Abstract
Clostridium difficile, an anaerobic, spore-forming, gram-positive, rod-shaped bacterium, is the most common nosocomial pathogen causing pseudomembranous colitis. C. difficile is not intrinsically invasive and rarely infects extraintestinal sites. The bacterium, therefore, is not commonly detected in blood cultures. Here, we report a case of C. difficile bacteremia in a patient who had underwent loop ileostomy because of rectal obstruction following metastatic colon cancer originated from prostate cancer.
Keywords: Clostridium difficile, Bacteremia, Loop ileostomy
INTRODUCTION
Clostridium difficile is an anaerobic, spore-forming, gram-positive, rod-shaped bacterial species and the most common pathogen causing nosocomial pseudomembranous colitis, a severe inflammation of the colon that often results because of an alteration or elimination of the normal gut flora. C. difficile is not intrinsically invasive, and pseudomembranous colitis occurs because of severe inflammatory response to the C. difficile toxins [1]. The clinical features, disease spectrum, pathogenesis, and optimal treatment of C. difficile in pseudomembranous colitis have been well documented [2]. Since this microorganism is not normally detected at locations other than the gut, reports of C. difficile isolation in blood cultures are extremely rare [3, 4]. We report a case of C. difficile bacteremia in a patient with loop ileostomy because of rectal obstruction following metastatic colon cancer of prostate cancer.
CASE REPORT
A 60-yr-old Korean man had been diagnosed with prostate cancer 4 yr ago and was followed up after chemotherapy. The cancer recurred after 2 yr and eventually metastasized to multiple sites, causing hydronephrosis, obstructive acute kidney injury, and rectal stricture. External compression of the rectum due to metastasis was suspected as the cause of rectal stricture. Consequently, the patient underwent nephrostomy in the pelvic region in conjunction with insertion of a stent in the colon. On hospitalization day (HD) 98, the patient developed fever, and antibiotic therapy with vancomycin/meropenem was initiated. On HD 113, loop ileostomy was performed, and antibiotic therapy involving ticarcillin was initiated. On HD 141, the fever recurred and blood culture revealed the presence of Escherichia coli. As a result, the antibiotic therapy was changed to piperacillin/metronidazole. On HD 146, the patient showed hematochezia through the site of ileostomy. On HD 157, the patient's blood culture showed the presence of Enterococcus faecium, and the patient was again treated with meropenem. Subsequently, blood culture on HD 163 showed the absence of pathogens in the patient's blood.
Blood cultures on HD 186 revealed the presence of anaerobic gram-positive bacilli, which were subcultured on blood agar and incubated anaerobically. They were identified as C. difficile by using the VITEK 2 system (bioMerieux, Marcy-I'Etoil, France), with 99% probability. The presence of these microorganisms was further confirmed with commercial 16S rRNA gene sequencing (Macrogen, Seoul, Korea), for which 2 primers were used: 518F (5'-CCAGCAGCCGCGGTAATACG-3') and 800R (5'-TACCAGGGTATCTAATCC-3'). The sequence thus obtained (1,466 bp) was compared with published sequences in the Gen-Bank database by using the basic local alignment search tool (BLAST)n algorithm (www.ncbi.nlm.nih.gov/blast), and the isolate showed 99.05% similarity to C. difficile and 95.47% to C. irregulare. A Clostridium toxin assay was performed for a stool specimen by using the VIDAS system (bioMerieux), and the results was negative. Subsequently, the patient's general condition worsened, and his family wished to have him close to home. He was discharged with no hope for recovery, and we were unable to investigate the C. difficile infection (CDI) further.
DISCUSSION
C. difficile is rarely found at sites outside the bowel. There are a few cases of extracolonic CDI, the manifestations of which include bacteremia, osteomyelitis, visceral abscess, empyema, reactive arthritis, and small bowel disease [3, 4]. The risk factors for CDI include the use of antibiotics, presence of chronic lung disease or ileus, prolonged stay at an intensive care unit, history of surgery, and a long-term stay at a care facility [5]. We think that our patient's exposure to risk factors made him vulnerable to CDIs.
To the best of our knowledge, our patient was the first case of C. difficile bacteremia in South Korea. Most of the CDI cases were associated with pseudomembranous colitis, except 1 that was accompanied by reactive arthritis [6]. In that particular instance, the patient had undergone appendectomy following the development of perforating inflammation due to acute appendicitis.
There are 7 cases of postsurgical C. difficile bacteremia in the English literature [7-10]. The manifestations of the infection in the 7 patients have been compared with those of our patient in Table 1. Hematochezia was observed in 2 patients, including our patient; the condition is a rare symptom of pseudomembranous colitis, but some such cases have been reported in Korea [11]. Four patients had undergone colon operations, and the others had undergone different operations. The history of surgery is one of the risk factors of CDI; thus, the patients who had surgery at sites other than the colon were already vulnerable to CDI. Clostridium toxin assay was performed on 7 patients, and the assay methods for each were variable, such as cytotoxicity analysis, enzyme immunoassay (EIA), tandem repeats analysis, polymerase chain reaction, and enzyme linked fluorescent assay (ELFA). The assay results of 3 patients were further analyzed for determining the toxinotype, with 1 showing a non-toxigenic strain and the other 2 having toxigenic strains. All of the patients recovered irrespective of the toxigenicity of the strains. Four patients showed negative results for EIA or ELFA, and recovered from the CDI. The negative result of the toxin assay was insubstantial for ruling out CDI, and the prognosis was not related to the toxigenicity. Four patients, including our patient, exhibited monomicrobial (C. difficile) bacteremia, 2 patients were negative for EIA or ELFA, and 1 showed a toxigenic strain. The negative results of EIA or ELFA did not imply a non-toxigenic nature of the infection, and the 2 patients with negative toxin assay may have had a toxigenic strain. To reveal the relationship between monomicrobial bacteremia and toxigenicity, further evaluations to define the toxigenicity at the molecular level should be carried out.
Table 1.
Reported cases with Clostridium difficile bacteremia
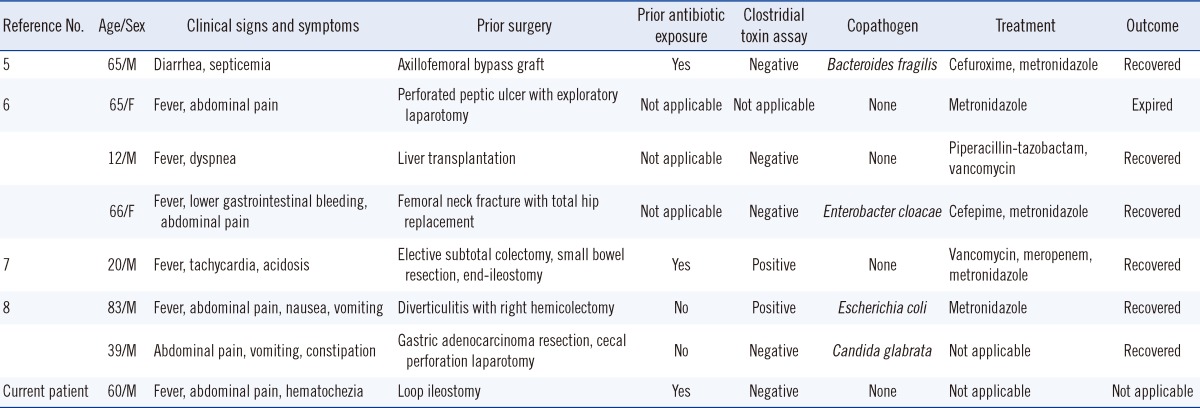
Abbreviations: M, male; F, female.
Recent incidences of CDI have been increasing, and the epidemic nature of the infection has been changing because of the prevalence of the B1/NAP1/027 strain in North America and Europe. Clinical manifestations of CDI caused by the B1/NAP1/027 strain are relatively severe and result in high mortality [10]. The epidemiology of CDI in Korea has not yet been analyzed well, but since the incidences have begun increasing [13, 14], the B1/NAP1/027 strain has emerged in Korea as well [15]. Since it is anticipated that more severe forms of CDI such as fulminant colitis or bacteremia will increase in Korea in the future, we think that further studies would be necessary to assess the role of C. difficile in the pathogenesis of bacteremia and the resulting mortality in the cases that are being currently reported.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonna I, Welsby PD. Pathogenesis and treatment of Clostridium difficile infection. Postgrad Med J. 2005;81:367–369. doi: 10.1136/pgmj.2004.028480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Lechuz JM, Hernangómez S, Juan RS, Peláez T, Alcalá L, Bouza E. Extra-intestinal infections caused by Clostridium difficile. Clin Microbiol Infect. 2001;7:453–457. doi: 10.1046/j.1469-0691.2001.00313.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs A, Barnard K, Fishel R, Gradon JD. Extracolonic manifestations of Clostridium difficile infections. Presentation of 2 cases and review of the literature. Medicine. 2001;80:88–101. doi: 10.1097/00005792-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Cho SM, Lee JJ, Yoon HJ. Clinical risk factors for Clostridium difficile-associated diseases. Braz J Infect Dis. 2012;16:256–261. doi: 10.1590/s1413-86702012000300007. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Kim GT, Yang MJ, Seo JH. A case of reactive arthritis in a patient with Clostridium difficile diarrhea. J Korean Rheum Assoc. 2009;16:43–47. [Google Scholar]
- 7.Spencer RC, Courtney SP, Nicol CD. Polymicrobial septicaemia due to Clostridium difficile and Bacteroides fragilis. Br Med J (Clin Res Ed) 1984;289:531–532. doi: 10.1136/bmj.289.6444.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee NY, Huang YT, Hsueh PR, Ko WC. Clostridium difficile bacteremia, Taiwan. Emerg Infect Dis. 2010;16:1204–1210. doi: 10.3201/eid1608.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGill F, Fawley WN, Wilcox MH. Monomicrobial Clostridium difficile bacteraemias and relationship to gut infection. J Hosp Infect. 2011;77:170–171. doi: 10.1016/j.jhin.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Hemminger J, Balada-Llasat JM, Raczkowski M, Buckosh M, Pancholi P. Two case reports of Clostridium difficile bacteremia, one with the epidemic NAP-1 strain. Infection. 2011;39:371–373. doi: 10.1007/s15010-011-0115-7. [DOI] [PubMed] [Google Scholar]
- 11.Kang HJ, Kwak DH, Choi MH, Kim HT, Kwak TY, Lee HC, et al. Three Cases of Pseudomembranous Colitis with Hematochezia. Korean J Gastrointest Endosc. 2011;42:410–414. [Google Scholar]
- 12.Pai H. Current epidemiology and treatment of Clostridium difficile infection. Infect Chemother. 2010;42:362–368. [Google Scholar]
- 13.Lee YJ, Choi MG, Lim CH, Jung WR, Cho HS, Sung HY, et al. Change of Clostridium difficile colitis during recent 10 years in Korea. Korean J Gastroenterol. 2010;55:169–174. doi: 10.4166/kjg.2010.55.3.169. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Lee SY, Kim YS, Park SW, Park SW, Jo SY, et al. The incidence and clinical features of Clostridium difficile infection; Single center study. Korean J Gastroenterol. 2010;55:175–182. doi: 10.4166/kjg.2010.55.3.175. [DOI] [PubMed] [Google Scholar]
- 15.Kim H, Lee Y, Moon HW, Lim CS, Lee K, Chong Y. Emergence of Clostridium difficile ribotype 027 in Korea. Korean J Lab Med. 2011;31:191–196. doi: 10.3343/kjlm.2011.31.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]


