Abstract
Pulmonary permeability oedema is a frequent complication in a number of life-threatening lung conditions, such as ALI and ARDS. Apart from ventilation strategies, no specific therapy yet exists for treatment of these potentially fatal illnesses. The oedema-reducing capacity of the lectin-like domain of TNF (TIP) and of synthetic peptides, mTIP and hTIP, which mimic the TIP domain of mouse and human TNF, have been demonstrated in various studies in rodents. Cell-based electrophysiological studies have revealed that the alveolar fluid clearing capacity of TNF and the TIP peptides is due to activation of the amiloride-sensitive Na+ current in alveolar epithelial cells and that the primary site of action is on the apical side of these cells.
AP301, a synthetic cyclic peptide mimicking the TIP domain of human TNF is currently undergoing clinical trials as a therapy for pulmonary permeability oedema. AP301 has been shown to improve alveolar liquid clearance and lung function in a porcine model of ALI.
For non-clinical regulatory assessment, dog, pig and rat are standard animal models; accordingly, pre-clinical toxicological and pharmacological safety studies have been conducted with AP301 in dogs and rats. Hitherto, no studies have assessed the pharmacodynamic effect of AP301 on primary canine or porcine type II AEC. The current study describes the effect of AP301 on the amiloride-sensitive Na+ current in type II AEC isolated from dog, pig and rat lungs. In whole cell patch clamp experiments with dog type II AEC, an increase in the amiloride-sensitive Na+ current from 3.7 pA to 49.4 pA was observed in the presence of AP301; in pig type II AEC, an increase from 10.0 pA to 159.6 pA was observed, and in rat AEC, from 6.9 pA to 62.4 pA. In whole cell patch clamp experiments in A549 cells, AP301-induced enhancement of the amiloride-sensitive current was eliminated when Na+ in the bath solution was replaced with N-methyl-D-glucamine (NMDG), and when the cells were pre-incubated with 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), an inhibitor of ENaC, but enhancement was unaffected by addition of cyclic nucleotide-gated (CNG) channel inhibitors Zn2+ or L-cis-diltiazem prior to AP301.
These results provide strong evidence that AP301 activates the amiloride-sensitive Na+ current through ENaC in type II AEC from dog, pig and rat. To our knowledge, this is the first cell-based analysis of the oedema-clearing effect of AP301 observed in the porcine model of pulmonary oedema. Furthermore, the results validate the dog and pig models in non-clinical assessment of AP301.
Keywords: AP301, ENaC, synthetic cyclic peptide, lectin-like domain, alveolar fluid clearance, type II alveolar cells, patch clamp
1. Introduction
Pulmonary permeability oedema is a frequent complication in a number of severe and life-threatening lung conditions, such as ALI, ARDS or ischaemia reperfusion injury, the latter of which can cause primary graft failure following lung transplantation. In ALI/ARDS patients, higher rates of alveolar fluid clearance (AFC) have been associated with better clinical outcome [1]. Currently there is no specific therapy available for pulmonary permeability oedema apart from ventilation strategies.
A wealth of evidence shows that the amiloride-sensitive epithelial Na+ channel, ENaC, plays a critical role in maintaining lung fluid balance in both healthy, physiological conditions as well as in cases of lung injury [2, 3], although evidence does exist for amiloride-insensitive fluid reabsorption in the mature lung [4, 5]. The current consensus hypothesis for the mechanism of AFC is that water is reabsorbed from the alveolar space through the epithelium and into the underlying interstitium, along the osmotic gradient created by the uptake of Na+ ions, which passively enter the epithelium through apically-located ENaC [6, 7], although there is ongoing discussion regarding the relative role of ENaC compared to the contribution of other Na+ permeable cation channels, such as cyclic nucleotide-gated (CNG) channels in this process [5]; the electrochemical gradient is maintained across the epithelium by the basolaterally-located Na+-K+-ATPase, which actively pumps Na+ ions out of alveolar epithelial cells through the basal membrane. Water is subsequently removed from the lungs via the lymphatic or capillary system.
The synthetic, cyclic peptide, AP301, which mimics the lectin-like domain (TIP) of human TNF [8] is being developed as a therapy for pulmonary oedema. Several early studies using rodent models have indicated that TIP peptide is an important regulator of alveolar fluid balance in healthy and injured lungs. Specifically, 1) TIP peptide derived from the murine TNF sequence (mTIP), administered intratracheally, improved AFC in an in situ flooded mouse lung and an ex vivo model of flooded rat lungs, the improvement being absent when amiloride was administered concomitantly with the peptide, [9]; 2) TIP peptide derived from the human TNF sequence (hTIP) activated fluid reabsorption in in situ and in vivo flooded rat lung models [10]; 3) mTIP decreased pulmonary oedema in isolated, endotoxin-injured rabbit lungs, but not when the lungs were pretreated with amiloride [11]. Moreover, hTIP, instilled intratracheally into rats prior to lung transplantation, significantly improved lung function, indicating its use as a potential therapy for ischaemia reperfusion injury associated with lung transplantation; the beneficial effect of TIP on oxygenation in these experiments was completely inhibited by cotreating the animals with amiloride, demonstrating that the effect of the TIP peptide is mediated by its effect on amiloride-sensitive Na+ uptake [12]. In a recent study using a porcine bronchoalveolar lavage (BAL) model of ALI, inhalation of nebulised AP301 (hTIP) resulted in an increased PaO2/FiO2 ratio and reduced EVLWI (extravascular lung water index) [13]. Cell-based, electrophysiological studies have demonstrated that the mTIP enhances the amiloride-sensitive Na+ current in microvascular endothelial cells from mouse lungs [14] and also in A549 cells [15], a human lung carcinoma cell line resembling type II alveolar epithelial cells. Furthermore, experiments with monolayers of rat alveolar epithelial type II cells in Ussing chambers have indicated that hTIP exerts an ENaC-enhancing effect from the apical side of these cells [12]. Hitherto, there are no reports of the effect of the TIP peptide on amiloride-sensitive Na+ current in primary alveolar type II cells from the dog or the pig.
Dog, pig and rat models are amongst the most widely used in non-clinical development of pharmaceutical substances. In the present study, the effect of AP301 on amiloride-sensitive Na+ current in a whole cell voltage-clamped patch clamp assay using freshly isolated canine and porcine alveolar epithelial type II cells is investigated, with the aim of establishing the relevance of non-rodent animal models such as dog and pig for toxicity and pharmacological safety testing during non-clinical development of AP301. Moreover, electrophysiological testing of the effect of AP301 on freshly-isolated porcine alveolar type II cells aims to investigate at the cellular level the mechanism underlying the observed improvement in alveolar fluid balance and lung function following inhalation of nebulised AP301 in the porcine model of ALI.
2. Materials and Methods
2.1. Chemicals and reagents
All chemicals and reagents and culture media were obtained from Sigma-Aldrich (Vienna, Austria), unless otherwise stated.
AP301 peptide
The cyclic peptide AP301 was synthesised by solid-phase peptide synthesis according to the fluorenylmethyloxycarbonyl/t-butyl protection strategy on 2-chlorotritylchloride resin, by Senn Chemicals AG, Dielsdorf, Switzerland. Details of the synthesis have been previously described [16]. Cyclisation in AP301 was achieved by oxidation of the terminal cysteine residues to form a disulphide bridge. The purity of the peptide was 96.3%. m/z (ESI) 1924.2 (M++1); theoretical average molecular mass 1923.1.
2.2. Harvesting of lung tissue
Canine lung parenchymal tissue, obtained from the Clinic for Small Animal Surgery, University of Veterinary Medicine in Vienna, was harvested from a dog immediately following euthanasia due to a splenic tumour with abdominal metastasis; no heart or lung metastasis was visible on heart ultrasound, lung X-rays and macroscopic examination of the lungs; histological examination showed no metastasis to lung tissue. Porcine lung tissue was obtained from the Department of Thoracic Surgery at the Medical University Vienna; during an operation (left thoracotomy through the sixth intercostal space) a small piece of parenchymal tissue was harvested from the lung periphery; the experiment had been approved by the Ethics Committee of the Medical University of Vienna. Canine and porcine lung parenchymal tissue was kept for up to 1 hour at 0–4 °C in Perfadex (Vitrolife Sweden AB) solution, pH 7.4, prior to execution of the cell isolation protocol. Whole lungs from laboratory rats were used as a source of rat lung tissue.
2.3. Isolation of alveolar type II cells
Dog, rat and pig alveolar epithelial type II cells were isolated as previously described [17, 18]. Elastase digestion of minced lung tissue with some modifications [19] was used to isolate alveolar cells. Enrichment for dog, pig or rat alveolar type II cells was achieved by removal of macrophages and lymphocytes using differential adherence on bacteriological plates (37 °C, 10% CO2) coated with dog IgG (Fitzgerald, MA, US), pig or rat IgG, respectively. Cells thus isolated were used in the patch clamp protocol as described below.
2.4. Cell culture
Cells of the human alveolar carcinoma cell line, A549 (ATTAC no. CCL-185) in passages 80–90 were used in experiments to test the effect of nebulisation on the potency of AP301 as well as in ion substitution and other experiments performed to further characterise the nature of the current elicited by AP301. Cells were grown in Dulbecco’s modified Eagle’s medium/nutrient mixture Ham’s F-12K (Life Technologies, Life Tech Austria), supplemented with 10% fetal bovine serum and containing 1% penicillin-streptomycin.
2.5. Effect of nebulisation on the potency of AP301
In a separate series of experiments designed to test whether the nebulisation process altered the potency of AP301 in solution, the effect on the amiloride-sensitive Na+ current of different concentrations of nebulised and untreated solutions of AP301 was measured in a whole cell patch clamp assay using A549 cells. A stock solution of AP301 in distilled water was nebulised in an Aeroneb Solo nebuliser chamber (Aerogene, Ireland) and the nebulised spray was trapped and condensed in a centrifuge tube held in an ice-bath. The condensate was used in a whole cell patch clamp assay using A549 cells; untreated AP301 stock solution was used as control. Aliquots of the stock solution were cumulatively added to the bath solution, resulting in final concentrations ranging from 3.5 to 480 nM nebulised and untreated AP301, respectively. Concentration-response curves were plotted for the nebulised and untreated solutions of AP301. Concentration-response curves and EC50 values were fitted and estimated for currents recorded at a holding potential, Ehof -100 mV with SigmaPlot 9.0.
2.6. Patch clamp protocol
Measurement of the amiloride-sensitive sodium ion current in enzymatically isolated alveolar epithelial type II cells from dog, pig and rat lungs was carried out using the patch clamp technique in whole cell mode [20].
Glass cover slips with the freshly isolated type II cells settled on the surface, were transferred to the assay chamber of capacity 1 mL, secured on the stage of a microscope (Axiovert 100, 400x magnification). The assay chamber contained 1 mL of the bath solution of the following composition (in mM): 145 NaCl, 2.7 KCl, 1.8 CaCl2, 2 MgCl25.5 glucose and 10 HEPES, adjusted to pH 7.4 with 1 M NaOH solution. Cells were totally submerged throughout. Alveolar epithelial type II cells could be distinguished from other cell types present by their small size, irregular surface appearance and lamellar inclusion bodies. With the aid of the microscope one suitable cell (fixed to the glass cover slip) was chosen and the microelectrode, a fire-polished glass pipette was pressed against the surface of the cell. Micropipettes were pulled from thin-walled borosilicate glass capillaries (World Precision Instruments, Inc., FL, USA) with a Flaming Brown micropipette puller (P87, Sutter Instruments, CA, USA) and polished on a microforge (Narishige, Tokyo, Japan) to obtain electrode resistances ranging from 2.0 to 3.5 MΩ. The pipette contained solution of the following composition (in mM): 135 potassium methane sulphonate, 10 KCl, 6 NaCl, 1 Mg2ATP, 2 Na3ATP, 10 HEPES and 0.5 EGTA, adjusted to pH 7.2 with 1 M KOH solution. A gentle suction was applied so as to obtain a “gigohm seal” between the glass pipette and the cell membrane. For the whole-cell recordings performed in the present study, the membrane under the electrode tip was subsequently ruptured by application of continued suction, such that current flowing through all the ion channels in one cell could be measured. The cell, whose interior comes into contact with the pipette solution, is voltage-clamped to membrane potential via a pre-amplifier (CV-203BU Headstage, Axon Instruments) and amplifier (Axopatch 200B, Axon Instruments). At the beginning of each measurement, the holding potential, Ehwas set to zero by application of the appropriate current and after equilibration, the cell membrane was clamped at a holding potential of −100 (Eh = −100 mV). Currents were filtered at 5 kHz and recorded at 10 kHz. Acquisition, storage and analysis of data were carried out by pCLAMP 10.0 software (Axon Instruments, CA, USA).
After GΩ seal formation, and an equilibrium phase of 5 min, the voltage was clamped at −100mV for a further 5 min. After recording current, AP301 dissolved in distilled water was added directly to the bath solution in the assay chamber. The solution of AP301 was of such composition that addition to the bath solution of 1 µL resulted in a final concentration of peptide in the bath solution of 240 nM. The −100 mV holding potential was chosen as observations from previous experiments with cells of the human alveolar carcinoma cell line, A549, had indicated that at this potential a maximum increase in the inward Na+ current was obtained [16]. The inward Na+ current was measured following addition of AP301 and results analysed using the computer program pCLAMP, version 10.0. Amiloride (up to 1 mM) was added to the bath solution after recording the current with AP301, in order to prove that the current affected was indeed that flowing through the amiloride-sensitive sodium ion channel (ENaC). Amiloride-sensitive current was calculated by subtracting the current in the presence of amiloride from the current recorded before amiloride was added to the bath solution.
2.7. Ion substitution experiments
Ion substitution experiments were performed using A549 cells in order to further characterise the current elicited by AP301. In one set of experiments, Na+-aspartate (140 mM) was used in place of NaCl in the bath solution of the whole cell patch clamp assay and in second set of experiments, N-methyl-D-glucamine (NMDG)-Cl (140 mM) was used in place of NaCl, in order to test whether AP301 affects primarily the Cl− or the Na+ current, respectively. AP301 was added to a final concentration of 120 nM to the bath solution as it was known from previous work that a maximum response is obtained with this concentration of AP301 in A549 cells.
2.8. Investigation of effect of CNG inhibitors
The effect of the CNG channel blockers Zn2+ [5] and L-cis-diltiazem [21] on the amiloride-sensitive current elicited by AP301 was tested separately in a whole cell patch clamp assay using A549 cells. ZnSO4 (1 mM) or L-cis-diltiazem (300 µM) was added to the bath solution after the equilibrium phase and before the addition of AP301 (120 nM). Finally, amiloride (100 µM) was added to the bath solution to distinguish the amiloride-sensitive current.
2.9. Investigation of effect of ENaC inhibitor AICAR
The effect of the AMP mimetic drug, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), previously shown to inhibit amiloride-sensitive Na+-permeable channels in H441 cells [21], was tested in a whole cell patch clamp assay in A549 cells. The cells were pre-incubated with AICAR (2 mM) for one hour prior to the patch clamp assay. After the control phase to establish a steady baseline current, AP301 (120 nM) was added to the bath solution and the current recorded for a further five minutes.
3. Results
For each species, dog, pig and rat, alveolar epithelial type II cells were successfully isolated from freshly harvested lung parenchymal tissue. The type II cells were subjected to a patch clamp assay in whole cell mode in order to measure the effect of AP301 on the amiloride-sensitive Na+ current.
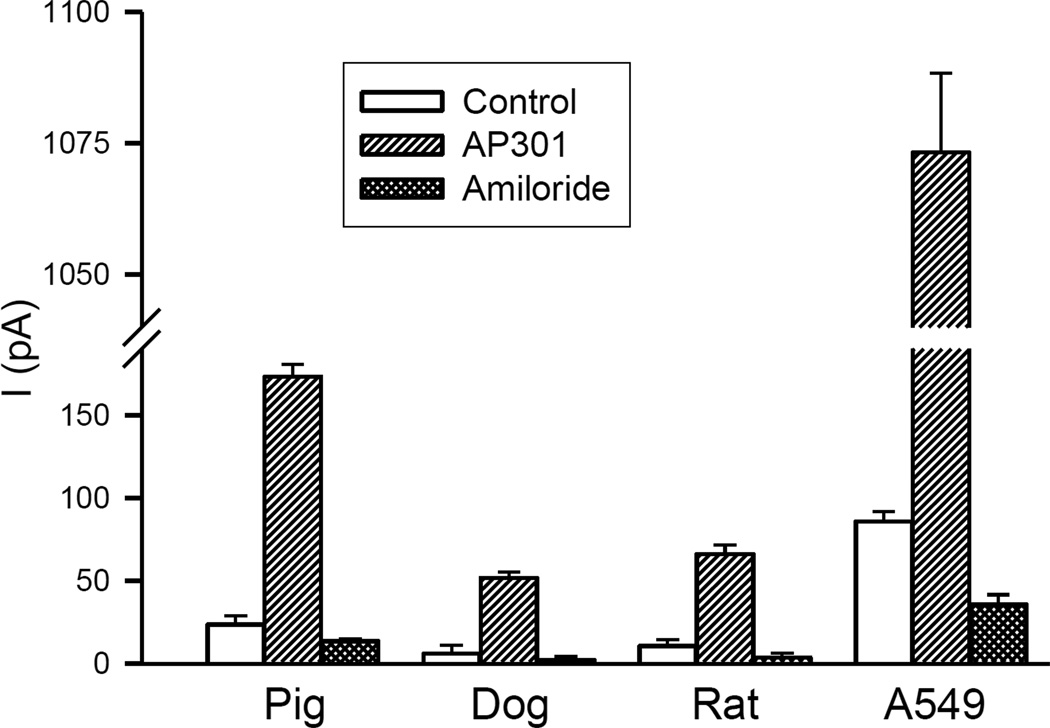
Following addition of AP301 (240 nM final concentration) to the bath solution, a steady increase in the inward Na+ current was observed with all cell species types. Subsequent addition of amiloride, starting at a concentration of 10 µM with gradual addition up to a maximum concentration of 1 mM, caused this enhanced Na+ current to drop to levels lower than the control recordings taken in the absence of AP301. In control experiments without addition of AP301, the concentration of amiloride required to achieve complete blockage was 10 µM. Thus, AP301 enhanced an amiloride-sensitive Na+ current in dog, pig and rat type II cells (Table 1).
Table 1.
Effect of AP301 on amiloride-sensitive Na+ current (pA) in dog (n=3), pig (n=5) and rat (n=3) alveolar type II cells in whole cell patch clamp assay at −100 mV holding potential. Values are mean +/− SE.
| Dog | Pig | Rat | |
|---|---|---|---|
| Control | 6.1 ± 4.9 | 23.6 ± 1.3 | 10.7 ± 3.8 |
| AP301, 240 nM | 51.8 ± 3.7 | 173.2 ± 7.4 | 66.2 ± 5.3 |
| Amiloride, 1 mM | 2.4 ± 2.0 | 13.6 ± 1.3 | 3.8 ± 2.5 |
The Na+ current-enhancing effect of AP301 varied between the different cell species types both quantitatively and qualitatively (Figures 1–3). Thus, AP301 caused an increase in the Na+ current from a mean control value of 6.1 ± 4.9 pA to 51.8 ± 3.7 pA in the dog, compared to an increase from 23.6 ± 1.3 pA to 173.2 ± 7.4 pA in the pig and an increase from 10.7 ± 3.8 pA to 66.2 ± 5.3 pA in the rat type II cells (Table 1). The effect of AP301 on the Na+ current in the primary type II cells of all species types was approximately an order of magnitude lower than the response observed in a previous study using A549 cells, in which a maximal response to AP301 had been observed at a current level of 1073 ± 15 pA [16].
Figure 1.
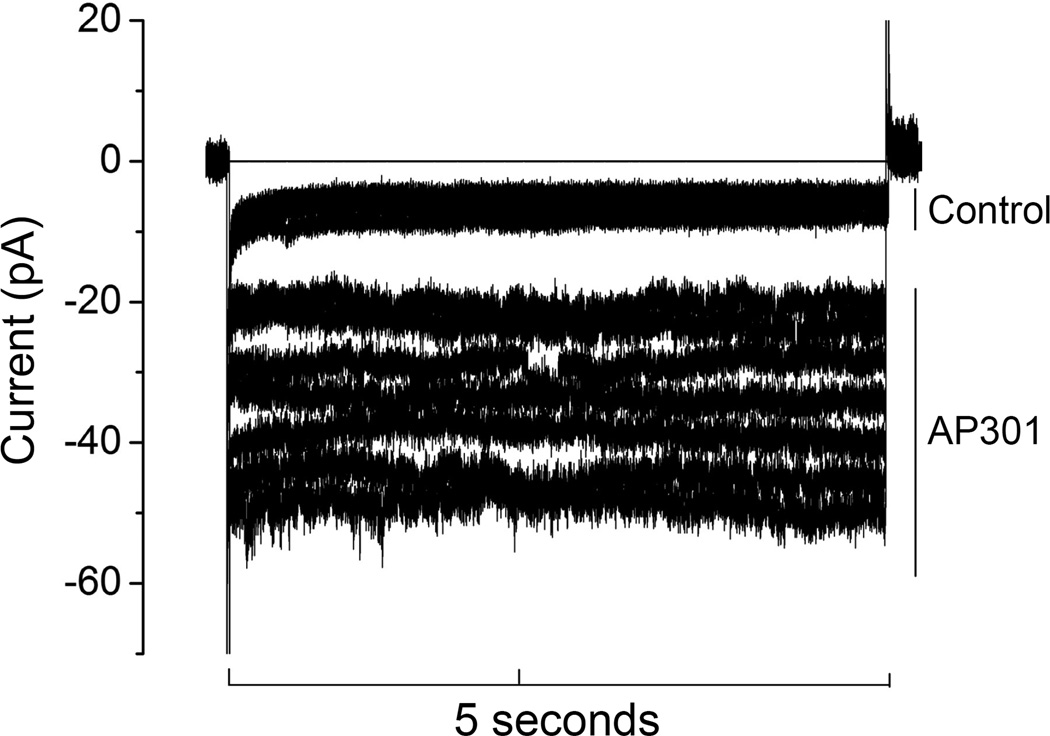
Effect of AP301 on Na+ current in a dog alveolar epithelial type II cell patched in the whole cell mode. Representative original recording from a cell clamped at a holding potential of - 100 mV during control phase and following addition of AP301 (240 nM) to the bath solution.
Figure 3.
Mean values of inward Na+ currents in dog (n= 3), pig (n=5) and rat (n=3) primary alveolar epithelial type II cells and A549 cells (n=5), clamped at −100 mV in a whole cell patch, during control phase, following addition of AP301 (240 nM) and after final addition of amiloride (1 mM) to the bath solution. Values are mean +/−SE.
The time taken for the current-enhancing effect to manifest itself also varied between the different species. Namely, in dog and rat alveolar type II cells the inward Na+ current began to increase within one minute of adding AP301 to the bath solution (Figure 2), whereas in the case of the pig type II cells an increase was first seen after 15 minutes of adding AP301. Previous experiments have shown that AP301 elicits an immediate increase in the amiloride sensitive Na+ current in A549 cells [16], in a similar manner to the effect observed in the present study with dog and rat primary type II cells.
Figure 2.
Effect of AP301 on Na+ current in a dog, pig and rat alveolar epithelial type II cells patched in the whole cell mode. Inward current was elicited by application of a pulse of −100 mV. Addition of AP301 (240 nM) and amiloride (1 mM) to the bath solution was performed in each case at the time point indicated by the arrow.
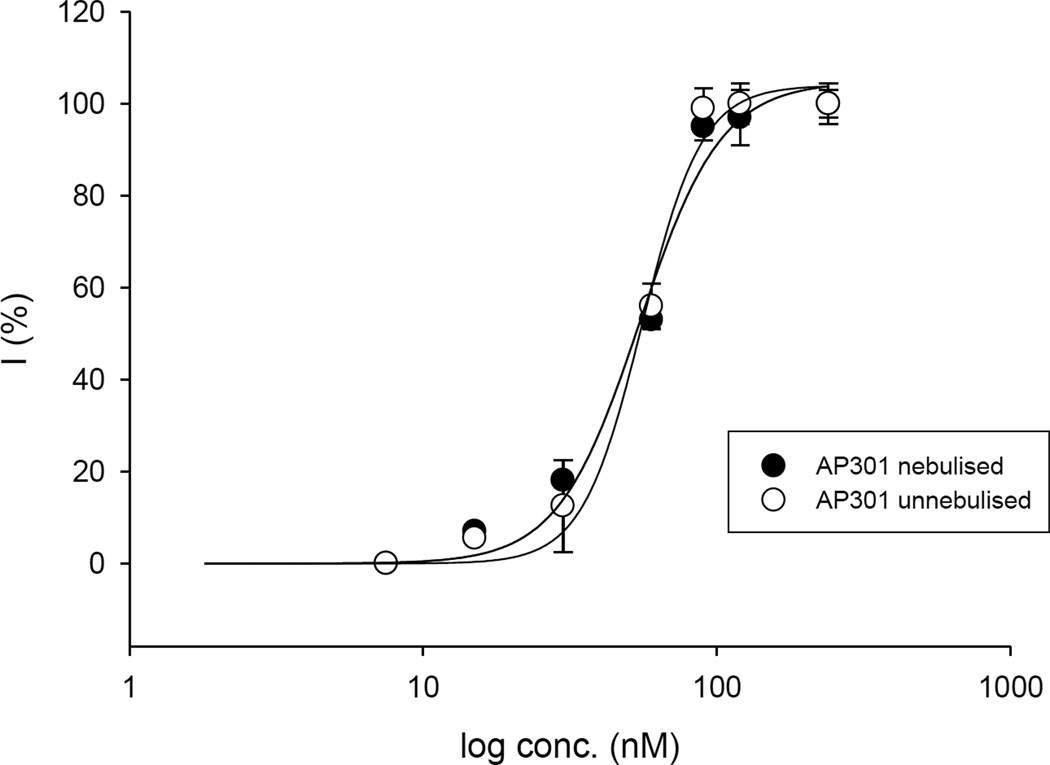
The dose response curves obtained for untreated and nebulised solutions of AP301 are shown in Figure 4.
Figure 4.
Concentration response curves for nebulised and untreated AP301 in A549 cells; mean values +/−SE for three to five experiments are given.
Nebulisation was found to have no effect on the potency of AP301 to increase the Na+ current in A549 cells in a whole cell patch clamp assay. Specifically, the EC50 for non-nebulised AP301 was 54.3 ± 0.8 nM and for nebulised AP301, 53.5 ± 1.0 nM (Figure 4).
Ion substitution experiments
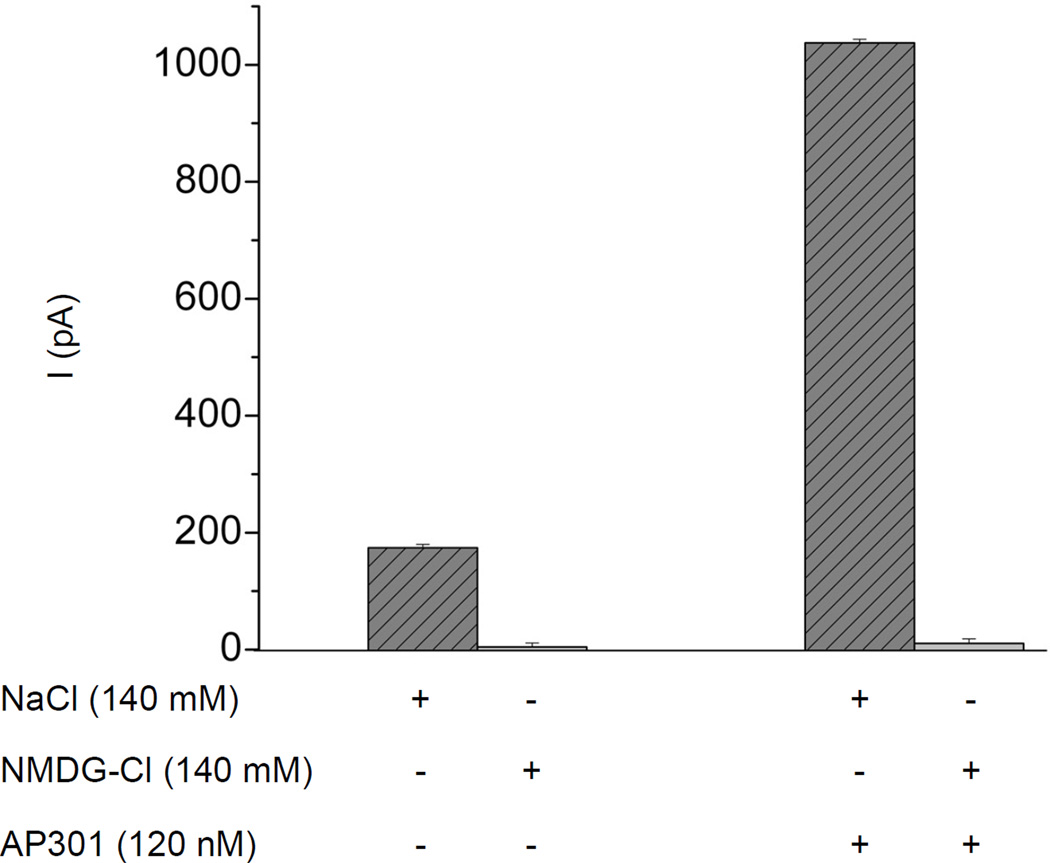
Replacement of the Cl− counterion with aspartate (140 mM) in the bath solution of a whole cell patch clamp assay in A549 cells, had no effect on the current enhancing effect of AP301. In contrast, replacement of Na+by substituting NaCl with NMDG-Cl (140 mM), resulted in abolition of the current enhancing effect of AP301 (Figure 5).
Figure 5.
Effect on AP301-induced current of replacing NaCl in the bath solution with equimolar concentration of NMDG-Cl (140 mM) in a whole cell patch clamp assay using A549 cells (n=5). Mean values (+/−SE) of inward currents in cells clamped at −100 mV.
Effect of CNG channel inhibitors
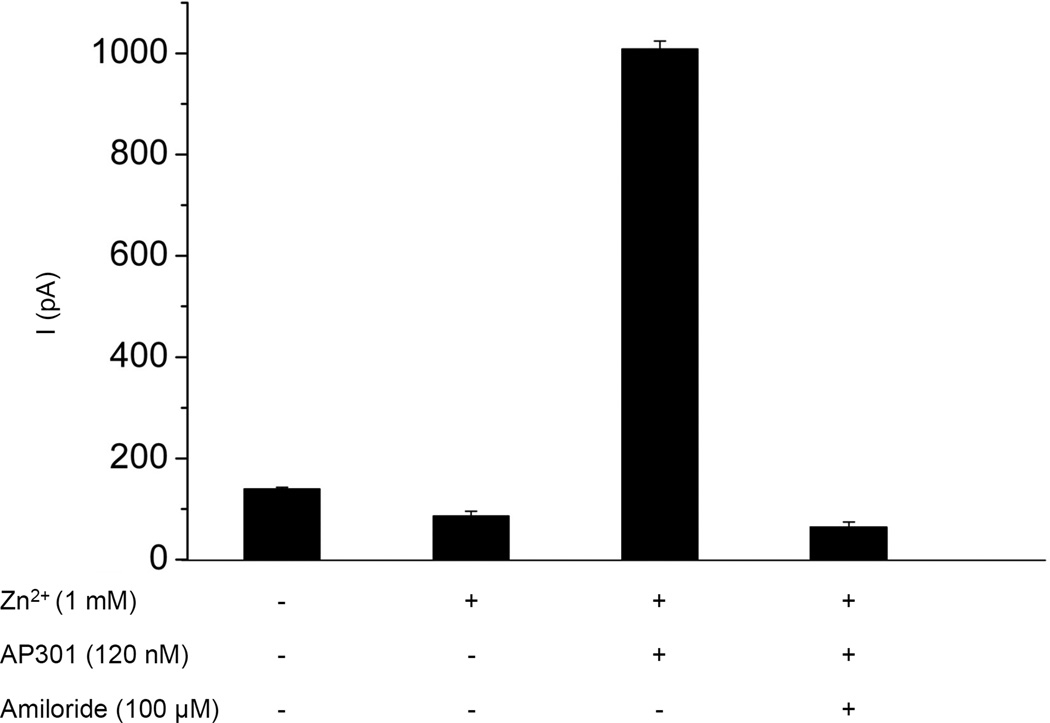
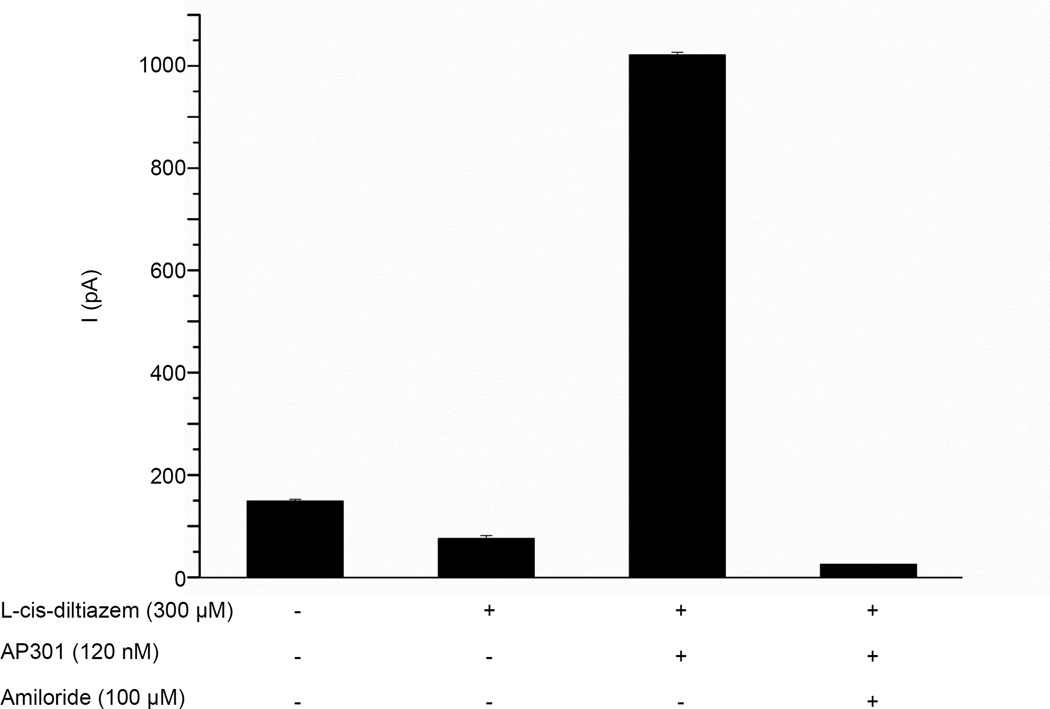
Nonselective cyclic nucleotide-gated cation channels (CNG) have been shown to be functionally expressed in alveolar epithelial type II cells, suggesting that these channels may contribute to alveolar liquid clearing capacity in the lung [5]. In order to investigate whether this channel might be involved in the current enhancing effect of AP301 observed in pneumocytes, whole patch clamp experiments were carried out in A549 cells using the CNG channel inhibitors L-cis-diltiazem and Zn2+. Addition of 1 mM Zn2+ to the bath solution resulted in a reduction in the current from a control value of 139.8 ± 3.1 pA to 86.1 ± 9.6 pA and subsequent addition of AP301 (120 nM) brought about an increase in the current to 1008.5 ± 15.8 pA; final additon of amiloride (100 µM) reduced the current to 63.5 ± 10.7 pA (Figure 6a). Thus, CNG channels are likely to be present in the A549 cells as evidenced by the initial fall in current by ~53.7 pA following addition of Zn2+but they are not responsible for the current enhancing effect of AP301, since the effect was still observed in the presence of Zn2+ at concentrations found elsewhere to be inhibitory to CNG channels. Similarly, addition of 300 µM L-cis-diltiazem to the bath solution resulted in an initial reduction of the current from a control value of 148.8 ± 3.5 pA to 76.0 ± 5.8 pA and subsequent addition of AP301 (120 nM) resulted in an increase in the current to 1021.3 ± 4.9 pA; final addition of amiloride (100 µM) resulted in a reduction of the current to 24.7 ± 3.7 pA (Figure 6b). Again this reinforces the results obtained with Zn2+ and suggests that the current enhancing effect of AP301 is due to its effect on amiloride-sensitive Na+ channels.
Figure 6.
Effect of inhibitors of CNG channels on amiloride-sensitive Na+ current induced by AP301 in A549 cells patched in whole cell mode. Inward current elicited by a pulse of −100 mV. a) Mean values of inward currents during control phase, following sequential addition of Zn2+ (1 mM), AP301 (120 nM) and final addition of amiloride (100 µM) to the bath solution (n=5). b) Mean values of inward currents during control phase, following sequential addition of L-cis-diltiazem (300 µM), AP301 (120 nM) and final addition of amiloride (100 µM) to the bath solution (n=3). Values are mean +/−SE.
Effect of AICAR an inhibitor of amiloride-sensitive transepithelial Na+ conductance
Pre-incubation of A549 cells with AICAR (2 mM), which is known to decrease the activity of amiloride-sensitive Na+-permeable channels in H441 cells [21], resulted in an almost complete abolition of the current-enhancing effect of AP301 (120 nM) when added to the bath solution in a whole cell patch clamp experiment. This result suggests that AP301 exerts its current-enhancing effect by activating Na+ current through amiloride-sensitive Na+ channels.
4. Discussion
The amiloride-sensitive epithelial sodium channel (ENaC), located apically in cells of the alveolar epithelium, plays a dominant role in uptake of Na+ from alveolar fluid and its involvement in alveolar fluid clearance has been demonstrated in many studies [3, 6, 22–24]. In this study we show that the synthetic cyclic peptide, AP301, which mimics the lectin-like domain (TIP) of human TNF, enhances the amiloride-sensitive Na+ current in primary alveolar epithelial type II cells from dog, pig and rat lungs. Previous studies have documented the capacity of the lectin-like domain of murine TNF-α to enhance amiloride-sensitive Na+ current in cells of the human lung carcinoma cell line, A549 [15], and of mTIP peptide to increase the membrane conductance in murine microvascular endothelial cells [14], and to enhance Na+ uptake in monolayers of rat type II cells [12]. Experiments using rat alveolar type II cell monolayers in Ussing chambers indicated that the hTIP peptide enhances the amiloride-sensitive Na+ current from the apical side of these cell layers [12] and the hTIP peptide, AP301, has been shown to enhance amiloride-sensitive Na+ current in A549 cells [16]. To our knowledge, this is the first time that the ability of a TNF lectin-like domain derived peptide to enhance the amiloride-sensitive Na+ current has been demonstrated in primary dog and pig alveolar epithelial type II cells in vitro. The results indicate that the dog and pig are suitable and relevant species in which to carry out toxicological and pharmacological safety studies of AP301. In addition, the study demonstrates that nebulisation of a solution of the therapeutic compound does not diminish its potency.
The present study provides strong evidence that the amiloride-sensitive Na+ current enhanced by AP301 is indeed that which flows through ENaC. The apical membranes of type II cells have been shown to contain nonselective cation channels [5] and these could also be responsible for the increase in current elicited by AP301. The cyclic nucleotide-gated (CNG) nonselective cation channels responsible for amiloride-resistant Na+ conductance described in type II cells by Kemp and co-workers [5] could be possible candidates. However, since the current-enhancing effect of AP301 was not perturbed by the presence of the CNG blocking agents L-cis-diltiazem and Zn2+ (1 mM) in the bath solution in whole cell patch clamp experiments with A549 cells (Figure 6a and b), the possibility that AP301 activates current through these channels seems rather unlikely. Furthermore, the experiments described here were conducted on type II cells immediately after isolation from freshly harvested lung tissue, and so CNG channel expression, which is usually only seen in type II cells after two or more days in culture [5], would not be expected.
Evidence from several independent studies suggests that Na+ transport through lung epithelia is mediated by a diversity of types of amiloride-sensitive channels, differing in their Na+/K+ selectivity as well as in the degree to which they are sensitive to amiloride [3]. Single channel studies have indicated that there are at least four different types of Na+ conducting channels in epithelial apical membranes which are blockable by amiloride [25]. These are: 1) Highly Na+ selective cation (HSC) channels with single-channel conductance of 4–6 pS and a Na+/K+ selectivity of >40 [26, 27]; 2) Moderately selective (Type I) channels with single channel conductance of 8–9 pS and a Na+/K+ selectivity of 5–8; 3); Moderately selective (Type II) channels with single channel conductance of 56 pS and a Na+/K+ selectivity of 7; 4) Nonselective cation (NSC) channels with single-channel conductance of 19–24 pS and an Na+/K+ selectivity of ~1.4. The abundance and relative distribution of HSC and NSC channels is determined by culture conditions and environment [27]. Both HSC and NSC channels with conductances of ~ 8.8 and 22.5 pS, respectively, have been implicated in Na+ transport during single channel patch clamp analysis of type II cells in live lung tissue slices [28]. The same study also identified the presence of anion channels with 10 pS conductance in the apical membrane of type II cells [28]. In adult type II alveolar cells grown on permeable support at an air interface and in the presence of steroids, the predominant channel was found to be HSC with a conductance of ~7 pS and a Na+/K+ selectivity of >80 which was inhibited by submicromolar concentrations of amiloride [27].
The HSC channel in the lung resembles the classical ENaC cloned from rat colon, which comprises three homologous subunits alpha, beta and gamma [29]. Heterologous expression studies using various combinations of these subunits and experiments using antisense oligonucleotides to differentially block their synthesis, have shown that HSC channels are composed of all three different subunits, and that the NSC channels comprise purely alpha subunits [27, 30]. Results from such studies have led to the hypothesis that the diverse range of amiloride-sensitive Na+ channels observed in lung epithelia results from expression of ENaCs with different combinations of alpha, beta and gamma subunits and that the moderately selective channels are some combination of alpha with beta or gamma [25].
In the present and previous studies we have used cells from the A549 cell line originating from a human alveolar cell carcinoma, as a model for alveolar type II cells. Ion substitution studies have characterised the amiloride-sensitive Na+ channels in A549 cells as being moderately selective for Na+ (Na+/K+ permeability ratio, 6:1) with a unitary conductance of ~ 8.6 pS [22]. In a previous study by our group, single channel experiments in A549 cells revealed that the amiloride-sensitive Na+ current elicited by AP301 and similar peptides was characterised by a conductance of ~ 9.4 pS and that the peptides were highly specific for amiloride-sensitive Na+ current [16]. Thus amiloride-sensitive Na+ current which is enhanced by AP301 in A549 cells is of a conductance corresponding to that of moderately selective ENaC and is blockable by a concentration of amiloride slightly higher than that required to block HSC channels. In the present study on freshly-harvested type II cells, no current-enhancing effect was observed with AP301 when Na+ was replaced by NMDG in the bath solution (Figure 5). Taken together these results suggest that in type II cells as well as in cultured A549 cells, the amiloride-sensitive current enhanced by AP301 is that which flows through the amiloride-sensitive Na+ channel, ENaC of the moderately selective (Type I) type.
The possibility that AP301 exerts its effect via alteration of the chloride current, Cl−flowing through anion channels was eliminated in our study by the observation that AP301 continued to elicit a current enhancing effect in ion substitution experiments using A549 cells, in which Cl− in the bath solution had been replaced with aspartate. On the other hand, substituting NaCl in the bath solution with NMDG-Cl, completely abolished the AP301-elicited enhancement of the amiloride-sensitive current, as mentioned previously. Thus the results of the ion substitution experiments presented here show that the primary effect of AP301 is due to alterations in the amiloride-sensitive Na+ current rather than the Cl− current.
Since amiloride can block different channels with similar affinities [31], we carried out experiments using AICAR, which has been shown to inhibit amiloride-sensitive transepithelial Na+ transport and apical Na+ entry through highly Na+ selective ENaC-like channels (HSC) and nonselective cation channels (NSC) in human H441 Clara-like airway epithelial cells [21]. Pre-incubation of A549 cells with AICAR (2 mM) for one hour prior to a whole cell patch clamp assay resulted in an almost total abolition of the AP301 induced current-enhancing effect. This is convincing evidence that AP301 exerts its current enhancing effect by activation of ENaC.
Thus the work presented here together with that of previous studies with lectin-like domain derived peptides in A549 cells, leads us to the conclusion that AP301 activates the amiloride-sensitive current through ENaC in type II alveolar cells from dog, pig and rat.
The findings of the present study are consistent with the ameliorative effect of inhaled, nebulised AP301 observed in the pig model of ALI [13], and provide further evidence that enhanced activity of ENaC in cells of the alveolar epithelium leads to improved fluid reabsorption from the alveolar space.
The quantitative increase in the amiloride-sensitive Na+ current brought about by AP301 varied between the different cell types. Specifically, AP301 elicited an increase in the amiloride-sensitive Na+ current in primary type II alveolar epithelial cells from the pig from 10.0 pA to 159.6 pA, an approximately 16-fold increase. Similar fold increases were elicited by AP301 in primary cells from the dog and rat although the absolute amount of current was lower in these two species (Figure 3). However the increases observed in primary cells were one order of magnitude lower than the previously recorded increase in amiloride-sensitive Na+ current from 50 pA to 1037.3 pA, elicited by AP301 in A549 cells [16]. These differences might be due to a differing number of ENaC channels active in the various cell types, since the 20-fold increase in amiloride-sensitive Na+ current brought about by AP301 in A549 cells is of the same order as the 16-fold increase seen in the primary cells in the present study. The differences may also be attributable to different subunit composition of ENaC channels and relative abundance of the resulting ENaC types in A549 compared to freshly isolated type II alveolar cells (according to the hypothesis of Eaton and co-workers, outlined above). A549 is an adenocarcinoma cell line and the cells may differ slightly in their biochemistry from freshly isolated type II cells. A549 cells are used frequently in electrophysiological experiments as a model for alveolar type II cells [22], but the results of the present study suggest that they may respond differently from physiologically normal cells in the presence of substances which influence ion-channel activity.
An intriguing feature of the responses of the different cell types to AP301 was the delayed increase in amiloride-sensitive Na+ current elicited by AP301 in the pig type II cells compared to all the other cell types. In primary type II cells from the dog and rat as well as in A549 cells, an immediate increase in the Na+ current was observed upon addition of AP301 to the bath solution in the whole cell patch clamp assay. In the case of the primary type II cells from the pig, an increase in the Na+ current was first seen 15 minutes after addition of AP301 to the bath solution. This could be related to species-dependent activity of membrane-bound, channel-activating proteases involved in ENaC activation and trafficking (for recent reviews see [32] and [33]).
The lectin-like or TIP domain which comprises residues C101 to E116 in human TNF [8] specifically binds oligosaccharides such as N,N’-diacetylchitobiose and branched trimannoses [8, 34]. This interaction has been associated with the trypanolytic properties of the lectin-like domain [8] as well as with its capacity to bring about an amiloride-sensitive increase in sodium transport in microvascular epithelial cells [14]. Similarly, administration of of N,N’-diacetylchitobiose together with hTIP in an in vivo flooded rat lung model blocked the alveolar fluid clearance effect observed when hTIP alone was used [9]. Equally critical for the ENaC-activating effect of the lectin-like domain are three residues, a threonine and two glutamic acids, as both mutant TNF and mutant TIP peptide in which these residues have been replaced with alanines, showed neither the amiloride-sensitive Na+ current-enhancing effect in A549 cells [16] nor the improved alveolar fluid clearance in in situ and in vivo flooded rat lungs brought about by the wild type counterparts [10, 15]. A multiple sequence alignment of the TNF TIP region in the species of relevance to the present study, shows that these critical residues, corresponding to T6, T8 and T11 in AP301, are completely conserved, whereas there are interspecies differences at other positions (Figure 7). Namely, the rat and mouse sequences differ at four and the dog at only one position from the human sequence. The TIP domain of TNF is identical in the pig and humans. The high degree of sequence similarity of the TNF TIP regions of these species, underpins the use of dog, pig and rodent models in non-clinical studies.
Figure 7.
Multiple sequence alignment of TIP domain in human, pig, dog, rat and mouse TNF, mTIP peptide and hTIP peptide/AP301. The human and pig sequences are identical, the dog differs at one position, and the mouse and rat sequences, which are identical, differ at four positions from the human TNF sequence. Three residues which have been shown to be essential for the ENaC-enhancing activity of the TIP domain are conserved throughout all species (highlighted and underlined); in AP301 these residues are T6, E8 and E11 [8]. Both human and mouse TIP peptides are derived from the corresponding native sequences by replacing the N-terminal (P) and C-terminal (E) residues with cysteine residues and replacing the native cysteine with a glycine residue. Cyclisation of the TIP peptides is effected by oxidation of the terminal cysteine residues [8]. Alignment carried out manually using Accelrys DS Visualiser 2.0.
4.1. Conclusion
The results of the present study show that AP301, a synthetic, cyclic peptide mimicking the lectin-like domain of human TNF, is effective at enhancing the amiloride-sensitive Na+ current, in primary dog, pig and rat alveolar type II cells. The study provides direct experimental evidence that dog, pig and rat models are relevant species for non-clinical assessment of ion channel modulators such as AP301. In addition, the study demonstrates that nebulisation of AP301 solution has no effect on its potency and is therefore suitable as a therapeutic mode of administration.
Acknowledgements
W.S. and P.H. received financial support from the Austrian Research Promotion Agency (FFG). This work was partially supported by a research grant from NHBLI, NIH (Grant RO1HL094609 to R.L.).
Abbreviations
amino acids and designation of peptides according to the IUPAC-IUB Joint Commission on Biochemical Nomenclature Recommendations 1983 (IUPAC-IUB, 1984).
- AEC
alveolar epithelial cell
- AFC
alveolar fluid clearance
- ALI
acute lung injury
- ARDS
acute respiratory distress syndrome
- ENaC
epithelial sodium channel
- EVLWI
extravascular lung water index
- TIP
lectin-like domain of TNF
- hTIP
synthetic peptide mimicking the TIP domain of human TNF
- mTIP
synthetic peptide mimicking the TIP domain of murine TNF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 2.Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol. 2006;35:10–19. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annual review of physiology. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- 4.Junor RW, Benjamin AR, Alexandrou D, Guggino SE, Walters DV. A novel role for cyclic nucleotide-gated cation channels in lung liquid homeostasis in sheep. The Journal of physiology. 1999;520(Pt 1):255–260. doi: 10.1111/j.1469-7793.1999.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp PJ, Kim KJ, Borok Z, Crandall ED. Re-evaluating the Na(+) conductance of adult rat alveolar type II pneumocytes: evidence for the involvement of cGMP-activated cation channels. The Journal of physiology. 2001;536:693–701. doi: 10.1111/j.1469-7793.2001.t01-1-00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthiaume Y, Matthay MA. Alveolar edema fluid clearance and acute lung injury. Respir Physiol Neurobiol. 2007;159:350–359. doi: 10.1016/j.resp.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Althaus M, Clauss WG, Fronius M. Amiloride-sensitive sodium channels and pulmonary edema. Pulm Med. 2011;2011:830320. doi: 10.1155/2011/830320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas R, Magez S, De Leys R, Fransen L, Scheerlinck JP, Rampelberg M, et al. Mapping the lectin-like activity of tumor necrosis factor. Science. 1994;263:814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- 9.Elia N, Tapponnier M, Matthay MA, Hamacher J, Pache JC, Brundler MA, et al. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor-alpha. American journal of respiratory and critical care medicine. 2003;168:1043–1050. doi: 10.1164/rccm.200206-618OC. [DOI] [PubMed] [Google Scholar]
- 10.Braun C, Hamacher J, Morel DR, Wendel A, Lucas R. Dichotomal role of TNF in experimental pulmonary edema reabsorption. J Immunol. 2005;175:3402–3408. doi: 10.4049/jimmunol.175.5.3402. [DOI] [PubMed] [Google Scholar]
- 11.Vadasz I, Schermuly RT, Ghofrani HA, Rummel S, Wehner S, Muhldorfer I, et al. The lectin-like domain of tumor necrosis factor-alpha improves alveolar fluid balance in injured isolated rabbit lungs. Crit Care Med. 2008;36:1543–1550. doi: 10.1097/CCM.0b013e31816f485e. [DOI] [PubMed] [Google Scholar]
- 12.Hamacher J, Stammberger U, Roux J, Kumar S, Yang G, Xiong C, et al. The lectin-like domain of tumor necrosis factor improves lung function after rat lung transplantation--potential role for a reduction in reactive oxygen species generation. Crit Care Med. 2010;38:871–878. doi: 10.1097/CCM.0b013e3181cdf725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann EK, Boehme S, Duenges B, David M, Markstaller K. Effect of the TNF-alpha derived Peptide AP301 in porcine acute lung injury. American Society of Anesthesiologists Annual Meeting. 2011 [Google Scholar]
- 14.Hribar M, Bloc A, van der Goot FG, Fransen L, De Baetselier P, Grau GE, et al. The lectin-like domain of tumor necrosis factor-alpha increases membrane conductance in microvascular endothelial cells and peritoneal macrophages. European journal of immunology. 1999;29:3105–3111. doi: 10.1002/(SICI)1521-4141(199910)29:10<3105::AID-IMMU3105>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda N, Jayr C, Lazrak A, Wang Y, Lucas R, Matalon S, et al. Mechanisms of TNF-alpha stimulation of amiloride-sensitive sodium transport across alveolar epithelium. American journal of physiology Lung cellular and molecular physiology. 2001;280:L1258–L1265. doi: 10.1152/ajplung.2001.280.6.L1258. [DOI] [PubMed] [Google Scholar]
- 16.Hazemi P, Tzotzos SJ, Fischer B, Andavan GS, Fischer H, Pietschmann H, et al. Essential structural features of TNF-alpha lectin-like domain derived peptides for activation of amiloride-sensitive sodium current in A549 cells. J Med Chem. 2010;53:8021–8029. doi: 10.1021/jm100767p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. The American review of respiratory disease. 1986;134:141–145. doi: 10.1164/arrd.1986.134.1.141. [DOI] [PubMed] [Google Scholar]
- 18.Dobbs LG. Isolation and culture of alveolar type II cells. The American journal of physiology. 1990;258:L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 19.Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest. 2011;91:363–378. doi: 10.1038/labinvest.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archiv : European journal of physiology. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 21.Albert AP, Woollhead AM, Mace OJ, Baines DL. AICAR decreases the activity of two distinct amiloride-sensitive Na+-permeable channels in H441 human lung epithelial cell monolayers. American journal of physiology Lung cellular and molecular physiology. 2008;295:L837–L848. doi: 10.1152/ajplung.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazrak A, Samanta A, Matalon S. Biophysical properties and molecular characterization of amiloride-sensitive sodium channels in A549 cells. American journal of physiology Lung cellular and molecular physiology. 2000;278:L848–L857. doi: 10.1152/ajplung.2000.278.4.L848. [DOI] [PubMed] [Google Scholar]
- 23.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiological reviews. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 24.Sartori C, Matthay MA. Alveolar epithelial fluid transport in acute lung injury: new insights. European Respiratory Journal. 2002;20:1299–1313. doi: 10.1183/09031936.02.00401602. [DOI] [PubMed] [Google Scholar]
- 25.Eaton DC, Chen J, Ramosevac S, Matalon S, Jain L. Regulation of Na+ channels in lung alveolar type II epithelial cells. Proc Am Thorac Soc. 2004;1:10–16. doi: 10.1513/pats.2306008. [DOI] [PubMed] [Google Scholar]
- 26.Chen XJ, Eaton DC, Jain L. Beta-adrenergic regulation of amiloride-sensitive lung sodium channels. American journal of physiology Lung cellular and molecular physiology. 2002;282:L609–L620. doi: 10.1152/ajplung.00356.2001. [DOI] [PubMed] [Google Scholar]
- 27.Jain L, Chen XJ, Ramosevac S, Brown LA, Eaton DC. Expression of highly selective sodium channels in alveolar type II cells is determined by culture conditions. American journal of physiology Lung cellular and molecular physiology. 2001;280:L646–L658. doi: 10.1152/ajplung.2001.280.4.L646. [DOI] [PubMed] [Google Scholar]
- 28.Helms MN, Jain L, Self JL, Eaton DC. Redox regulation of epithelial sodium channels examined in alveolar type 1 and 2 cells patch-clamped in lung slice tissue. The Journal of biological chemistry. 2008;283:22875–22883. doi: 10.1074/jbc.M801363200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 30.Jain L, Chen XJ, Malik B, Al-Khalili O, Eaton DC. Antisense oligonucleotides against the alpha-subunit of ENaC decrease lung epithelial cation-channel activity. The American journal of physiology. 1999;276:L1046–L1051. doi: 10.1152/ajplung.1999.276.6.L1046. [DOI] [PubMed] [Google Scholar]
- 31.Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiological reviews. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 32.Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Current topics in developmental biology. 2007;78:23–46. doi: 10.1016/S0070-2153(06)78002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaillard EA, Kota P, Gentzsch M, Dokholyan NV, Stutts MJ, Tarran R. Regulation of the epithelial Na+ channel and airway surface liquid volume by serine proteases. Pflugers Archiv : European journal of physiology. 2010;460:1–17. doi: 10.1007/s00424-010-0827-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marquardt A, Bernevic B, Przybylski M. Identification, affinity characterisation and biological interactions of lectin-like peptide-carbohydrate complexes derived from human TNF-alpha using high-resolution mass spectrometry. J Pept Sci. 2007;13:803–810. doi: 10.1002/psc.902. [DOI] [PubMed] [Google Scholar]