Abstract
ABO discrepancy refers to an inconsistency between red cell and serum typings and has various causes, including hypogammaglobulinemia. IgM deficiency is a rare disorder that may accompany several conditions such as infection and autoimmune disorders. Here, we describe a case of IgM deficiency discovered during the evaluation of an ABO discrepancy in a 16-yr-old Korean boy. ABO blood grouping showed that while his cell type was O+, serum typing detected only anti-A (3+). Anti-B was not detectable at room temperature but was graded at 1+ at 4℃. ABO genotyping revealed an O/O genotype. His serum IgG, IgA, and IgM concentrations were 770 mg/dL (reference range: 800-1,700 mg/dL), 244 mg/dL (reference range: 100-490 mg/dL), and 13.5 mg/dL (reference range: 50-320 mg/dL), respectively. He was diagnosed with acute osteomyelitis on the basis of clinical presentation and imaging studies. The symptoms gradually improved within 3 weeks of treatment. However, the ABO discrepancy and IgM deficiency persisted even 6 months after recovery and lymphocyte subset analysis revealed CD19+ B cell deficiency. To the best of our knowledge, IgM deficiency detected by ABO discrepancy in a patient with acute osteomyelitis has not been reported before.
Keywords: IgM deficiency, B cell deficiency, ABO discrepancy, Acute osteomyelitis
INTRODUCTION
Selective IgM deficiency is defined as a dysgammaglobulinemia characterized by an isolated low level of serum IgM (less than 20 mg/dL in infants and children or less than 2 SDs or 10% below age-adjusted means in children and adults). It is a rare immunodeficiency, with the prevalence ranging from 0.03% in a community-based study to 3% in hospitalized patients. This immunodeficiency displays various clinical presentations, from asymptomatic cases to variable diseases [1-3].
ABO grouping requires both red cell and serum typings. When the results of red cell and serum tests do not agree, it is referred to as ABO discrepancy. ABO discrepancy can be caused by various factors, including hypogammaglobulinemia. In Korea, a few cases of ABO discrepancy induced by primary or secondary hypogammaglobulinemia have been reported, but a case associated with selective IgM deficiency has not been reported before [4-8].
Here, we report a case of IgM deficiency detected by ABO discrepancy in a 16-yr-old Korean boy. He was diagnosed with acute osteomyelitis, which might be related to IgM deficiency. To the best of our knowledge, this is the first case of IgM deficiency detected by ABO discrepancy in a patient with acute osteomyelitis.
CASE REPORT
A 16-yr-old boy presented at our hospital with a 5-day history of intermittent and diffused pain in his right knee after exercise. He had no history of previous trauma or infection. On physical examination, he experienced pain upon motion of the right knee, with mild swelling and anterior knee tenderness.
Laboratory examination revealed leukocytosis (11.7×109/L), a hemoglobin level of 13.1 g/dL, and a platelet count of 297×109/L. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were elevated at 76 mm/hr (reference range: 2-10 mm/hr) and 51.7 mg/L (reference range: 0-5 mg/L), respectively. The rheumatoid factor (RF) level was 24.6 IU/mL (reference range: 0-14 IU/mL). The results of liver and kidney function, serum electrolyte, alkaline phosphatase, and antinuclear antibody testing were unremarkable. Culture studies from blood specimens showed negative results for bacteria, fungus, and tuberculosis.
During routine ABO blood grouping, cell type by the slide method was O+, but serum typing by the tube method detected only anti-A (3+). Anti-B was not detectable at room temperature but was graded at 1+ at 4℃. We performed several studies to define the cause of this discrepancy. Antibody screening test using LISS/Coombs gel (DiaMed-ID; Bio-Rad Laboratories, DiaMed GmbH, Cressier FR, Switzerland) did not detect any antibodies in the patient's serum. Adsorption and elution testing revealed no A or B antigens, and transferase test was negative. The isoagglutinin titers for anti-A and anti-B by the tube technique were 32 (control: 128) and 2 (control: 1,024), respectively. To confirm his blood group type, ABO genotyping was performed. The result revealed an O/O allele using the SNaPshot multiplex kit (Applied Biosystems, Foster City, CA, USA) according to a previously described method [9]. Serum IgG, IgA, and IgM concentrations were 770 mg/dL (reference range: 800-1,700 mg/dL), 244 mg/dL (reference range: 100-490 mg/dL), and 13.5 mg/dL (reference range: 50-320 mg/dL), respectively. Total protein and albumin were 70 g/L and 43 g/L, and the results of protein electrophoresis were unremarkable.
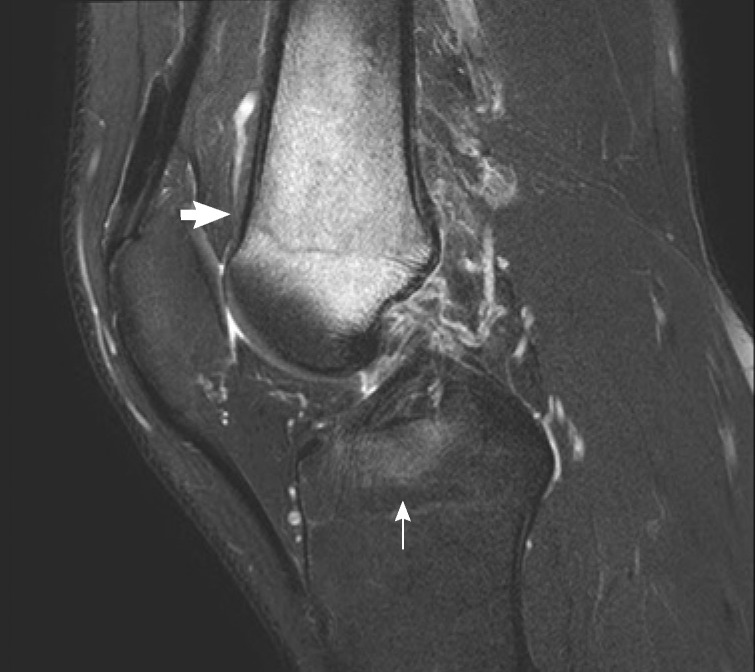
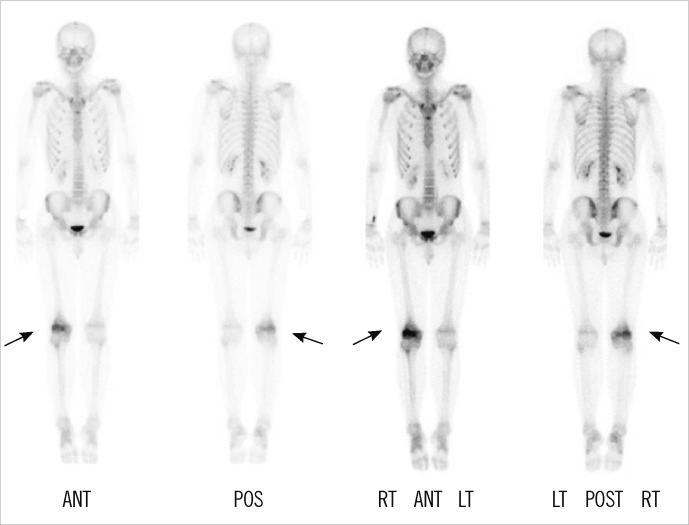
Magnetic resonance imaging (MRI) showed decreased bone marrow signal at the distal femoral metaphysis and anterior crucial ligament tibial attachment (Fig. 1). The technetium 99m-hydroxymethylene diphosphonate (Tc-99m HDP) 30mCi bone scan showed increased uptake only in the metaphysis of right femur (Fig. 2). The symptoms gradually improved within 3 weeks after treatment with antibiotics (ceftizoxime), nonsteroidal anti-inflammatory drugs, splinting, and bed rest. His ESR and CRP levels retested at the end of this period and displayed normal values.
Fig. 1.
T2-weighted magnetic resonance image of the right knee, sagittal section. The image demonstrated bone marrow signal deterioration at the distal femoral metaphysis (short arrow) and anterior crucial ligament tibial attachment (long arrow), suggesting chronic recurrent multifocal osteomyelitis or acute osteomyelitis.
Fig. 2.
Bone scan revealed increased uptake only in the metaphysis of the right femur (arrows).
Abbreviations: ANT, anterior; POS, posterior; RT, right; LT, left.
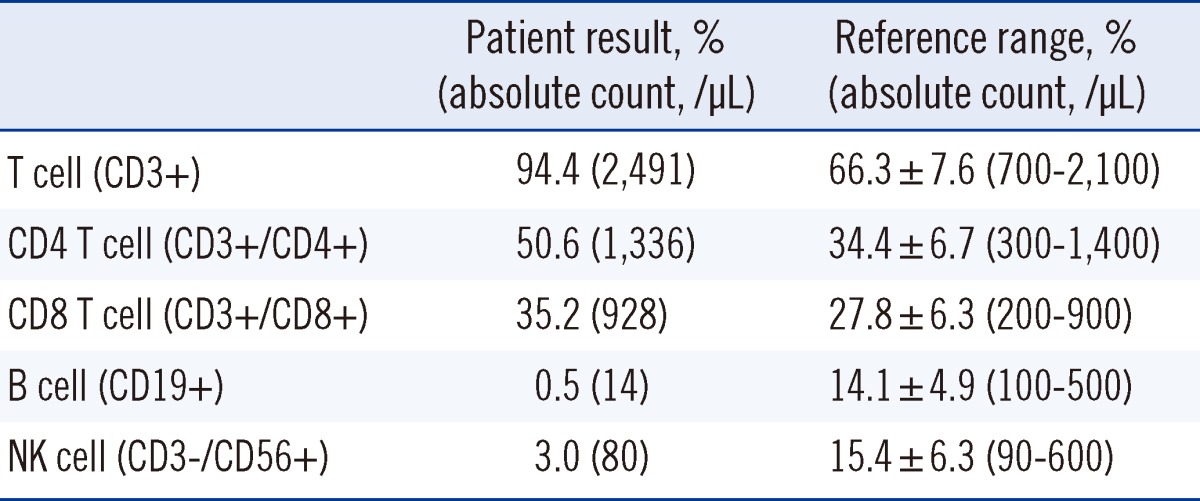
Six months after recovery, ABO discrepancy and low levels of serum IgM persisted. Specifically, serum IgG, IgA, and IgM concentrations were 721 mg/dL (reference range: 700-1,600 mg/dL), 183 mg/dL (reference range: 70-400 mg/dL), and <16.8 mg/dL (reference range: 40-230 mg/dL), respectively. Complete blood count (CBC) was within the normal limit and RF level was 22.9 IU/mL (Table 1). Additionally, lymphocyte subset analysis was performed by flow cytometry (Beckman Coulter, Fullerton, CA, USA) and the results showed CD19+ B cell deficiency (Table 2).
Table 1.
Patient laboratory data
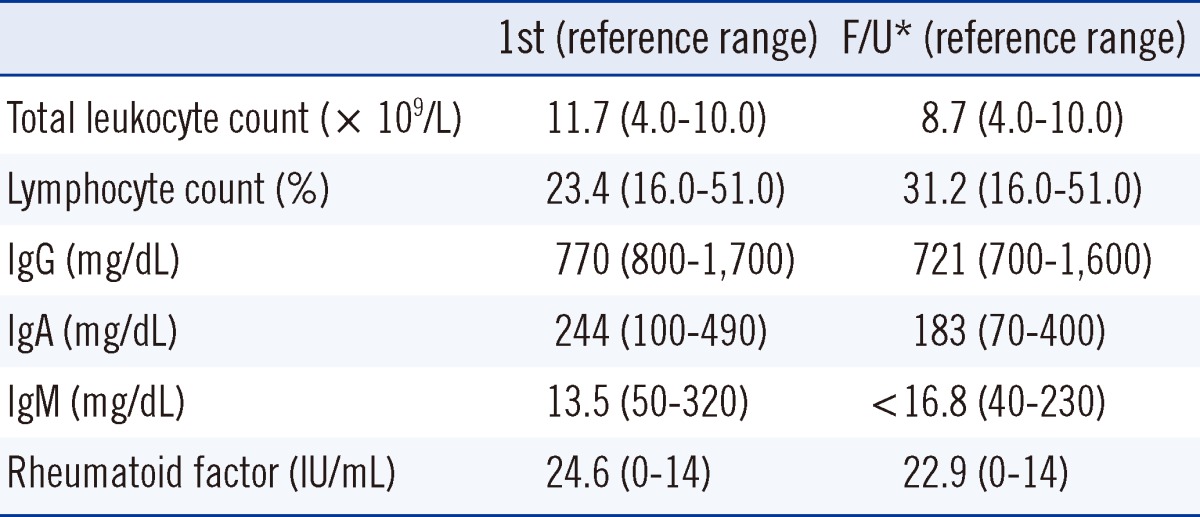
*Follow-up (F/U) measurement performed 6 months after the patient had recovered from acute osteomyelitis.
Table 2.
Lymphocyte subset analysis
DISCUSSION
Primary immunodeficiencies (PIDs) are diverse disease entities caused by one or more abnormalities of the immune system. To date, more than 130 phenotypically different PIDs have been identified on account of recent advances in medical research [10]. Nevertheless, our understanding of PIDs is still limited due to its low prevalence and unfamiliarity, leading to underdiagnosis. Selective IgM deficiency is a primary B cell immunodeficiency [11]. It is generally considered to be an unusual disorder and is frequently underestimated in the literature [12].
Selective IgM deficiency may develop as a primary or acquired condition. Before diagnosis of a primary selective IgM deficiency, secondary causes should be excluded [1]. No obvious secondary causes were found in this patient. There were no abnormal laboratory findings except for the RF level, and the patient presented no evidence of infection, gastrointestinal disease, allergy, or malignancy. A slightly increased RF level may be observed in some healthy people, but we could not completely rule out the possibility that it was due to undetected immunologic causes. Follow-up and further investigations such as molecular study for genetic mutations might be helpful to ensure that it is a primary deficiency.
Wiskott-Aldrich syndrome (WAS) is a representative disease that should be considered in patients with low IgM concentrations. It is an X-linked recessive disease characterized by eczema, thrombocytopenia, and recurrent infections. However, our patient had a normal platelet count and did not exhibit other symptoms related to WAS. In addition, dedicator of cytokinesis 8 (DOCK8) deficiency is a very rare hyper-IgE syndrome and also associated with low levels of IgM. It is an autosomal recessive PID with characteristic laboratory findings, such as lymphopenia, especially in CD4 T cells, and eosinophilia [13, 14]. Although the IgE level was not measured in this patient, he revealed no T cell lymphopenia or eosinophilia.
Selective IgM deficiency is known to be associated with several diseases, including recurrent infections, resulting in respiratory tract infections, skin infections, and meningitis. It may also be associated with autoimmune diseases such as systemic lupus erythematosus, allergic disorders such as asthma, gastrointestinal diseases such as celiac disease, and malignancies [1-3]. Until now, selective IgM deficiency associated with acute osteomyelitis has not been reported.
Chronic recurrent multiple osteomyelitis (CRMO) is characterized by a prolonged, wavering course with recurrent flares of bone pain occurring over several years that is not responsive to antibiotic therapy [15]. Our patient responded to antibiotics within 3 weeks and disease did not relapse until 10 months after discharge. Although it is difficult to distinguish between CRMO and acute osteomyelitis on the basis of imaging studies, he was more likely to have acute osteomyelitis rather than CRMO in terms of his clinical manifestations.
Makay et al. [16] described a patient with CRMO coexisting with selective IgM deficiency. CRMO is a rare disorder of unknown etiology and regarded as an autoinflammatory disorder, because there are no attested infectious causes. Thus, the authors speculated that IgM deficiency could be associated with CRMO. MRI findings from our patient were suggestive of CRMO or acute osteomyelitis, but, as described previously, he was diagnosed with acute osteomyelitis on the basis of his clinical manifestations. Acute osteomyelitis is an inflammation of the bone caused by pyogenic organisms, and the spectrum varies depending on causative agents and the immune state of the host [17]. IgM is involved in the primary antibody response and it is important for host defense against diverse organisms. Therefore, IgM deficiency can predispose some people to infections. However, because immunodeficiency states are not always consistent with infectious conditions, it is assumed that there are many factors involved in infection susceptibility [2].
The specific etiology of IgM deficiency remains poorly understood. Various mechanisms have been proposed, including impaired terminal differentiation of B cells into IgM-secreting cells, defective helper T-cell functions, increased suppressor T-cell functions, and failure of secreted µ mRNA synthesis [1, 2]. In our case, the number of peripheral B cells was markedly low. Among the patients with IgM deficiency, the proportion of CD19+ B cells has been reported to be highly variable, ranging from very low to normal levels [2, 18]. The relationship between B cell deficiency and infection susceptibility is not clear, since several immunologic function studies have yielded different results, regardless of the number of B cells [18].
The ABO discrepancy in our patient showed O+ by cell typing and B+ by serum typing at room temperature. However, his serum displayed weak anti-B (1+) reaction at low temperature (4℃). He was confirmed as having the O/O allele by genetic study. These findings indicate a weakening of serum antibodies, suggesting that hypogammaglobulinemia was a possible cause of the discrepancy.
Although isoagglutinin titers may differ according to ethnic populations, ages, and test methods, those in our case appear to be low [19, 20]. Particularly, the anti-B titer (1:2) was lower than the anti-A titer (1:32), in parallel with serum typing. Two studies by other investigators also noted a tendency similar to our result among patients with IgM deficiency. Goldstein et al. [1] described that all tested patients had antibodies detectable at low titers and Makay et al. [16] reported low levels of anti-B titer. In a person with type O blood, the anti-B titer seems to be lower than anti-A titer. A relatively lower titer of anti-B may account for the reason why anti-B was not detected in serum typing for this case [5].
In the present case, selective IgM deficiency with CD19 cell deficiency was discovered during the evaluation of an ABO discrepancy. This case contributes to our understanding of the etiology of IgM deficiency and ABO discrepancy. IgM deficiency is a rare disorder, which may correlate with several disorders, and longer follow-up periods are needed to clarify the relationship between IgM deficiency and acute osteomyelitis.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Selective IgM immunodeficiency: retrospective analysis of 36 adult patients with review of the literature. Ann Allergy Asthma Immunol. 2006;97:717–730. doi: 10.1016/S1081-1206(10)60962-3. [DOI] [PubMed] [Google Scholar]
- 2.Yel L, Ramanuja S, Gupta S. Clinical and immunological features in IgM deficiency. Int Arch Allergy Immunol. 2009;150:291–298. doi: 10.1159/000222682. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Pediatric selective IgM immunodeficiency. Clin Dev Immunol. 2008;2008:624850. doi: 10.1155/2008/624850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh IB, Chang EA, Kim HJ, Kang CD, Lee SJ, Kim TS, et al. Two cases of ABO discrepancy due to hypogammaglobulinemia. Korean J Blood Transfus. 2003;14:240–245. [Google Scholar]
- 5.Oh SH, Kang CI, Kim J, Park TS. ABO discrepancy in a young Korean serviceman with common variable immunodeficiency. Ann Hematol. 2010;89:629–630. doi: 10.1007/s00277-009-0840-5. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Jung KH, Han KS, Han BY, Yoon JH, Chun SA. A case of group O without anti-A,B due to hypogammaglobuluinemia. Korean J Blood Transfus. 1996;7:269–273. [Google Scholar]
- 7.Park TS, Oh SH, Choi JC, Kim HH, Park JH, Lee EY, et al. A case of agammaglobulinemia detected by ABO discrepancy in a 13-yr-old girl. Korean J Lab Med. 2002;22:364–366. [Google Scholar]
- 8.Ha JS, Kim EJ, Chun HJ, Kim JR, Jeon DS. Three cases of multiple myeloma showing ABO discrepancy. Korean J Blood Transfus. 1998;9:289–293. [Google Scholar]
- 9.Hyun J, Chang HE, Heo SR, Song SH, Park KU, Song J, et al. ABO genotyping using a multiplex single-base primer extension reaction. Korean J Blood Transfus. 2007;18:79–88. [Google Scholar]
- 10.Gathmann B, Binder N, Ehl S, Kindle G ESID Registry Working Party. The European internet-based patient and research database for primary immunodeficiencies: update 2011. Clin Exp Immunol. 2012;167:479–491. doi: 10.1111/j.1365-2249.2011.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Moise A, Nedelcu FD, Toader MA, Sora SM, Tica A, Ferastraoaru DE, et al. Primary immunodeficiencies of the B lymphocyte. J Med Life. 2010;3:60–63. [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Herz W, Bousfiha A, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol. 2011;2:54. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Q, Davis JC, Dove CG, Su HC. Genetic, clinical, and laboratory markers for DOCK8 immunodeficiency syndrome. Dis Markers. 2010;29:131–139. doi: 10.3233/DMA-2010-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wipff J, Adamsbaum C, Kahan A, Job-Deslandre C. Chronic recurrent multifocal osteomyelitis. Joint Bone Spine. 2011;78:555–560. doi: 10.1016/j.jbspin.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Makay B, Unsal E, Anal O, Güne D, Men S, Cakmakçi H, et al. Chronic recurrent multifocal osteomyelitis in a patient with selective immunoglobulin M deficiency. Rheumatol Int. 2009;29:811–815. doi: 10.1007/s00296-008-0788-0. [DOI] [PubMed] [Google Scholar]
- 17.Offiah AC. Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children. Eur J Radiol. 2006;60:221–232. doi: 10.1016/j.ejrad.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Agrawal S, Gollapudi S. Selective IgM deficiency with T cell defects and Mycobacterium Avium Complex (MAC) infection. Open Immunol J. 2012;5:8–12. [Google Scholar]
- 19.Mazda T, Yabe R, NaThalang O, Thammavong T, Tadokoro K. Differences in ABO antibody levels among blood donors: a comparison between past and present Japanese, Laotian, and Thai populations. Immunohematology. 2007;23:38–41. [PubMed] [Google Scholar]
- 20.Cho CH, Kim HN, Yun SG, Choi GR, Choi JY, Kim JS, et al. Evaluation of ABO antibody titration using tube and column agglutination techniques. Lab Med Online. 2011;1:57–63. [Google Scholar]