Abstract
Diastolic dysfunction (DD) with preserved left ventricular (LV) ejection fraction (EF) has been linked to obesity. Adiponectin is a cytokine related to obesity and obesity-linked cardiovascular complications. The authors aimed to determine the independent association of DD with adiponectin. Fifty patients with impaired relaxation DD and a normal EF and age-matched normal controls were recruited. Plasma levels of total and high molecular weight (HMW) adiponectin were measured. Mid and low molecular weight (MMW+LMW) fractions of adiponectin were calculated by subtracting HMW fraction from total adiponectin. The DD group had significantly lower total (median, 4.4 vs 12.7 μg/mL; P=.001), HMW fraction (median, 1.3 vs 3.4 μg/mL; P=.02), and MMW+LMW fraction of adiponectin (median, 3.8 vs 7.2 μg/mL; P=.01). Body mass index (BMI) negatively correlated with total (r:−0.46, P=.003), HMW (r:−0.32, P=.038), and MMW+LMW (r:−0.40, P=.006) fractions of adiponectin. DD had an independent association with both BMI (P<.05) and total adiponectin (P<.001) in linear regression model using sex, BMI, blood pressure, and total adiponectin as covariates. DD was associated with BMI (P=.02), HMW fraction (P=.03), and MMW+LMW fraction (P=.004) in similar linear regression analyses. Adiponectin deficiency may be one explanation for the adiposity-related cardiac oxidation known to be involved in the pathogenesis of DD.
Diastolic dysfunction (DD) with preserved ejection fraction (EF) accounts for half of all heart failure (HF) presentations.1,2 Morbidity and mortality from DD are similar to HF with reduced EF,3 yet therapies that show benefit in the latter have not been found effective in the former.4,5
DD is characterized by impairment in ventricular relaxation and chamber stiffness during diastole.6 The pathogenesis of DD is still incompletely understood; however, it is well known that DD is associated with obesity, hypertension, and insulin resistance,7-9 conditions associated with oxidative stress. Recently, it has been shown that nitric oxide (NO) and NO synthase (NOS) have a role in cardiac relaxation, with a reduction in cardiac NO contributing to DD.10
Adiponectin, an adipocyte-derived cytokine, is exclusively expressed in adipose tissue and is believed to be regulated by adiposity.11 It circulates in the plasma as different oligomers: low molecular weight (LMW), medium molecular weight (MMW), and high molecular weight (HMW) forms.12 Hypoadiponectinemia has been shown to be associated with obesity and obesity-related disorders such as diabetes, insulin resistance, and coronary artery disease.13-15 Hypo-adiponectinemia has also been linked to endothelial dysfunction and oxidative stress. Adiponectin stimulates NO production through AMP-activated protein kinase (AMPK)–dependent and AMPK–independent phosphorylation of endothelial NOS.16 It is postulated that adiponectin further decreases NO inactivation by blocking superoxide production.17
Adiponectin can modify cardiac hypertrophy, fibrosis, and remodeling.18,19 Shibata colleagues20 demonstrated severe concentric cardiac hypertrophy and increased mortality in adiponectin knockout mice in response to pressure overload when compared with wild-type and diabetic mice. It has also been shown that infusion of adiponectin attenuates cardiac hypertrophy.18
We aimed to determine whether there was an association of plasma adiponectin levels with DD in humans when compared with age-matched controls.
METHODS
In a cross-sectional, case-control design, 25 patients with New York Heart Association (NYHA) class I or II HF symptoms and echocardiographic evidence of early DD and 25 age-matched controls were recruited from the outpatient clinics at the Atlanta Veterans Affairs Medical Center and Emory University Hospital. DD was defined by preserved LVEF of >50% and abnormal echocardiographic parameters consistent with diastolic dysfunction as determined by 2-dimensional echocardiography with tissue Doppler imaging.21 Early diastolic dysfunction was described as impaired relaxation with an E/A wave ratio of <1.21 Tissue Doppler imaging was used for measuring mitral valvular motion.22 The protocol was approved by the Emory University institutional review board. Early DD was chosen to minimize changes in adiponectin known to occur with acute HF exacerbations.23 No patient in our study had greater than NYHA class II HF, and it is unclear what factors predict who will go on to develop signs and symptoms of HF with DD. Nevertheless, reduced cardiac relaxation is thought to be a prerequisite to HF with preserved LVEF, and our aim was to study early biomarkers of reduced cardiac relaxation. Moreover, since adiponectin levels can be altered during the clinical syndrome of HF, we focused on relatively asymptomatic patients.
Eligibility criteria for both groups included age 18 years and older, an echocardiogram with mitral valve inflow velocities and tissue Doppler measurements within 6 months of enrollment, normal sinus rhythm, LVEF between 50% and 70%, and normal systolic and diastolic cardiac dimensions on qualifying echocardiogram. Exclusion criteria included systemic inflammatory disease, malignant neoplasm, severe valvular heart disease, HF NYHA class III or IV, untreated hyperthyroidism or hypothyroidism, greater than mild cardiac hypertrophy, cardiomyopathy of any etiology, blood pressure (BP) >180/100 mm Hg while taking medications, any concurrent illness resulting in a life expectancy <1 year, and current illicit drug or alcohol abuse.
Demographic and clinical data were collected from review of medical records, history and physical examination upon enrollment, and the qualifying echocardiogram. A single blood draw was obtained from patients in both groups. Plasma adiponectin was quantitatively determined using multimeric enzyme-linked immunosorbent assay (ALPCO Diagnostics, Salem, NH). LMW and MMW adiponectin levels were obtained by subtracting the HMW adiponectin from the total adiponectin.
Statistical analyses were performed using SPSS version 16 (SPSS Inc, Chicago, IL). A sample size of 25 patients in each group was sufficient to detect a 2-μg/mL difference in the adiponectin levels at a power >85%, keeping the 2-tailed level of significance at .05. Categorical data are presented as numbers (percentages). Continuous data with normal distribution are presented as mean ± standard deviation (SD) while those with a skewed distribution are presented as median (interquartile range [IR]). General linear models were used to evaluate univariate association of baseline variables with DD. Linear regression analysis was performed to evaluate the independent association of predictive variables with DD. Linear regression model 1 evaluated the independent association of baseline variables found to be significant on univariate analysis. Linear regression model 2 evaluated the independent association of adiponectin or its fractions using age, sex, BP, and body mass index (BMI) as predictive covariates. All investigators had direct access to the primary data.
RESULTS
The baseline demographic and clinical characteristics of patients with early DD and age-matched controls are shown in Table I. Univariate analysis using general linear models showed that male sex (P=.04) and a higher mean BMI (P=.003) were the only two baseline variables associated with early DD (Table I). There was no association of DD with race, hypertension, hypercholesterolemia, use of different classes of anti-hypertensive agents and the use of statins. Only BMI retained significant association with DD in a linear regression analysis using variables significant on univariate analysis as covariates (model 1, r2=0.46; t score=3.4; P=.006).
TABLE I.
Comparison of Baseline Characteristics Between Patients With and Without Diastolic Dysfunctiona
| Cases | Controls | Univariate Analysis |
||
|---|---|---|---|---|
| Variables | (n=25) | (n=25) | F Score | P Value |
| Demographic | ||||
| Age, y | 64.8±10.8 | 65.0±11.3 | 0.003 | .9 |
| Male sex, No. (%) | 18 (72) | 11 (44) | 4.2 | .04 |
| White race, No. (%) | 13 (54) | 12 (48) | 0.08 | .8 |
| Clinical | ||||
| BMI, kg/m2 | 29.6±4.8 | 25.3±4.7 | 9.9 | .003 |
| SBP, mm Hg | 135.4±19.2 | 127.4±16.7 | 2.5 | .1 |
| DBP, mm Hg | 75.8±13.0 | 74.0±10.3 | 0.3 | .6 |
| Smoking, No. (%) | 10 (40) | 10 (40) | 0.0 | 1.0 |
| Diabetes, No. (%) | 8 (32) | 9 (36) | 0.09 | .8 |
| Hypercholesteremia, No. (%) |
14 (56) | 10 (40) | ||
| Medications, No. (%) | ||||
| β-Blocker | 15 (60) | 10 (40) | 2.0 | .2 |
| ACE inhibitor | 12 (48) | 9 (36) | 0.7 | .4 |
| ARB | 4 (16) | 1 (4) | 2.0 | .2 |
| Diuretic | 4 (16) | 8 (32) | 1.7 | .2 |
| Statin | 14 (56) | 16 (64) | 0.3 | .6 |
Univariate association of baseline characteristics with diastolic dysfunction shown by general linear models.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure. Bold values indicate significance.
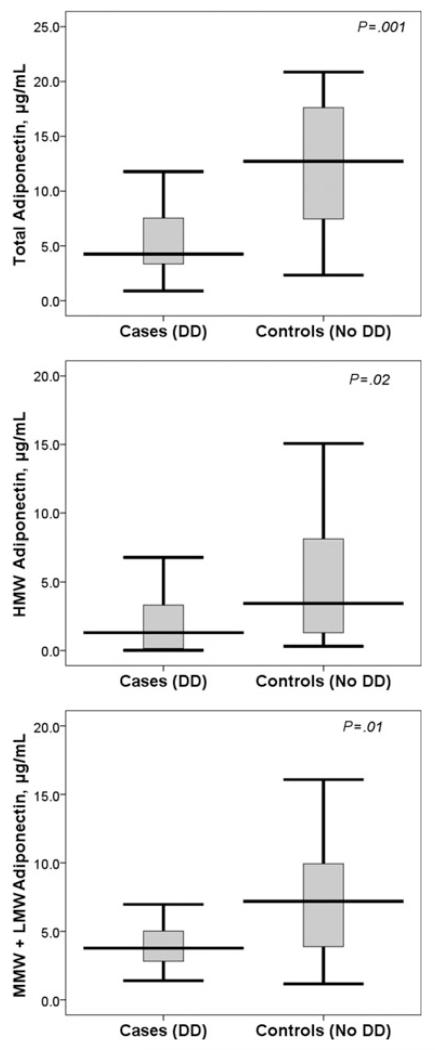
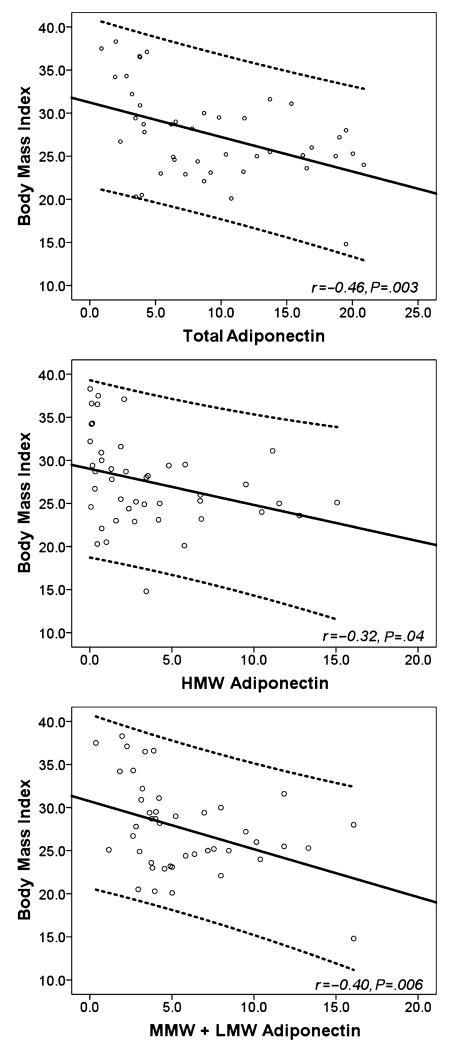
Figure 1 compares total, HMW, and MMW+LMW adiponectin levels among the cases and controls. Patients with DD had a significantly lower total adiponectin (median [IR], 4.4 [3.4–8.0] vs 12.7 [6.2–18.7] μg/mL, P=.001), lower HMW fraction of adiponectin (median [IR], 1.3 [0.04–3.4] vs 3.4 [1.0–9.5] μg/mL, P=.02), and lower MMW+LMW fraction of adiponectin (median [IR], 3.8 [2.7–5.1] vs 7.2 [3.8–10.4] μg/mL, P=.01). There was a moderately negative correlation of BMI with total (r:−0.46, P=.003), HMW (r:−0.32, P=.038), and MMW+LMW (r:−0.40, P=.006) adiponectin levels in the study sample (Figure 2).
FIGURE 1.
Comparison of (top) total, (center) high molecular weight (HMW), and (bottom) mid molecular weight (MMW) + low molecular weight (LMW) adiponectin levels between patients with and without diastolic dysfunction. DD indicates diastolic dysfunction. Horizontal bars show median levels, boxes show interquartile range, and vertical error bars show 95% confidence intervals for the median; P value derived from nonparametric comparison.
FIGURE 2.
Correlation of body mass index with (top) total, (center) high molecular weight (HMW), and (bottom) mid molecular weight (MMW) + low molecular weight (LMW) adiponectin levels. DD indicates diastolic dysfunction. Dotted lines show 95% confidence intervals for individual cases.
Patients with DD had an independent association with both BMI (P<.05) and total adiponectin (P<.001) in linear regression analysis (model 2A) using age, sex, BMI, systolic and diastolic BPs, and total adiponectin as covariates (Table II). DD was independently associated with BMI (P=.02), HMW adiponectin (P=.03), and MMW+LMW adiponectin (P=.004) in linear regression analyses (models 2B and 2C, respectively). DD had a stronger association with total adiponectin than BMI (model 2A), similar association with both HMW adiponectin and BMI (model 2B), and was only associated with MMW+LMW adiponectin (model 2C).
TABLE II.
Linear Regression Model (Model 2) Evaluating Independent Association of Adiponectin With Diastolic Dysfunction Using Age, Sex, Blood Pressure, and BMI as Covariates
| Model | Method | R 2 | Variable | t Score | P Value |
|---|---|---|---|---|---|
| 2A | Enter | 0.51 | Age | 0.75 | .46 |
| Sex | 1.94 | .07 | |||
| SBP | 0.70 | .49 | |||
| DBP | −0.28 | .78 | |||
| BMI | 2.11 | <.05 | |||
| Total adiponectin | −3.84 | <.001 | |||
| 2B | Enter | 0.40 | Age | 0.47 | .64 |
| Sex | 1.01 | .13 | |||
| SBP | 0.85 | .40 | |||
| DBP | −0.47 | .64 | |||
| BMI | 2.43 | .02 | |||
| Total adiponectin | −2.32 | .03 | |||
| 2C | Enter | 0.45 | Age | 0.29 | .77 |
| Sex | 1.87 | .09 | |||
| SBP | 1.63 | .11 | |||
| DBP | −0.74 | .47 | |||
| BMI | 1.55 | .13 | |||
| Total adiponectin | −3.11 | .004 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HMW, high molecular weight fraction; MMW+LMW, mid and low molecular weight fractions; SBP, systolic blood pressure. Bold values indicate significance.
DISCUSSION
This study shows an association of low plasma adiponectin with DD. This association is independent of age, BMI, and the existence of diabetes or hypertension. Our results are consistent with recent literature. In a recent study, Fukuta and colleagues24 have shown that decreased adiponectin levels are associated with LV DD in patients with known or suspected coronary artery disease. We confirmed this result in a more diverse cohort studying subfractions of adiponectin. Sam and colleagues25 showed that hypoadiponectinemia in hypertension-induced diastolic HF exacerbates LV hypertrophy and DD in mice. In observational studies in humans, it has been shown that low levels of adiponectin are associated with progression of LV hypertrophy and LV remodeling.18,19 McManus and colleagues,26 in a cross-sectional epidemiologic study derived from the Framingham offspring cohort, found that higher levels of adiponectin were associated with lower LV mass.
Mechanisms of cardioprotective effects of adiponectin in DD have been conjectured. The most plausible explanation is the effect of reduced adiponectin on cardiac oxidation. It has been reported that the relaxation defect seen in DD is, at least in part, associated with cardiac oxidation and reduced NO production by NOS.27 It is believed that reduced NO leads to phospholamban changes that are consistent with increased cytosolic calcium, impaired myocardial relaxation, and DD.25 Adiponectin increases NO production from endothelial NOS under physiologic conditions,26 possibly contributing to its cardioprotective effects in DD. In pathologic conditions where inducible NOS expression is stimulated, adiponectin inhibits NO overproduction by inhibition of inducible NOS expression and protects tissues from nitrative stress.28 In summary, the lack of adiponectin can contribute to DD by facilitating cardiac oxidative/nitrative stress.
In our study, we observed an association between obesity and DD. Obesity has been linked to hypoadiponectinemia.13 It has been shown that visceral rather than subcutaneous fat is more closely related to low adiponectin levels.29 DD has been associated similarly with obesity and epicardial fat accumulation.30 Epicardial fat is a pathologic determinant of cardiovascular disease and DD in the metabolic syndrome.31 These effects of visceral and epicardial fat are believed to be mediated by the lack of adiponectin.32 This may explain the observed associations. Nevertheless, adiponectin was associated with DD even when BMI was included in the statistical models, suggesting that adiponectin may be regulated by other factors aside from obesity.
Systolic HF and HF with preserved EF are different pathologic entities that may clinically present in a similar fashion,33 but their response to conventional HF therapies and outcomes are different.34 It is known that chronic systolic HF leads to up-regulation of plasma adiponectin levels,35 and higher BMI is protective in this population. Thus, the finding of a low adiponectin level might be useful in differentiating the 2 types of HF when echocardiography is unavailable.
Study Limitations
Our study had some limitations. The sample size was small. Nevertheless, we were able to show statistically significant association of low adiponectin levels with DD, and the results are similar to a recent report in patients with DD and coronary artery disease.24 The blood samples were obtained in a nonfasting state. It is shown that fasting and postprandial adiponectin levels do not differ significantly in healthy individuals; however, it is not known whether this is true in diabetic patients.36 In addition, we did not follow the patients over time to determine the association of adiponectin levels with clinical outcomes in either group. These findings will need to be further confirmed in larger clinical trials.
Conclusions and Future Directions
Deficiency of adiponectin may be a link between adiposity and DD. The mechanism could be through adiponectin deficiency–related cardiac oxidation known to be involved in the pathogenesis of DD. In chronic HF, it may be possible to use adiponectin levels to differentiate diastolic from systolic HF, and raising adiponectin levels may form a potential pharmacologic target in DD.
Acknowledgments
Funding Sources: The study was supported by the National Institute of Heart and Lung (R01 HL085558, R01 HL073753, P01 HL058000) and a Veterans Affairs MERIT grant.
Footnotes
Both first and second authors have contributed equally to the study.
Disclosures: Dr Dudley has patents pending, Methods and Compositions for Treating Diastolic Dysfunction (60/840,368) Sodium Current Blockade for Treatment of Diastolic Heart Failure (61/241,585 and 61/263,920), and Metabolic modulators improve cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity (61/348,105).
References
- 1.Owan TE, Hodge DO, Herges RM, et al. Trends in the prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 4.Massie BM, Carson PE, McMurray JJ, I-PRESERVE Investigators Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Pfeffer MA, Swedberg K, et al. The CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 6.Westermann D, Kasner M, Steendijk P, et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 7.Movahed MR, Saito Y. Obesity is associated with left atrial enlargement, E/A reversal and left ventricular hypertrophy. Exp Clin Cardiol. 2008;13:141–143. [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975;56:56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JE, Palmieri V, Roman MJ, et al. The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol. 2001;37:1943–1949. doi: 10.1016/s0735-1097(01)01230-x. [DOI] [PubMed] [Google Scholar]
- 10.Takimoto E, Champion HC, Li M, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 12.Pajvani UB, Du X, Combs TP, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:90739085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 13.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 14.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 15.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 16.Shimano M, Ouchi N, Shibata R, et al. Adiponectin deficiency exacerbates cardiac dysfunction following pressure overload through disruption of an AMPK-dependent angiogenic response. J Mol Cell Cardiol. 2010;49:210–220. doi: 10.1016/j.yjmcc.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng G, Long Y, Yu YR, et al. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes. 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 18.Fujita K, Maeda N, Sonoda M, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2008;28:863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- 19.Fujioka D, Kawabata K, Saito Y, et al. Role of adiponectin receptors in endothelin-induced cellular hypertrophy in cultured cardiomyocytes and their expression in infarcted heart. Am J Physiol Heart Circ Physiol. 2006;290:240–16. doi: 10.1152/ajpheart.00987.2005. [DOI] [PubMed] [Google Scholar]
- 20.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulus WJ, Tschope C, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Association of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 22.Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426–440. doi: 10.1016/0735-1097(88)90416-0. [DOI] [PubMed] [Google Scholar]
- 23.Dieplinger B, Gegenhuber A, Haltmayer M, et al. Evaluation of novel biomarkers for the diagnosis of acute destabilised heart failure in patients with shortness of breath. Heart. 2009;95(18):1508–1513. doi: 10.1136/hrt.2009.170696. [DOI] [PubMed] [Google Scholar]
- 24.Fukuta H, Ohte N, Wakami K, et al. Relation of plasma levels of adiponectin to left ventricular diastolic dysfunction in patients undergoing cardiac catheterization for coronary artery disease. Am J Cardiol. 2011;108:1081–1085. doi: 10.1016/j.amjcard.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Sam F, Duhaney TA, Sato K, et al. Adiponectin deficiency, diastolic dysfunction, and diastolic heart failure. Endocrinology. 2010;151:322–331. doi: 10.1210/en.2009-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McManus DD, Lyass A, Ingelsson E, et al. Relationship of circulating resistin and adiponectin and cardiac structure and function: the Framingham Offspring Study. Obesity (Silver Spring) 2011 Feb 24; doi: 10.1038/oby.2011.32. [Epub ahead of print]. doi 10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silberman GA, Fan TH, Liu H, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao L, Gao E, Jiao X, et al. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–1416. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 29.Yatagai T, Nagasaka S, Taniguchi A, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G, Ribaudo MC, Zappaterreno A, et al. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;15:1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 31.Aydin H, Toprak A, Deyneli O, et al. Epicardial fat tissue thickness correlates with endothelial dysfunction and other cardiovascular risk factors in patients with metabolic syndrome. Metab Syndr Relat Disord. 2010;8:229–234. doi: 10.1089/met.2009.0080. [DOI] [PubMed] [Google Scholar]
- 32.Karastergiou K, Evans I, Ogston N, et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 33.Ezekowitz JA, Lee DS, Tu JV, et al. Comparison of one-year outcome in hospitalized heart failure patients with left ventricular ejection fraction <50% versus those with ejection fraction 50% Am J Cardiol. 2008;102:79–83. doi: 10.1016/j.amjcard.2008.02.102. [DOI] [PubMed] [Google Scholar]
- 34.Zile MR, Gaasch WH, Anand IS, et al. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 2010;121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 35.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 36.Imbeault P, Pomerleau M, Harper ME, et al. Unchanged fasting and postprandial adiponectin levels following a 4-day caloric restriction in young healthy men. Clin Endocrinol. 2004;60:429–433. doi: 10.1111/j.1365-2265.2004.01997.x. [DOI] [PubMed] [Google Scholar]