Abstract
This study aimed to investigate whether CHROMagar Acinetobacter medium (CHROMagar, France) in combination with an antimicrobial supplement (modified CHROMagar Acinetobacter; CHROMagar, France) can be used for detecting and isolating multidrug-resistant Acinetobacter species (MRA) in nasal and rectal surveillance cultures. Nasal and rectal swab samples were collected from patients in an intensive care unit at a teaching hospital. The samples were used to inoculate modified CHROMagar Acinetobacter plates, which were examined after 24 and 48 hr of incubation at 37℃. Their susceptibility against the antimicrobial agents meropenem, imipenem, ciprofloxacin, and amikacin was analyzed using the Etest (bioMerieux, France). A total of 406 paired samples (406 nasal swabs and 406 rectal swabs) were obtained from 226 patients, and 120 samples (28 nasal and 28 rectal cultures, 47 nasal cultures only, and 17 rectal cultures only) yielded MRA. Seventy-five MRA isolates (18.5%) were recovered from the 406 nasal samples, and 45 MRA isolates (11.1%) were recovered from the 406 rectal samples. Of the 120 MRA isolates, 3 (2.5%) were detected only after 48 hr of incubation. The use of modified CHROMagar Acinetobacter together with nasal and rectal swabs and 1-day incubation is an effective surveillance tool for detecting MRA colonization.
Keywords: Modified CHROMagar Acinetobacter, Multidrug-resistant Acinetobacter spp., Nasal swab, Rectal swab
Acinetobacter species has emerged as an important cause of healthcare-associated infections [1-3]. In many parts of the world, strains resistant to almost all available antimicrobial agents, including carbapenems, have been reported and implicated in numerous hospital outbreaks. These outbreaks usually occur in intensive care units (ICUs). Acinetobacter spp. are extremely difficult to eradicate from the environment [4-7]. Increased morbidity and mortality associated with these outbreaks have prompted some hospitals to begin active surveillance in high-risk patients to control the spread of Acinetobacter spp. [8].
CHROMagar Acinetobacter (CHROMagar, Paris, France) is a recently developed medium for selective and rapid identification of Acinetobacter spp. It contains agents that inhibit the growth of most yeasts and gram-positive cocci and uses a color-change method that permits identification of Acinetobacter spp. as red colonies within 18-24 hr of incubation. Previous studies have reported that CHROMagar Acinetobacter is highly sensitive (91.7%) and specific (89.7%) for multidrug-resistant Acinetobacter baumannii (MRAB) [9]. Although CHROMagar Acinetobacter cannot distinguish carbapenem-resistant from carbapenem-susceptible A. baumannii [10], it may be a useful medium for diagnostic screening and for improving strategies to control infections and limit the spread of MRAB. However, this requires improvement of the medium for better selection for MRAB [11]. The objective of this study was to analyze the performance of CHROMagar Acinetobacter modified by the addition of an antimicrobial supplement for the detection and isolation of MRA in nasal and rectal surveillance cultures.
Modified CHROMagar Acinetobacter was prepared from dehydrated powder and liquid supplement added in the form of the antimicrobial selective supplement (CR 102; CHROMagar, Paris, France), according to the manufacturer's instructions. Nasal and rectal swab samples were collected from ICU patients as part of active surveillance screening for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci, respectively. The samples were used to inoculate the modified CHROMagar Acinetobacter plates, which were then incubated at 37℃ and examined after 24 and 48 hr for oxidase-negative and bright salmon-red colonies indicative of MRA. Isolates identified as Acinetobacter spp. were tested using the Vitek 2 system (bioMerieux Vitek, Hazelwood, MO, USA) to confirm their identity. Antimicrobial susceptibilities were analyzed using Etest strips (bioMerieux, Marcy-I'Etoile, France) and interpreted on the basis of the current Clinical and Laboratory Standards Institute guidelines [12]. Resistance of the Acinetobacter spp. isolates was tested against meropenem, imipenem, ciprofloxacin, and amikacin, and multidrug resistance (MR) was defined as resistance against 3 or all of these antimicrobial agents.
A total of 406 paired samples (406 nasal swabs and 406 rectal swabs) were obtained from 226 patients admitted to the medical and surgical ICUs at a teaching hospital in Korea during a 2-month study period (February-March 2012). A total of 120 samples (28 nasal and 28 rectal cultures, 47 nasal cultures only, 17 rectal cultures only) yielded MRA (Table 1). In a previous study that had assessed the MRA colonization rates of 3 body sites, including skin, pharynx, and digestive tract, the detection rates of Acinetobacter spp. were similar (75-77%) for armpit, pharynx, and rectum [13]. However, our findings demonstrated that the use of the nasal swab resulted in higher sensitivity (nasal, 18.5% vs. rectal, 11.1%) for the detection of MRA colonization than the rectal swab, and that a combination of nasal and rectal swabs increased the detection rates (22.7%) of MRA colonization. The identity of all 120 isolates as Acinetobacter spp., as diagnosed with the modified CHROMagar Acinetobacter medium, was confirmed using the Vitek 2 system (bioMerieux Vitek).
Table 1.
Samples used and the minimum inhibitory concentration (MIC) of four antimicrobial drugs for 120 multidrug-resistant Acinetobacter strains identified with modified CHROMagar Acinetobacter
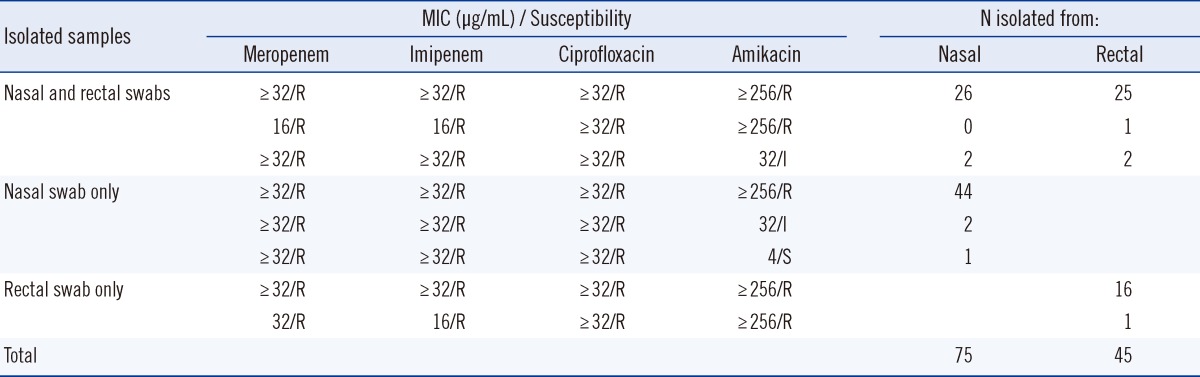
Abbrebiations: R, resistant; I, intermediate; S, susceptible.
All the 120 Acinetobacter spp. isolates were confirmed to be MR by the Etest (Table 1). In another study that yielded similar results, addition of the KPC supplement (CHROMagar, Paris, France) to CHROMagar Acinetobacter enabled selective recovery of carbapenem-resistant A. baumannii [14]. In the current study, 75 MRA isolates (18.5%) were recovered from 406 nasal samples, and 45 MRA isolates (11.1%) were recovered from 406 rectal samples (Table 1). Three (2.5%) of the 120 MRA isolates were detected only after 48 hr of incubation. Another 10 samples appeared as pinpoint-sized, colorless colonies at 24 hr that turned red at 48 hr. These 10 isolates tested oxidase positive and were identified as Pseudomonas spp. (data not shown). Therefore, extension of the incubation time does not improve the detection of MRA.
The limitations of this study are that these evaluations were performed only in a clinical setting and in the absence of a comparable medium. However, these results show that the use of modified CHROMagar Acinetobacter together with nasal and rectal swabs and 1-day incubation is an effective active surveillance tool for the detection of MRA colonization.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, Kim SW, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother. 2007;59:525–530. doi: 10.1093/jac/dkl499. [DOI] [PubMed] [Google Scholar]
- 2.Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden H, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wareham DM, Bean DC, Khanna P, Hennessy EM, Krahe D, Ely A, et al. Bloodstream infection due to Acinetobacter spp.: epidemiology, risk factors and impact of multi-drug resistance. Eur J Clin Microbiol Infect Dis. 2008;27:607–612. doi: 10.1007/s10096-008-0473-y. [DOI] [PubMed] [Google Scholar]
- 4.Agustí C, Pujol M, Argerich MJ, Ayats J, Badía M, Domínguez MA, et al. Short-term effect of the application of selective decontamination of the digestive tract on different body site reservoir ICU patients colonized by multi-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2002;49:205–208. doi: 10.1093/jac/49.1.205. [DOI] [PubMed] [Google Scholar]
- 5.Héritier C, Dubouix A, Poirel L, Marty N, Nordmann P. A nosocomial outbreak of Acinetobacter baumannii isolates expressing the carbapenem-hydrolysing oxacillinase OXA-58. J Antimicrob Chemother. 2005;55:115–118. doi: 10.1093/jac/dkh500. [DOI] [PubMed] [Google Scholar]
- 6.Kohlenberg A, Brümmer S, Higgins PG, Sohr D, Piening BC, de Grahl C, et al. Outbreak of carbapenem-resistant Acinetobacter baumannii carrying the carbapenemase OXA-23 in Germany university medical centre. J Med Microbiol. 2009;58:1499–1507. doi: 10.1099/jmm.0.012302-0. [DOI] [PubMed] [Google Scholar]
- 7.Lolans K, Rice TW, Munoz-Price LS, Quinn JP. Multicity outbreak of carbapenem-resistant Acinetobacter baumannii isolates producing the carbapenemase OXA-40. Antimicrob Agents Chemother. 2006;50:2941–2945. doi: 10.1128/AAC.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maragakis LL. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 9.Gordon NC, Wareham DW. Evaluation of CHROMagar Acinetobacter for detection of enteric carriage of multidrug-resistant Acinetobacter baumannii in samples from critically ill patients. J Clin Microbiol. 2009;47:2249–2251. doi: 10.1128/JCM.00634-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akers KS, Barsoumian A, Beckius ML, Murray CK, Mende K. CHROMagar Acinetobacter is not selective for carbapenem-resistant Acinetobacter baumannii-clacoaceticus complex. Diagn Microbiol Infect Dis. 2010;67:209–211. doi: 10.1016/j.diagmicrobio.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Ajao AO, Robinson G, Lee MS, Ranke TD, Venezia RA, Furuno JP, et al. Comparison of culture media for detection of Acinetobacter baumannii in surveillance cultures of critically-ill patients. Eur J Clin Microbiol Infect Dis. 2011;30:1425–1430. doi: 10.1007/s10096-011-1237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing. Wayne, PA: CLSI; 2012. 22nd informational supplement, M100-S22. [Google Scholar]
- 13.Ayats J, Corbella X, Ardanuy C, Domínguez MA, Ricart A, Ariza J, et al. Epidemiological significance of cutaneous, pharyngeal, and digestive tract colonization by multiresistant Acinetobacter baumannii in ICU patients. J Hosp Infect. 1997;37:287–295. doi: 10.1016/s0195-6701(97)90145-6. [DOI] [PubMed] [Google Scholar]
- 14.Wareham DW, Gordon NC. Modifications to CHROMagar Acinetobacter for improved selective growth of multi-drug resistant Acinetobacter baumannii. J Clin Pathol. 2011;64:164–167. doi: 10.1136/jcp.2010.083469. [DOI] [PubMed] [Google Scholar]


