176–184. doi: 10.1016/j.mrrev.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Lisker-Melman M, Sayuk GS. Defining optimal therapeutic outcomes in chronic hepatitis. Arch Med Res. 2007;38:652–660. doi: 10.1016/j.arcmed.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 9.El-Zayadi AR, Attia M, Barakat EM, Badran HM, Hamdy H, El-Tawil A, El-Nakeeb A, Selim O, Saied A. Response of hepatitis C genotype-4 naïve patients to 24 weeks of Peg-interferon-alpha2b/ribavirin or induction-dose interferon-alpha2b/ribavirin/amantadine: a non-randomized controlled study. Am J Gastroenterol. 2005;100:2447–2452. doi: 10.1111/j.1572-0241.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- 10.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 11.Poli G, Parola M. Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997;22:287–305. doi: 10.1016/s0891-5849(96)00327-9. [DOI] [PubMed] [Google Scholar]
- 12.Abalea V, Cillard J, Dubos MP, Anger JP, Cillard P, Morel I. Iron-induced oxidative DNA damage and its repair in primary rat hepatocyte culture. Carcinogenesis. 1998;19:1053–1059. doi: 10.1093/carcin/19.6.1053. [DOI] [PubMed] [Google Scholar]
- 13.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 14.Zaher KS, Ahmed WM, Zerizer SN. Observations on the Biological Effects of Black Cumin Seed (Nigella sativa) and Green Tea (Camellia sinensis) Global Veterinaria. 2008;2:198–204. [Google Scholar]
- 15.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Ramadan MF, Kroh LW, Mörsel JT. Radical scavenging activity of black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.), and niger (Guizotia abyssinica Cass.) crude seed oils and oil fractions. J Agric Food Chem. 2003;51:6961–6969. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- 17.Swamy SM, Tan BK. Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella sativa L. seeds. J Ethnopharmacol. 2000;70:1–7. doi: 10.1016/s0378-8741(98)00241-4. [DOI] [PubMed] [Google Scholar]
- 18.Ali BH, Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- 19.Al-Naggar TB, Gómez-Serranillos MP, Carretero ME, Villar AM. Neuropharmacological activity of Nigella sativa L. extracts. J Ethnopharmacol. 2003;88:63–68. doi: 10.1016/s0378-8741(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 20.Salem ML, Hossain MS. In vivo acute depletion of CD8(+) T cells before murine cytomegalovirus infection upregulated innate antiviral activity of natural killer cells. Int J Immunopharmacol. 2000;22:707–718. doi: 10.1016/s0192-0561(00)00033-3. [DOI] [PubMed] [Google Scholar]
- 21.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Meral I, Kanter M. Effects of Nigella sativa L. and Urtica dioica L. on selected mineral status and hematological values in CCl4-treated rats. Biol Trace Elem Res. 2003;96:263–270. doi: 10.1385/BTER:96:1-3:263. [DOI] [PubMed] [Google Scholar]
- 23.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 24.Ciccone E, Viale O, Pende D, Malnati M, Biassoni R, Melioli G, Moretta A, Long EO, Moretta L. Specific lysis of allogeneic cells after activation of CD3- lymphocytes in mixed lymphocyte culture. J Exp Med. 1988;168:2403–2408. doi: 10.1084/jem.168.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson DR. The immunopathogenesis of hepatitis C virus infection. Clin Liver Dis. 2001;5:931–953. doi: 10.1016/s1089-3261(05)70202-6. [DOI] [PubMed] [Google Scholar]
- 26.Joyce MA, Tyrrell DL. The cell biology of hepatitis C virus. Microbes Infect. 2010;12:263–271. doi: 10.1016/j.micinf.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Yildiz F, Coban S, Terzi A, Ates M, Aksoy N, Cakir H, Ocak AR, Bitiren M. Nigella sativa relieves the deleterious effects of ischemia reperfusion injury on liver. World J Gastroenterol. 2008;14:5204–5209. doi: 10.3748/wjg.14.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enomoto S, Asano R, Iwahori Y, Narui T, Okada Y, Singab AN, Okuyama T. Hematological studies on black cumin oil from the seeds of Nigella sativa L. Biol Pharm Bull. 2001;24:307–310. doi: 10.1248/bpb.24.307. [DOI] [PubMed] [Google Scholar]
- 29.Muting D, Wohlgemuth D, Dorsett R. Liver cirrhosis and diabetes mellitus. Geriatrics. 1969;24:91–99. [PubMed] [Google Scholar]
- 30.Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256–1262. doi: 10.1056/NEJM198511143132004. [DOI] [PubMed] [Google Scholar]
- 31.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 32.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 33.Fraser GM, Harman I, Meller N, Niv Y, Porath A. Diabetes mellitus is associated with chronic hepatitis C but not chronic hepatitis B infection. Isr J Med Sci. 1996;32:526–530. [PubMed] [Google Scholar]
- 34.Grimbert S, Valensi P, Lévy-Marchal C, Perret G, Richardet JP, Raffoux C, Trinchet JC, Beaugrand M. High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case-control study. Gastroenterol Clin Biol. 1996;20:544–548. [PubMed] [Google Scholar]
- 35.Ozyilkan E, Arslan M. Increased prevalence of diabetes mellitus in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1996;91:1480–1481. [PubMed] [Google Scholar]
- 36.Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: therapeutic strategies for insulin sensitization. World J Gastroenterol. 2010;16:1943–1952. doi: 10.3748/wjg.v16.i16.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamosa AO, Kaatabi H, Lebdaa FM, Elq AM, Al-Sultanb A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J Physiol Pharmacol. 2010;54:344–354. [PubMed] [Google Scholar]
- 38.Srinivasan R. Autoimmune hemolytic anemia in treatment-naïve chronic hepatitis C infection. J Clin Gastroenterol. 2001;32:245–247. doi: 10.1097/00004836-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Chao TC, Chen CY, Yang YH, Chen PM, Chang FY, Lee SD. Chronic hepatitis C virus infection associated with primary warm-type autoimmune hemolytic anemia. J Clin Gastroenterol. 2001;33:232–233. doi: 10.1097/00004836-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Moccia F, Tognoni E, Boccaccio P. Autoimmune hemolytic anemia in chronic hepatitis C virus infection: an unusual extrahepatic autoimmune manifestation. Ann Ital Med Int. 2001;16:256–259. [PubMed] [Google Scholar]
- 41.Spivak JL. The blood in systemic disorders. Lancet. 2000;355:1707–1712. doi: 10.1016/S0140-6736(00)02249-2. [DOI] [PubMed] [Google Scholar]
- 42.Streiff MB, Mehta S, Thomas DL. Peripheral blood count abnormalities among patients with hepatitis C in the United States. Hepatology. 2002;35:947–952. doi: 10.1053/jhep.2002.32486. [DOI] [PubMed] [Google Scholar]
- 43.Dieterich DT, Spivak JL. Hematologic disorders associated with hepatitis C virus infection and their management. Clin Infect Dis. 2003;37:533–541. doi: 10.1086/376971. [DOI] [PubMed] [Google Scholar]
- 44.Meral I, Donmez N, Baydas B, Belge F, Kanter M. Effect of Nigella sativa L. on heart rate and some haematological values of alloxan-induced diabetic rabbits. Scand J Lab Anim Sci. 2004;31:49–53. [Google Scholar]
- 45.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 46.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41:1211–1219. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 47.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 48.Nagao Y, Sata M. Serum albumin and mortality risk in a hyperendemic area of HCV infection in Japan. Virol J. 2010;7:375. doi: 10.1186/1743-422X-7-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotoh K, Nakamuta M, Fukushima M, Matsuzaki C, Enjoji M, Sakai H, Nawata H. High relative fat-free mass is important for maintaining serum albumin levels in patients with compensated liver cirrhosis. World J Gastroenterol. 2005;11:1356–1360. doi: 10.3748/wjg.v11.i9.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan MH, Farrell GC, Byth K, Lin R, Weltman M, George J, Samarasinghe D, Kench J, Kaba S, Crewe E, et al. Which patients with hepatitis C develop liver complications? Hepatology. 2000;31:513–520. doi: 10.1002/hep.510310236. [DOI] [PubMed] [Google Scholar]
- 51.Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol. 1997;50:693–703. doi: 10.1016/s0895-4356(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 52.Corti MC, Salive ME, Guralnik JM. Serum albumin and physical function as predictors of coronary heart disease mortality and incidence in older persons. J Clin Epidemiol. 1996;49:519–526. doi: 10.1016/0895-4356(95)00562-5. [DOI] [PubMed] [Google Scholar]
- 53.al-Gaby AM. Amino acid composition and biological effects of supplementing broad bean and corn proteins with Nigella sativa (black cumin) cake protein. Nahrung. 1998;42:290–294. doi: 10.1002/(sici)1521-3803(199810)42:05<290::aid-food290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 54.Tollbaand AAH, Hassan MSH. Using some natural additives to improve physiological and productive performance of broiler chicks under high temperature conditions 2 - black cumin (Nigella Sativa) or garlic (Allium sativum) Egypt Poul Sci. 2003;23:327–340. [Google Scholar]
- 55.Shewita RS, Taha AE. Effect of Dietary Supplementation of Different Levels of Black Seed (Nigella Sativa L.) on Growth Performance, Immunological, Hematological and Carcass Parameters of Broiler Chicks. WASET. 2011;77:788–794. [Google Scholar]
- 56.Zaoui A, Cherrah Y, Lacaille-Dubois MA, Settaf A, Amarouch H, Hassar M. [Diuretic and hypotensive effects of Nigella sativa in the spontaneously hypertensive rat] Therapie. 2000;55:379–382. [PubMed] [Google Scholar]
- 57.Sayed-Ahmed MM, Nagi MN. Thymoquinone supplementation prevents the development of gentamicin-induced acute renal toxicity in rats. Clin Exp Pharmacol Physiol. 2007;34:399–405. doi: 10.1111/j.1440-1681.2007.04560.x. [DOI] [PubMed] [Google Scholar]
- 58.Akhtar MS, Riffat S. Field trial of Saussurea lappa roots against nematodes and Nigella sativa seeds against cestodes in children. J Pak Med Assoc. 1991;41:185–187. [PubMed] [Google Scholar]
- 59.Boskabady MH, Farhadi J. The possible prophylactic effect of Nigella sativa seed aqueous extract on respiratory symptoms and pulmonary function tests on chemical war victims: a randomized, double-blind, placebo-controlled trial. J Altern Complement Med. 2008;14:1137–1144. doi: 10.1089/acm.2008.0049. [DOI] [PubMed] [Google Scholar]
- 60.Najmi A, Nasiruddin M, Khan RA, Haque SF. Effect of Nigella sativa oil on various clinical and biochemical parameters of insulin resistance syndrome. Int J Diabetes Dev Ctries. 2008;28:11–14. doi: 10.4103/0973-3930.41980. [DOI] [PMC free article] [PubMed] [Google Scholar]


