Abstract
Extrahepatic portal vein (PV) stenosis has various causes, such as tumor encasement, pancreatitis and as a post-surgical complication. With regard to post-pancreaticoduodenectomy, intraoperative radiation therapy with/without PV resection is reported to be associated with PV stenosis. However, there has been no report of PV stenosis after pancreatectomy following neoadjuvant chemoradiation therapy (NACRT). Here we report the cases of three patients with PV stenosis after pancreatectomy and PV resection following gemcitabine-based NACRT for pancreatic cancer and their successful treatment with stent placement. We have performed NACRT in 18 patients with borderline resectable pancreatic cancer since 2005. Of the 15 patients who completed NACRT, nine had undergone pancreatectomy. Combined portal resection was performed in eight of the nine patients. We report here three patients with PV stenosis, and thus the ratio of post-operative PV stenosis in patients with PV resection following NACRT is 37.5% in this series. We encountered no case of PV stenosis among 22 patients operated with PV resection for pancreatobiliary cancer without NACRT during the same period. A relationship between PV stenosis and NACRT is suspected, but further investigation is required to determine whether NACRT has relevance to PV stenosis.
Keywords: Pancreatic cancer, Portal vein stenosis, Neoadjuvant chemoradiation therapy, Pancreatectomy, Expandable metallic stent
Core tip: Intraoperative radiation therapy for pancreatic cancer with/without portal vein (PV) resection is reported to be associated with PV stenosis. However, there has been no report of PV stenosis after pancreatectomy following neoadjuvant chemoradiation therapy (NACRT). Here we report the cases of three patients with PV stenosis after pancreatectomy and PV resection following gemcitabine-based NACRT for pancreatic cancer and their successful treatment with stent placement. We have performed pancreatectomy with PV resection following NACRT in 8 patients with borderline resectable pancreatic cancer since 2005. The ratio of post-operative PV stenosis is 37.5% in this series.
INTRODUCTION
Extrahepatic portal vein (PV) stenosis can occur due to tumor encasement[1], pancreatitis[2] and as a post-surgical complication-especially post-liver transplantation[3]. With regard to post-pancreaticoduodenectomy (PD), intraoperative radiation therapy (IORT) with/without PV resection is reported to be associated with PV stenosis[4-6]. The incidence rate for PV stenosis is reported to be 11% to 23%[5,6].
Portal hypertension secondary to PV stenosis causes gastrointestinal bleeding from gastroesophageal or jejunal varices, and refractory ascites[1]. Gastrointestinal bleeding is the most serious life-threatening complication. Refractory ascites is not fatal but affects the patient’s quality of life.
Here we provide the case reports of three patients with PV stenosis after pancreatectomy and PV resection following neoadjuvant chemoradiation therapy (NACRT) for pancreatic cancer, and we discuss the relationship between PV stenosis and NACRT and the indications for stent placement. To the best of our knowledge, PV stenosis after pancreatectomy following NACRT has not been described in the literature.
CASE REPORT
Case 1
A 54-year-old man underwent PD and PV resection for pancreatic cancer following NACRT. The protocol consisted of external-beam radiotherapy to the pancreatic bed and regional lymphatics for a total dose of 50.4 Gy in 28 fractions. Concomitant chemotherapy consisted of gemcitabine at a dose of 150 mg/m2 once weekly. The reconstruction between the PV and the superior mesenteric vein (SMV) was end-to-end anastomosis using a continuous running suture of 6-0 prolene. The splenic vein was not reconstructed.
Refractory ascites and malnutrition were recognized at 9 postoperative months (POMs). Computed tomography (CT) showed short segmental stenosis of the PV in the region of the anastomosis, severe ascites, and liver atrophy (Figure 1). Intraoperative portography through the catheter via the ileocolic vein and balloon dilation were performed but were not sufficiently effective because the region had elastic stenosis. Therefore, an expandable metallic stent (EMS; 1 cm diameter, 3 cm length) was inserted into the stenotic region. The portal venous pressure decreased from 14.5 to 8.6 cm H2O, and the pressure gradient of 6.5 cm H2O across the PV stenosis disappeared. Anticoagulant therapy was initiated immediately after stent placement. Heparin was administered at a dose of 10000 IU/d by intravenous infusion for 3 d initially, and then oral warfarin was administered. The warfarin was switched to aspirin 3 mo later. After the stent placement, a follow-up CT showed that the patient’s ascites decreased and his liver atrophy improved (Figure 2). Stent patency is maintained at present, 5 years after the placement.
Figure 1.
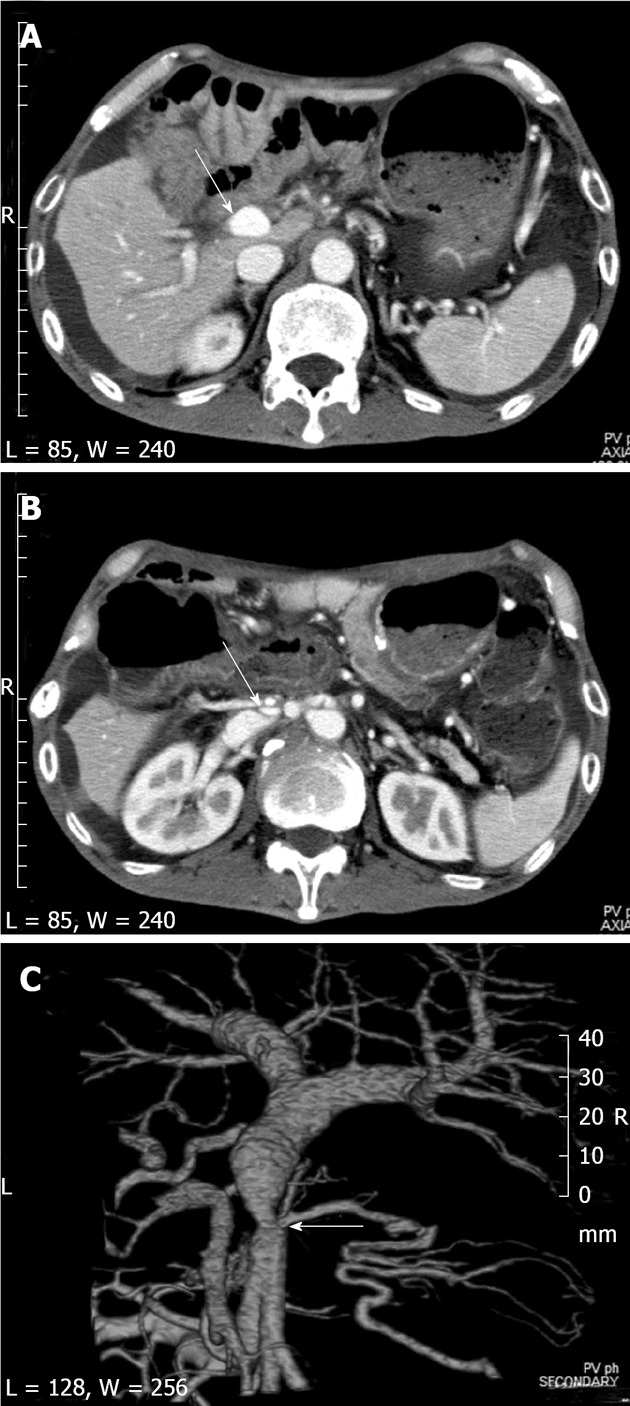
Computed tomography showed short segmental stenosis of the portal vein in the region of the anastomosis, severe ascites, and liver atrophy. A, B: Computed tomography (CT) scan shows severe ascites and liver atrophy (arrow) (A), and stenosis of the portal vein (arrow) behind the superior mesenteric artery (B); C: The image of the 3D reconstruction of the portal vein shows short segmental stenosis in the region of the anastomosis (arrow).
Figure 2.
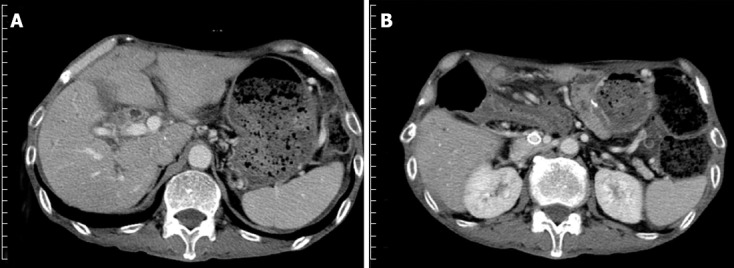
Computed tomography scan 3 mo after the expandable metallic stent placement. A: Shows that the ascites decreased and the liver atrophy improved; B: The stent placed in the portal vein remained patent.
Case 2
A 44-year-old man underwent distal pancreatectomy and PV resection simultaneously with splenectomy and total gastrectomy for pancreatic cancer following NACRT using the same protocol as that described for Case 1. The PV was preoperatively occluded by tumor thrombus, and a cavernous transformation was identified. The reconstruction between the PV and the SMV was end-to-end anastomosis using the same procedure as that described for Case 1. Refractory diarrhea, ascites and malnutrition were recognized at 5 POMs. Initially, malabsorption was suspected, and thus total parenteral nutrition support was initiated. There was no improvement in symptoms after the improvement in nutrition status. CT showed short segmental stenosis of the PV in the region of the anastomosis, collateral circulation through the cavernous transformation of the pancreatic head, severe ascites, and thickness of the intestinal wall (Figure 3). We suspected portal hypertension secondary to PV stenosis, even though the portal venous flow seemed to be sustained by the collateral circulation. Percutaneous transhepatic direct portography was performed (Figure 4A). The portal venous pressure was 31.0 cm H2O, and the pressure gradient across the PV stenosis was 21.0 cm H2O. An EMS (1 cm diameter, 4 cm length) was inserted into the stenotic region (Figure 4B). The portal venous pressure decreased to 17.0 cm H2O, and the pressure gradient decreased to 2.0 cm H2O. Anticoagulant therapy was performed as that described for Case 1 After a stent placement, the patient’s diarrhea and ascites improved. Stent patency is maintained at present, 1.5 years after the placement.
Figure 3.
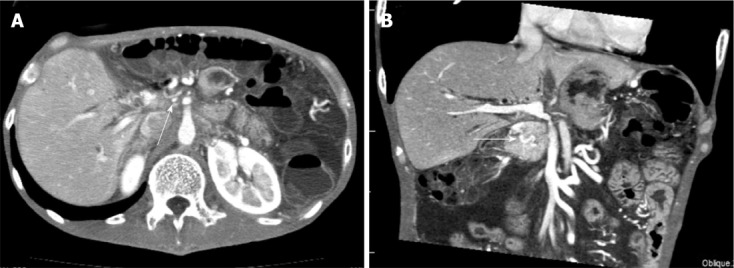
Computed tomography showed short segmental stenosis of the portal vein in the region of the anastomosis, collateral circulation through the cavernous transformation of the pancreatic head, severe ascites, and thickness of the intestinal wall. A: Computed tomography scan showing severe portal vein stenosis (arrow) in the region of the anastomosis; B: Multiplanar reconstruction revealed the collateral circulation through the cavernous transformation of the pancreatic head (arrow), severe ascites and thickness of the intestinal wall.
Figure 4.
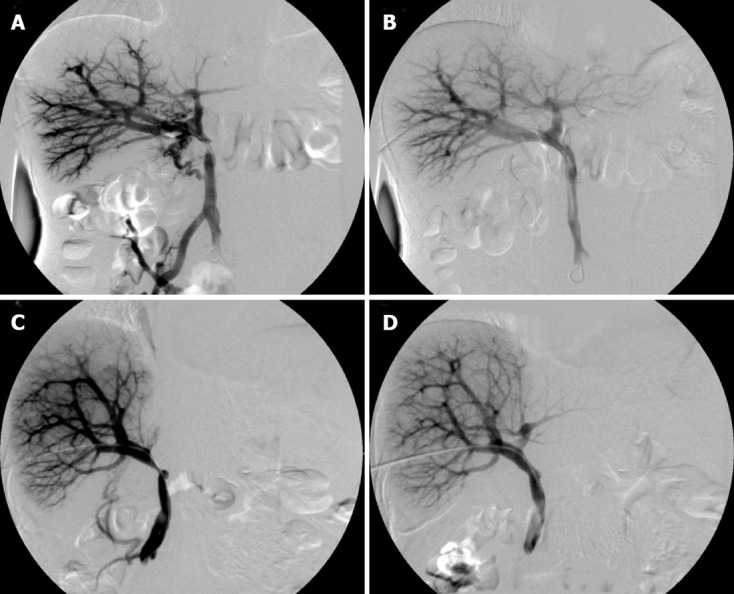
Percutaneous transhepatic direct portography showing short segmental stenosis of the portal vein in the region of the anastomosis. A: Collateral circulation of the pancreatic head; B: After the expandable metallic stent (EMS) placement, the stenosis was improved, and the collateral circulation disappeared; C: The blood flow of the umbilical portion of the left portal vein was unclear; D: The stenosis was improved, and the blood flow of the umbilical portion became clear after the EMS placement.
Case 3
A 68-year-old woman underwent PD and PV resection for pancreatic cancer following NACRT using the same protocol as that described for Case 1. The reconstruction between the PV and the SMV was end-to-end anastomosis using the same procedure as that described for Case 1. Malnutrition and ascites were recognized at 5 POMs. Total parenteral nutrition support and repeated drainage of ascites were performed, but there was no improvement of the ascites. Cytology of the ascites showed no evidence of malignancy. CT showed short segmental stenosis of the PV in the region of the anastomosis. Percutaneous transhepatic direct portography was performed (Figure 4C). The portal venous pressure was 24.5 cm H2O, and the pressure gradient across the PV stenosis was 12.0 cm H2O. An EMS (1 cm diameter, 3 cm length) was inserted into the stenotic region (Figure 4D). The portal venous pressure decreased to 11.5 cm H2O, and the pressure gradient disappeared. Anticoagulant therapy was performed as that described for Case 1. After the stent placement, the patient’s ascites improved. Stent patency is maintained at present, 4 mo after the placement.
DISCUSSION
Despite considerable research, the prognosis for pancreatic cancer remains poor. For all stages combined, the 1- and 5-year relative survival rates are 25% and 6%, respectively[7]. Complete surgical resection is the only therapy to afford a chance of cure[8], but patients with borderline resectable pancreatic cancer are at high risk of having positive surgical margins due to vascular involvement[9]. NACRT for borderline resectable pancreatic cancer is expected to increase the margin-negative resection rate and improve survival[10].
We have performed gemcitabine-based NACRT in 18 patients with borderline resectable pancreatic cancer since 2005. Of 15 patients who completed the NACRT, nine had pancreatectomy. Combined portal resection was performed in 8 of the 9 patients. We report here the cases of three patients with PV stenosis (Table 1), and thus the ratio of post-operative PV stenosis in patients with PV resection following NACRT increases to 37.5% in our patient series.
Table 1.
The clinical characteristics of three patients
| Pt. No. | Age (yr) | Sex | Operative procedure | Symptoms | Months before onset | Procedure of stent placement |
Pressure gradient (cmH2O) |
Improvement in symptoms | |
| Before | After | ||||||||
| 1 | 54 | M | PD | Ascites, malnutrition | 9 | Intraoperative, via ileocolic vein | 6.5 | 0 | Yes |
| 2 | 44 | M | DP | Ascites, malnutrition, diarrhea | 5 | Percutaneous transhepatic | 21.0 | 2.0 | Yes |
| 3 | 68 | F | PD | Ascites, malnutrition | 5 | Percutaneous transhepatic | 12.0 | 0 | Yes |
PD: Pancreaticoduodenectomy; DP: Distal pancreatectom; M: Male; F: Female.
There was no case of PV stenosis among 22 patients who underwent PV resection for pancreatobiliary cancer without NACRT in the same period in our department. The incidence of PV obstruction after PD with PV resection has been reported as 1.5%[11] and 25%[12]. The incidence of PV stenosis after PD with PV resection is rarely reported. Leach et al[12] reported the incidence 18% (5 of 29 patients), but the 18% includes 14 patients who received IORT. Mitsunaga et al[5] suggested that the mechanism of the development of extrahepatic PV occlusion after IORT is associated with the periportal changes induced by IORT and periportal fibrosis. We did not find any reports of PV stenosis after pancreatectomy following NACRT for pancreatic cancer because most papers about NACRT for pancreatic cancer report only perioperative complications[13,14]. However, it seems possible that the periportal changes are induced by neoadjuvant radiation, similar to those induced by IORT, and they increase the risk of the development of PV stenosis.
The first choice of treatment for PV stenosis after liver transplantation is balloon dilation[15]. The indication of stent placement is limited to elastic stenosis and recurrent stenosis. Case 1 had an elastic stenosis, and thus the EMS placement was done after the venoplasty. However, we placed the stents in Cases 2 and 3 before the venoplasty. Placing a stent for benign stenosis before a venoplasty is controversial. Takaki et al[16] speculated that stent placement is essential for the treatment of early anastomotic stenosis because such stenosis is caused by reactive edema or technical problems, and balloon angioplasty fails to dilate the vessel due to recoil. The onset of the stenosis in the three cases presented here was also early (5-9 POMs). Considering the poor prognosis of pancreatic cancer, early improvement of a patient’s symptoms and quality of life is important.
In Case 1, we placed the stent via ileocolic vein because massive ascites interfered with transhepatic puncture. However, in Cases 2 and 3, we could safely performed percutaneous transhepatic stent placement after draining the ascites.
In conclusion, we have reported the cases of three patients with PV stenosis after pancreatectomy and PV resection following NACRT for pancreatic cancer. A relationship between PV stenosis and NACRT is suspected, but further investigation is required to determine whether NACRT has relevance to PV stenosis.
Footnotes
P- Reviewer Aurello P S- Editor Wen LL L- Editor A E- Editor Xiong L
References
- 1.Novellas S, Denys A, Bize P, Brunner P, Motamedi JP, Gugenheim J, Caroli FX, Chevallier P. Palliative portal vein stent placement in malignant and symptomatic extrinsic portal vein stenosis or occlusion. Cardiovasc Intervent Radiol. 2009;32:462–470. doi: 10.1007/s00270-008-9455-9. [DOI] [PubMed] [Google Scholar]
- 2.Woodrum DA, Bjarnason H, Andrews JC. Portal vein venoplasty and stent placement in the nontransplant population. J Vasc Interv Radiol. 2009;20:593–599. doi: 10.1016/j.jvir.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Kawano Y, Mizuta K, Sugawara Y, Egami S, Hisikawa S, Sanada Y, Fujiwara T, Sakuma Y, Hyodo M, Yoshida Y, et al. Diagnosis and treatment of pediatric patients with late-onset portal vein stenosis after living donor liver transplantation. Transpl Int. 2009;22:1151–1158. doi: 10.1111/j.1432-2277.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu Y, Yasui K, Fuwa N, Arai Y, Yamao K. Late complication in patients undergoing pancreatic resection with intraoperative radiation therapy: gastrointestinal bleeding with occlusion of the portal system. J Gastroenterol Hepatol. 2005;20:1235–1240. doi: 10.1111/j.1440-1746.2005.03913.x. [DOI] [PubMed] [Google Scholar]
- 5.Mitsunaga S, Kinoshita T, Kawashima M, Konishi M, Nakagohri T, Takahashi S, Gotohda N. Extrahepatic portal vein occlusion without recurrence after pancreaticoduodenectomy and intraoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2006;64:730–735. doi: 10.1016/j.ijrobp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Hoffer EK, Krohmer S, Gemery J, Zaki B, Pipas JM. Endovascular recanalization of symptomatic portomesenteric venous obstruction after pancreaticoduodenectomy and radiation. J Vasc Interv Radiol. 2009;20:1633–1637. doi: 10.1016/j.jvir.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 7.American Cancer Society Atlanta. Cancer Facts and Figures 2010. Available from: http: //www.cancer.org/research/cancerfactsfigures/cancerfactsfigures/cancer-facts-and-figures-2010.
- 8.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, Zheng H, Szymonifka J, Wargo JA, Thayer SP, Lauwers GY, Deshpande V, Mino-Kenudson M, et al. Pancreatic ductal adenocarcinoma: long-term survival does not equal cure. Surgery. 2012;152:S43–S49. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Abrams RA, Lowy AM, O’Reilly EM, Wolff RA, Picozzi VJ, Pisters PW. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1751–1756. doi: 10.1245/s10434-009-0413-9. [DOI] [PubMed] [Google Scholar]
- 11.Yekebas EF, Bogoevski D, Cataldegirmen G, Kunze C, Marx A, Vashist YK, Schurr PG, Liebl L, Thieltges S, Gawad KA, et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg. 2008;247:300–309. doi: 10.1097/SLA.0b013e31815aab22. [DOI] [PubMed] [Google Scholar]
- 12.Leach SD, Lee JE, Charnsangavej C, Cleary KR, Lowy AM, Fenoglio CJ, Pisters PW, Evans DB. Survival following pancreaticoduodenectomy with resection of the superior mesenteric-portal vein confluence for adenocarcinoma of the pancreatic head. Br J Surg. 1998;85:611–617. doi: 10.1046/j.1365-2168.1998.00641.x. [DOI] [PubMed] [Google Scholar]
- 13.Evans DB, Varadhachary GR, Crane CH, Sun CC, Lee JE, Pisters PW, Vauthey JN, Wang H, Cleary KR, Staerkel GA, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 14.Ohigashi H, Ishikawa O, Eguchi H, Takahashi H, Gotoh K, Yamada T, Yano M, Nakaizumi A, Uehara H, Tomita Y, et al. Feasibility and efficacy of combination therapy with preoperative full-dose gemcitabine, concurrent three-dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg. 2009;250:88–95. doi: 10.1097/SLA.0b013e3181ad65cc. [DOI] [PubMed] [Google Scholar]
- 15.Shibata T, Itoh K, Kubo T, Maetani Y, Shibata T, Togashi K, Tanaka K. Percutaneous transhepatic balloon dilation of portal venous stenosis in patients with living donor liver transplantation. Radiology. 2005;235:1078–1083. doi: 10.1148/radiol.2353040489. [DOI] [PubMed] [Google Scholar]
- 16.Takaki H, Yamakado K, Nakatsuka A, Uraki J, Usui M, Sahurai H, Isaji S, Takeda K. Stent placement for portal venous stenosis following major abdominal surgery. Hepatogastroenterology. 2009;56:407–410. [PubMed] [Google Scholar]


