Abstract
Aims
Fractional flow reserve (FFR) is the reference standard for the assessment of the functional significance of coronary artery stenoses, but is underutilized in daily clinical practice. We aimed to study long-term outcomes of FFR-guided percutaneous coronary intervention (PCI) in the general clinical practice.
Methods and results
In this retrospective study, consecutive patients (n = 7358), referred for PCI at the Mayo Clinic between October 2002 and December 2009, were divided in two groups: those undergoing PCI without (PCI-only, n = 6268) or with FFR measurements (FFR-guided, n = 1090). The latter group was further classified as the FFR-Perform group (n = 369) if followed by PCI, and the FFR-Defer group (n = 721) if PCI was deferred. Clinical events were compared during a median follow-up of 50.9 months. The Kaplan–Meier fraction of major adverse cardiac events at 7 years was 57.0% in the PCI-only vs. 50.0% in the FFR-guided group (P = 0.016). Patients with FFR-guided interventions had a non-significantly lower rate of death or myocardial infarction compared with those with angiography-guided interventions [hazard ratio (HR): 0.85, 95% CI: 0.71–1.01, P = 0.06]; the FFR-guided deferred-PCI strategy was independently associated with reduced rate of myocardial infarction (HR: 0.46, 95% CI: 0.26–0.82, P = 0.008). After excluding patients with FFR of 0.75–0.80 and deferring PCI, the use of FFR was significantly associated with reduced rate of death or myocardial infarction (HR: 0.80, 95% CI: 0.66–0.96, P = 0.02).
Conclusion
In the contemporary practice, an FFR-guided treatment strategy is associated with a favourable long-term outcome. The current study supports the use of the FFR for decision-making in patients undergoing cardiac catheterization.
Keywords: Fractional flow reserve, Percutaneous coronary intervention, Outcome
See page 1321 for the editorial comment on this article (doi:10.1093/eurheartj/eht080)
Introduction
The benefits of percutaneous coronary intervention (PCI) are mainly attributable to reduction of myocardial ischaemia.1 Therefore, clinical practice guidelines currently recommend performing PCI only when symptoms and/or myocardial ischaemia are identified.2,3
The accuracy of fractional flow reserve (FFR) for assessments of the functional significance of a coronary stenosis has been well established.4 Improved clinical outcomes have been demonstrated in clinical trials when the decision to perform PCI was based on available FFR.5–7 Indeed, the randomized ‘Fractional Flow Reserve vs. Angiography for Multivessel Evaluation’ (FAME) study8 has recently shown a favourable 2-year clinical outcome of FFR-guided PCI compared with angiography-guided PCI in a broad population of patients. Moreover, the FAME II trial subsequently reported that combination of an FFR-guided treatment strategy and the best available medical therapy improved outcomes in patients with stable coronary disease, compared with the best available medical therapy alone.9 These important studies increased physicians' awareness to the benefits of FFR-guided PCI, and in the current guideline on coronary revascularization of the European Society of Cardiology, FFR has been upgraded to a class IA classification in multivessel PCI.3
Nevertheless, while evidence for the utility of FFR in different patient subsets has been mounting,6,10–18 FFR is assessed in <10% of PCI performed in the absence of clinical evidence of ischaemia.19,20 The operator's decision on the use of FFR in clinical practice is often based on angiographic findings, which frequently fail to provide an accurate and reproducible measure of the haemodynamic significance of a stenosis.21,22 Therefore, the ability of routine use of FFR in clinical practice to confer any benefit remains uncertain. This ambiguity was reflected in the design of the FAME study,8 in which FFR-guidance was mandated in patients randomized to the FFR arm.
The aim of this study was to test the hypothesis that the use of FFR is associated with improved outcome in contemporary clinical practice. For this purpose, we compared long-term outcomes of FFR-guided to angiography-guided PCI.
Methods
Study population
Fractional flow reserve measurements were first introduced at the Mayo Clinic in 1999, but patients were followed in a registry starting October 2002. Therefore, consecutive patients referred for coronary revascularization with or without adjunct FFR between October 2002 and December 2009 were included in this study. Exclusion criteria included presentation with ST-segment elevated myocardial infarction (MI) or cardiogenic shock; referral for coronary artery bypass surgery; or patient refusal to allow the use of their records for research purposes. The study followed the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Mayo Clinic.
Patients were divided into two groups: (i) PCI-only and (ii) FFR-guided PCI groups. The latter group was further divided into subgroups of patients who underwent PCI in all the lesions assessed by FFR (a FFR-Perform group), and those in whom after FFR measurement PCI was deferred in at least one vessel (a FFR-Defer group) (Figure 1). Medical records of all patients were reviewed to extract information on clinical, laboratory, and angiographic characteristics.
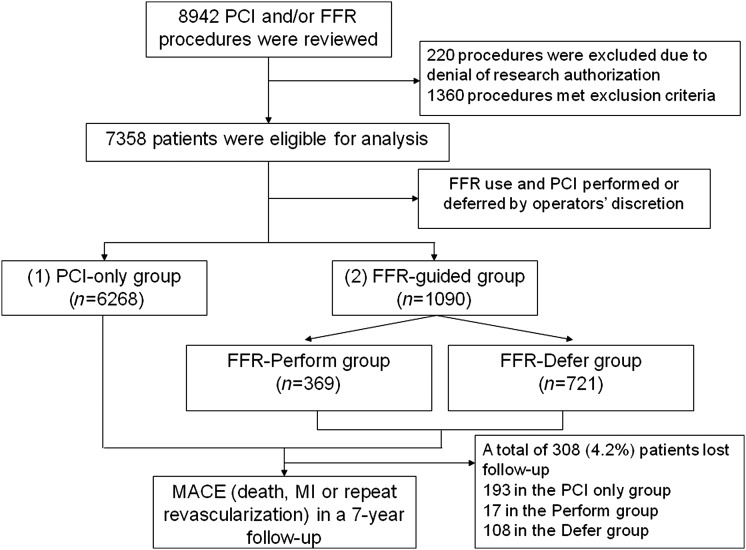
Figure 1.
Study flowchart. FFR, fractional flow reserve; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Coronary angiography
Diagnostic coronary angiography was performed using 4–7 French Judkins catheters through femoral or radial approaches.23 To avoid spasm and to achieve maximal epicardial vasodilatation, intracoronary (0.1–0.3 mg) or sublingual (0.4 mg) nitroglycerine was administered before angiography. All stenoses were assessed visually.
Intracoronary pressure measurements
Intracoronary pressure was measured using a 0.014-inch pressure-monitoring guidewire (Pressure Wire, Radi Medical, Uppsala, Sweden, or Wave Wire, Volcano, Rancho Cordova, CA, USA). The pressure wire was introduced via a 5F, 6F, or 7F guiding catheter, calibrated, advanced into the coronary artery, and placed distal to the assessed stenosis, as described previously.4 Fractional flow reserve was assessed after the administration of incremental doses of intracoronary adenosine to achieve maximal hyperaemia (up to 42 μg for the right coronary artery and up to 72 μg for the left coronary artery). In 19 (1.7%) of the 1090 patients with the use of FFR, adenosine was given i.v. (140 μg/kg/min). Fractional flow reserve was calculated as the ratio of the mean distal (trans-stenotic) coronary pressure measured by the pressure wire to the mean aortic pressure measured by the guiding catheter at maximal hyperaemia.24
Generally, PCI was performed in patients with FFR <0.75, and deferred in those with FFR >0.8. For FFR values ranging between 0.75 and 0.80, the decision was left to the operator's discretion.
Clinical follow-up
Patients that had undergone PCI were subsequently tracked via telephone calls at 6 , 12 months, and annually thereafter. Hospital records were obtained and reviewed to record follow-up events. Patients in whom PCI had been deferred were followed up by means of a single questionnaire and history review.
The primary endpoint during the follow-up was major adverse cardiac events (MACE), defined as composite of death, MI, and any repeat revascularization. The secondary endpoints were individual components of the MACE. Death encompassed all-cause mortality. Myocardial infarction was defined as (two out of three criteria): prolonged chest pain >20 min; levels of serum creatine kinase (or the MB fraction) or troponin over two-fold higher than the upper normal limit; and ST-T segment changes or new Q waves on serial electrocardiogram indicative of myocardial damage.25
Statistical analysis
Continuous variables are summarized as mean ± standard deviation for most variables, or median (25th, 75th percentile) where indicated. Discrete variables are summarized as frequency (group percentage). Group comparisons are tested using Student's two-sample t-test for most continuous variables, the rank sum test for FFR comparisons, and Pearson's χ2 test for discrete data. Kaplan–Meier estimates were used to estimate survival curves, and the log-rank test to test differences between groups. Cox proportional hazards multiple regression models were used to estimate association between FFR use vs. deferral on long-term outcomes, after adjusting for other patient characteristics that were significantly different between groups. All significance tests were two-tailed with a 0.05 significance level. All analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
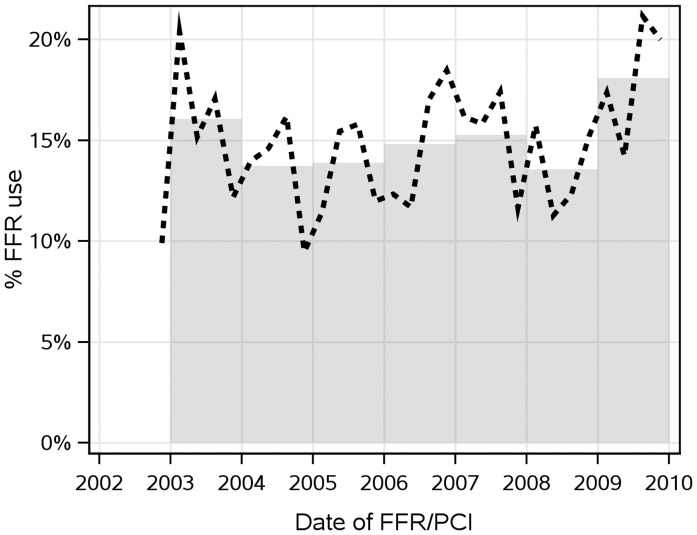
A total of 8942 PCI and/or FFR procedures were performed during the indicated time period. Of these, 220 patients were excluded due to denial of research authorization. We used only the first qualifying procedure for each unique patient, resulting in 7358 patients identified for analysis (Figure 1). Of these, 6268 (85.2%) underwent PCI without FFR assessment, while in the remaining 1090 patients (14.8%) FFR was performed. Among the latter, in 369 (33.9%) PCI was ultimately performed and in 721 (66.1%) PCI was deferred; in 115 patients (10.5%) PCI was performed in a vessel with FFR >0.80, while in 39 (3.6%) no PCI was performed in a vessel with FFR <0.75. The annual use rate of FFR was between 14 and 18% (Figure 2). Multivessel intervention was undertaken in 1186 (19.0%) of the PCI-only group, 73 (19.8%) of the FFR-Perform group, and 18 (2.5%) of the FFR-Defer group. In 135 (18.4%) patients of the FFR-Defer group, PCI was deferred in one vessel after the measurement of FFR, but was performed in another vessel. The clinical and angiographic characteristics of the different groups are shown in Tables 1 and 2. In-hospital outcomes are shown in Table 3.
Figure 2.
Utility rates of fractional flow reserve between 2002 and 2009. The annual rate of fractional flow reserve use was generally between 14 and 18%. The bar represents the rate of FFR use for that year and the bold-dashed line represents rate of fractional flow reserve use quarterly. FFR, fractional flow reserve; PCI, percutaneous coronary intervention.
Table 1.
Baseline characteristics of patients
| Variables | PCI-only (n = 6268) | FFR-guided group (n = 1090) |
||
|---|---|---|---|---|
| All (n = 1090) | FFR-Perform (n = 369) | FFR-Defer (n = 721) | ||
| Age (year) | 67.9 ± 11.6* | 65.7 ± 11.3 | 65.5 ± 11.3 | 65.8 ± 11.4 |
| Male gender, n (%) | 4416 (70.4)* | 683 (62.6) | 255 (69.1) | 428 (59.3)‡ |
| Body mass index | 30.2 ± 5.9 | 30.4 ± 5.8 | 30.4 ± 5.7 | 30.4 ± 5.8 |
| Current smoking, n (%) | 836 (13.3) | 140 (12.8) | 49 (13.2) | 91 (12.6) |
| Diabetes mellitus, n (%) | 1862 (29.7) | 306 (28.0) | 106 (28.7) | 200 (27.7) |
| Hypertension, n (%) | 4897 (78.1) | 864 (79.2) | 296 (80.2) | 568 (78.7) |
| Hypercholesterolaemia, n (%) | 5119 (81.6)* | 608 (55.7) | 312 (84.5) | 296 (41.0) |
| Chest pain, n (%) | 4529 (72.2)* | 753 (69.0) | 261 (70.7) | 492 (68.2) |
| Dyspnoea, n (%) | 168 (2.6)* | 89 (8.1) | 5 (1.3) | 84 (11.6)† |
| ACS (1–7 days), n (%) | 818 (13.0)* | 48 (4.4) | 19 (5.1) | 29 (4.0) |
| History of MI (>7 days), n (%) | 1882 (31) | 270 (25) | 99 (27) | 171 (24) |
| Prior PCI, n (%) | 1981 (31.6)* | 445 (40.8) | 168 (45.5) | 277 (38.4)‡ |
| CVD, n (%) | 682 (10.8) | 103 (9.4) | 35 (9.4) | 68 (9.4) |
| PAD, n (%) | 718 (11.4) | 106 (9.7) | 37 (10.0) | 69 (9.5) |
| Chronic obstructive pulmonary disease, n (%) | 704 (11.2) | 121 (11.1) | 32 (8.6) | 89 (12.3) |
| Renal dysfunction, n (%) | 285 (4.5) | 41 (3.7) | 13 (3.5) | 28 (3.8) |
| Stress test, n (%) | 1008 (16.0) | 112 (10.2) | 67 (18.1) | 45 (6.2) |
| LVEF ≤40%, n (%) | 705 (11.2)* | 82 (7.5) | 31 (8.4) | 51 (7.0) |
ACS, acute coronary syndrome; CVD, cerebral vascular disease; FFR, fractional flow reserve; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PCI, percutaneous coronary intervention; PAD, peripheral artery disease.
*P < 0.001 when compared with the FFR-guided group.
†P < 0.05 when compared with the FFR-Perform group.
‡P < 0.001 when compared with the FFR-Perform group.
Table 2.
Angiography and percutaneous coronary intervention characteristics
| Variables | PCI-only (n = 6268) | FFR-guided group (n = 1090) |
||
|---|---|---|---|---|
| All (n = 1090) | FFR-Perform (n = 369) | FFR-Defer (n = 721) | ||
| RCA stenosis ≥70%, n (%) | 3134 (50.0)* | 293 (26.8) | 125 (33.8) | 168 (23.0)‡ |
| LMCA stenosis ≥50%, n (%) | 205 (3.2)* | 9 (0.8) | 1 (0.3) | 8 (1.1) |
| LAD stenosis ≥70%, n (%) | 3474 (55.4)* | 419 (38.4) | 233 (63.1) | 186 (26.0)† |
| LCX stenosis ≥70%, n (%) | 2620 (41.7)* | 250 (22.9) | 104 (28.1) | 146 (20.2) |
| FFR in LMCA, n (%) | — | 37 (3.3) | 3 (0.8) | 34 (4.7)† |
| FFR 0.75–0.80, n (%) | 1 (0.1) | 0 | 1 (0.1) | |
| FFR in LAD, n (%) | — | 713 (65.4) | 244 (66.1) | 469 (65.0) |
| FFR 0.75–0.80, n (%) | 149 (13.7) | 87 (27.4) | 62 (8.6)† | |
| FFR in LCX, n (%) | — | 192 (17.6) | 51 (13.8) | 141 (19.5)† |
| FFR 0.75–0.80, n (%) | 22 (2.0) | 14 (3.8) | 8 (1.1)† | |
| FFR in RCA, n (%) | — | 213 (19.5) | 70 (18.9) | 143 (19.8) |
| FFR 0.75–0.80, n (%) | 37 (3.4) | 22 (6.0) | 15 (2.1)† | |
| FFR in graft, n (%) | — | 23 (2.1) | 4 (1.0) | 19 (2.6) |
| FFR 0.75–0.80, n (%) | 2 (0.2) | 0 | 2 (0.3) | |
| Median FFR value (IQR) | — | 0.77 (0.72, 0.82) | 0.87 (0.82, 0.91)† | |
| PCI in native LM, n (%) | 262 (4.1)*,‡ | 7 (0.6) | 6 (1.6) | 1 (0.1)‡ |
| PCI in native LAD, n (%) | 2846 (45.4)*,† | 299 (27.4) | 260 (70.4) | 39 (5.4)† |
| PCI in native LCX, n (%) | 1867 (29.7)*,† | 124 (11.3) | 79 (21.4) | 45 (6.2)† |
| PCI in native RCA, n (%) | 2118 (33.7)*,‡ | 147 (13.4) | 97 (26.2) | 50 (6.9)† |
| Vein graft intervention, n (%) | 475 (7.5)*,† | 6 (0.5) | 3 (0.8) | 3 (0.4) |
| Number of vessel treated, n (%) | ||||
| 1 | 5078 (81.0) | — | 296 (80.2) | — |
| 2 | 1087 (17.3) | — | 71 (19.2) | — |
| 3 | 99 (1.7) | — | 2 (0.6) | — |
| Use of DES, n (%) | 4417 (70.4)* | 361 (33.1) | 273 (73.9) | 88 (12.2)† |
| Use of BMS, n (%) | 1632 (26.0)* | 118 (10.8) | 88 (23.8) | 30 (4.2)† |
| Number of stents placed | 1.5 ± 1.0*,‡ | 0.6 ± 0.9 | 1.4 ± 0.7 | 0.2 ± 0.7† |
| Procedural success of stents placement, n (%) | 5953 (94.9)‡ | — | 359 (97.3) | — |
BMS, bare metal stent; DES, drug-eluting stent; FFR, fractional flow reserve; LAD, left anterior descending; LCX, left circumflex; LMCA, left main coronary artery; PCI, percutaneous coronary intervention; RCA, right coronary artery.
*P < 0.001 when compared with the FFR-guided group.
‡P < 0.05 when compared with the FFR-Perform group.
†P < 0.001 when compared with the FFR-Perform group.
Table 3.
In-hospital events
| Variables | PCI-only (n=6268) | FFR-guided group (n = 1090) |
||
|---|---|---|---|---|
| All (n = 1090) | Perform (n = 369) | Defer (n = 721) | ||
| In-hospital events | ||||
| Death, n (%) | 22 (0.3) | 3 (0.2) | 2 (0.5) | 1 (0.1) |
| Death/Q-wave MI/stroke/CABG, n (%) | 63 (0.9)§ | 4 (0.3) | 2 (0.5) | 2 (0.2) |
| Q-wave MI, n (%) | 9 (0.1) | 0 | 0 | 0 |
| Emergency CABG, n (%) | 18 (0.2) | 1 (0.09) | 0 | 1 (0.1) |
| In-hospital CVD, n (%) | 19 (0.3) | 0 (0) | 0 | 0 |
| In-hospital any MI, n (%) | 266 (4.2)* | 18 (1.6) | 12 (3.2) | 6 (1)‡ |
CABG, coronary artery bypass grafting; CVD, cerebral vascular disease; FFR, fractional flow reserve; MI, myocardial infarction; PCI, percutaneous coronary intervention.
§P < 0.05 when compared with the FFR-guided group.
*P < 0.001 when compared with the FFR-guided group.
‡P < 0.05 when compared with the FFR-Perform group.
Clinical outcome of patients with vs. without use of fractional flow reserve
Follow-up information was available in 7050 (95.8%) patients. The median follow-up duration was 44.9 months in the PCI-only group, 52.2 months in the FFR-Perform group, and 48.7 months in the FFR-Defer group, which were not significantly different.
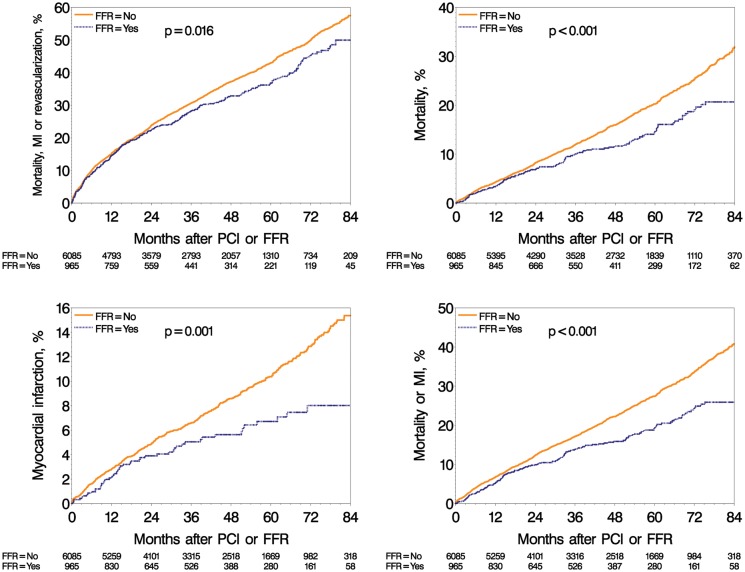
The unadjusted Kaplan–Meier estimates of MACE (50 vs. 57%, P = 0.016), mortality (21 vs. 32%, P < 0.001), MI (8 vs. 15%, P = 0.001), and mortality or MI (26 vs. 41%, P < 0.001) at 7 years were lower in the FFR-guided compared with the PCI-only group; on the other hand, the rate of repeat revascularization was comparable between the two groups (35 vs. 36%, P = 0.97) (Figure 3).
Figure 3.
Long-term adverse events in the percutaneous coronary intervention only group and fractional flow reserve-guided group. Unadjusted Kaplan–Meier curves during a 7-year follow-up for major adverse cardiac event (left top); death (right top); myocardial infarction (bottom left); and for death or myocardial infarction (bottom right). FFR, fractional flow reserve; PCI, percutaneous coronary intervention; MACE, major adverse cardiac events; MI, myocardial infarction.
Long-term outcomes in the fractional flow reserve -Perform and fractional flow reserve -Defer groups
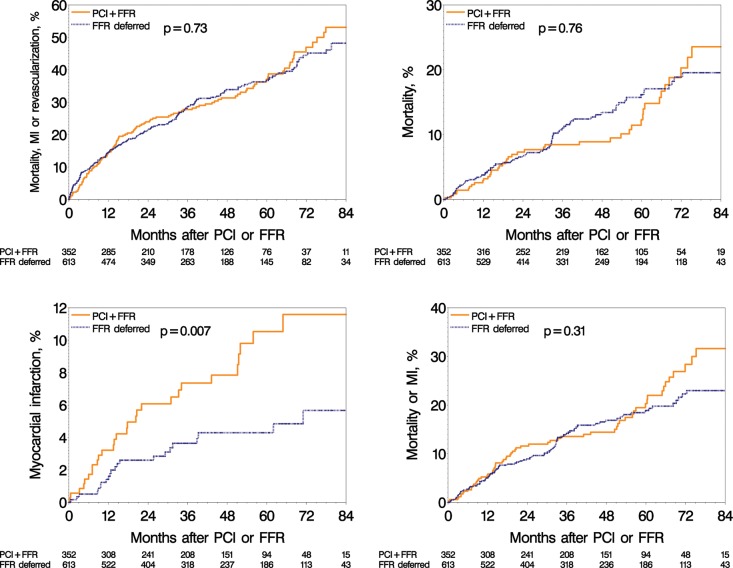
The unadjusted Kaplan–Meier fraction of MI estimated at 7 years reached 12% in the FFR-Perform vs. 6% in the FFR-Defer groups (P = 0.007) (Figure 4). Other outcome events showed no significant difference between the two groups.
Figure 4.
Long-term adverse events in the fractional flow reserve-Perform group and fractional flow reserve-Defer group. Unadjusted Kaplan–Meier curves during a 7-year follow-up for major adverse cardiac event (left top); death (right top); myocardial infarction (bottom left); and for death or myocardial infarction (bottom right). FFR, fractional flow reserve; PCI, percutaneous coronary intervention; MI, myocardial infarction
Cox multivariate models for follow-up events
After adjustment for baseline characteristics in a Cox multivariable model, patients undergoing FFR measurements tended to have lower rates of death/MI [hazard ratio (HR): 0.85, 95% confidential interval (CI): 0.71–1.01, P = 0.06]; specifically, deferral of PCI guided by FFR was significantly associated with a reduced rate of MI (HR: 0.46, 95% CI: 0.26–0.82, P = 0.008) (Table 4).
Table 4.
Cox multivariable models for determinants of outcome events
| Events | Adjusteda HR | 95% CI | P-value |
|---|---|---|---|
| FFR use vs. no FFR | |||
| MACE | 1.01 | 0.89–1.14 | 0.93 |
| Death | 0.89 | 0.73–1.10 | 0.28 |
| MI | 0.79 | 0.58–1.07 | 0.12 |
| Death/revascularization | 1.003 | 0.88–1.14 | 0.96 |
| Death/MI | 0.85 | 0.71–1.01 | 0.06 |
| Deferral of PCI after FFR vs. Perform PCI | |||
| MACE | 0.97 | 0.77–1.23 | 0.81 |
| Death | 0.84 | 0.56–1.24 | 0.37 |
| MI | 0.46 | 0.26–0.82 | 0.008 |
| Death/revascularization | 1.002 | 0.78–1.27 | 0.98 |
| Death/MI | 0.73 | 0.52–1.01 | 0.06 |
CI, confidential interval; FFR, fractional flow reserve; HR, hazard ratio; MACE, major adverse cardiac events; MI, myocardial infarction; PCI, percutaneous coronary intervention.
aAdjusted for age, sex, body mass index, smoking history (current, former, or never), chronic heart failure on presentation, diabetes, hypertension, hypercholesterolaemia, primary symptom, recent MI, prior PCI, prior coronary artery bypass grafting, history of myocardial infarction, heart failure, cerebral vascular disease, peripheral artery disease, chronic obstructive pulmonary disease, renal dysfunction, presence of tumour/lymphoma/leukaemia, metastatic cancer, ejection fraction ≤40%, ejection fraction unknown, level of stenosis in each coronary vessel (right coronary artery, left anterior descending, left circumflex, left main coronary artery).
After excluding patients with FFR of 0.75–0.80 and deferral of PCI, the incidence of death or MI was significantly lower in the FFR-guided group compared with the PCI-only group (HR: 0.80, 95% CI: 0.66–0.96, P = 0.02); deferral of PCI after FFR measurement was significantly associated with reduced rates of MI (HR: 0.39, 95% CI: 0.20–0.73, P = 0.004) and death or MI (HR: 0.64, 95% CI: 0.45–0.92, P = 0.02) (Table 5).
Table 5.
Model estimates after excluding patients with percutaneous coronary intervention deferred in a vessel with a 0.75–0.80 fractional flow reserve measure
| Events | Adjusteda HR | 95% CI | P-value |
|---|---|---|---|
| FFR use vs. no FFR | |||
| MACE | 0.95 | 0.83–1.08 | 0.42 |
| Death | 0.84 | 0.67–1.04 | 0.11 |
| MI | 0.75 | 0.54–1.03 | 0.08 |
| Death/revascularization | 0.94 | 0.82–1.08 | 0.38 |
| Death/MI | 0.80 | 0.66–0.96 | 0.02 |
| Deferral of PCI after FFR vs. Perform PCI | |||
| MACE | 0.86 | 0.67–1.11 | 0.24 |
| Death | 0.73 | 0.48–1.11 | 0.14 |
| MI | 0.39 | 0.20–0.73 | 0.004 |
| Death/revascularization | 0.89 | 0.69–1.15 | 0.36 |
| Death/MI | 0.64 | 0.45–0.92 | 0.02 |
CI, confidential interval; FFR, fractional flow reserve; HR, hazard ratio; MACE, major adverse cardiac events; MI, myocardial infarction; PCI, percutaneous coronary intervention.
aAdjusted for age, sex, body mass index, smoking history (current, former, or never), chronic heart failure on presentation, diabetes, hypertension, hypercholesterolaemia, primary symptom, recent MI, prior PCI, prior coronary artery bypass grafting, history of myocardial infarction, heart failure, cerebral vascular disease, peripheral artery disease, chronic obstructive pulmonary disease, renal dysfunction, presence of tumour/lymphoma/leukaemia, metastatic cancer, ejection fraction ≤40%, ejection fraction unknown, level of stenosis in each coronary vessel (right coronary artery, left anterior descending, left circumflex, left main coronary artery).
Discussion
In this registry-based study, we compared clinical outcomes of patients undergoing FFR-guided vs. angiography-guided PCI in a large non-selected population. Overall, the use of FFR (achieved in 14.8% of patients) was associated with a non-significantly lower incidence (P = 0.06) of death or MI. However, after excluding patients in whom PCI was deferred in a vessel with FFR between 0.75 and 0.80, the long-term outcome was better in the FFR-guided group compared with the angiography-guided group. Furthermore, the incidence of MI was significantly lower in the FFR-Defer group compared with the FFR-Perform group. These observations, therefore, support the use of FFR in routine clinical practice.
Fractional flow reserve-guided percutaneous coronary intervention in the general clinical practice
The current registry-based study, to our knowledge, examined the largest number of patients with FFR-guided interventions. In the overall population, the primary endpoint (MACE) was not different between the FFR-guided and angiography-guided groups and the secondary endpoint of death/MI reached only borderline significance levels. However, several disparities in the use of FFR between the routine clinical practice and clinical trials may diminish potential benefits from FFR-guided treatment.
First, in daily practice settings in many hospitals, the use of FFR is often entirely at the operator's discretion based on angiographic information. Alas, the relationship between the angiographic appearance of a coronary stenosis and its functional significance is notoriously poor.21,22 Consequently, when FFR is infrequently used, some functionally severe lesions that by visual assessment induce ostensibly mild to moderate vessel narrowing might have been excluded from PCI. Conversely, some haemodynamically insignificant lesions, which potentially have a better outcome, may be visually interpreted as severe, and therefore undergo unnecessary PCI. Indeed, Sant'Anna et al.26 have demonstrated that FFR routinely used for decision-making modifies treatment decisions for 32% of all stenoses and 48% of all patients, compared with decisions based on angiography alone. Therefore, low usage rate of functional assessment in general practice may lead to underestimation of its potential benefits. Since FFR has been validated in almost all clinical and anatomical subsets, its systematic use can render PCI an even more effective and appropriate treatment than it is currently.27
Moreover, FFR values ranging between 0.75 and 0.80 were previously considered to be in a ‘grey zone’, and required clinical judgment for decision-making regarding revascularization. However, Legalery et al.20 demonstrated that deferring PCI in lesions with FFR under 0.80 was harmful. More recent studies adopted the upper limit of this small transition zone as a threshold to perform PCI in order to limit the number of ischaemic lesion left untreated.7,11,12 In the current study, in 40.0% of the 220 patients with FFR values between 0.75 and 0.80 PCI was deferred. This might influence relative outcomes between the angiography-guided and FFR-guided groups, probably in favour of the angiography-guided strategy. After excluding those patients from analysis, the FFR-guided group had a significantly lower rate of death or MI when compared with the PCI-only group. This result is consistent with the 2-year follow-up of the FAME study8 and indicates a FFR value of 0.80 as an appropriate threshold to intervene.
In addition, a registy study reported that 9% of patients were treated despite an FFR ≥0.80.20 In the FFR-guided group of the current study, 10.5% of patients had PCI performed in a vessel with FFR >0.80 and 39 (3.6%) did not have PCI in a vessel with FFR <0.75. It is not uncommon to encounter a clinical or angiographic situation where the FFR result seems to disagree with visual assessment by angiography. Although its accuracy may be influenced by some pathological factors,28–30 when performed correctly, false negative or false positive FFR results are relatively rare.31 Obviously, FFR should only be measured when the operator plans to follow through on the result of this test. In fact, the FAME study suggests that stenting of lesions with an FFR >0.80 is detrimental.8 Thus, clinical benefits could be enhanced when patients are treated in accordance with the FFR criteria.
The safety of deferring percutaneous coronary intervention under guidance of fractional flow reserve
The benefit of functional evaluation is attributed to identification of ischaemia-causing coronary stenoses, and its contribution to judicious decision-making of revascularization, which in turn may reduce unexpected device-related diseases.2,3 Consistent with previous studies,32,33 our data demonstrate a favourable outcome in the FFR-Defer group compared with the FFR-Perform group, which strongly supports the use of FFR when evaluating whether PCI can be safely deferred. Moreover, although some patients underwent PCI in a vessel with FFR >0.80, the number of stents placed was still significantly lower in the FFR-guided group. Similarly, a recent analysis of the FAME study showed that the FFR-guided PCI resulted in significant cost-saving by reducing stent use, rehospitalizations, and MACE.34 Therefore, the FFR-guided treatment could have been more economical in daily practice if decision-making for PCI relies more strictly on FFR value.
Incidentally, the use of FFR between 2003 and 2009 showed a transient decline in the mid-years, followed by a sudden increase in 2009. This may reflect a change in clinical practice after publication of the landmark FAME study in January 2009.7
Limitations
This single-centre, observational study has limitations inherent to non-randomized trials. It was performed in a non-selected population of clinical practice with unequal baseline characteristics, and involved multiple operators. Multiple regression analysis may mitigate bias after adjustment of confounding factors, but unmeasured indicators leave room for residual bias.
In the current study, the adenosine protocol included both i.v. and intracoronary routes. Although we have previously demonstrated that incremental doses of intracoronary adenosine were valid to achieve maximum coronary hyperaemia,35 reports are inconsistent.36,37 Presently, central i.v. administration of adenosine remains the gold standard for FFR measurements. Moreover, the dose of intracoronary adenosine used in this study followed common clinical practice, but might be too low for some patients.
The coronary stenoses were assessed visually, rather than by quantitative coronary angiography (QCA) or by intravascular ultrasound. Nevertheless, visual assessment is the most common method to evaluate diameter stenoses in the catheterization laboratory. On the other hand, the accuracy of QCA or intravascular ultrasound for predicting functionally significant FFR is also limited.38,39
Conclusion
In this registry study, we found a favourable long-term outcome in an FFR-guided group. This result is in keeping with previous clinical trials, and therefore provides important evidence supporting the rationale for the use of FFR in routine practice.
Funding
National Institute of Health (NIH Grant HL-92954 and AG-31750 to A.L.). The study was also supported by an unrestricted grant from St Jude Medical. J.L. was supported by the China Scholarship Council (NO.2010811095), and ‘Beijing Nova program’ of Beijing Municipal Science & Technology Commission (A2007079), China. A.J.F. was supported by the Walter and Gertrud Siegenthaler Foundation, the Young Academics Support Committee of the University of Zurich, and the Swiss foundation for Medical-Biological Scholarships (SSMBS; SNSF No PASMP3_132551). L.O.L. has received financial support through an institutional grant from the NIH.
Conflict of interest: L.O.L. has also received honorarium from the NIH. She has also been employed by the NIH. With regard to financial activities outside the submitted work, she has been employed by the NIH and has received research grant support from Stealth Peptides, Inc. She has also been awarded a post doc fellowship by the AHA. In addition, she has received institutional financial support for pending patents, and has also received support for accommodation at international scientific meetings from other academic institutions. With regard to financial activities outside the submitted work, A.L. (corresponding author) has received remuneration as a board member of Itamar Medical as well as support from an NIH institutional grant.
References
- 1.Davies RF, Goldberg AD, Forman S, Pepine CJ, Knatterud GL, Geller N, Sopko G, Pratt C, Deanfield J, Conti CR. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation. 1997;95:2037–2043. doi: 10.1161/01.cir.95.8.2037. doi:10.1161/01.CIR.95.8.2037. [DOI] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. doi:10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. doi:10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 4.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604. doi:10.1056/NEJM199606273342604. [DOI] [PubMed] [Google Scholar]
- 5.Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928–2934. doi: 10.1161/01.cir.103.24.2928. doi:10.1161/01.CIR.103.24.2928. [DOI] [PubMed] [Google Scholar]
- 6.Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087. doi:10.1016/j.jacc.2007.01.087. [DOI] [PubMed] [Google Scholar]
- 7.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. doi:10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 8.Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–184. doi: 10.1016/j.jacc.2010.04.012. doi:10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 9.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Juni P, Fearon WF. Fractional flow reserve-guided pci versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. doi:10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 10.Hamilos M, Muller O, Cuisset T, Ntalianis A, Chlouverakis G, Sarno G, Nelis O, Bartunek J, Vanderheyden M, Wyffels E, Barbato E, Heyndrickx GR, Wijns W, De Bruyne B. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505–1512. doi: 10.1161/CIRCULATIONAHA.109.850073. doi:10.1161/CIRCULATIONAHA.109.850073. [DOI] [PubMed] [Google Scholar]
- 11.Puymirat E, Peace A, Mangiacapra F, Conte M, Ntarladimas Y, Bartunek J, Vanderheyden M, Wijns W, De Bruyne B, Barbato E. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary revascularization in patients with small-vessel disease. Circ Cardiovasc Interv. 2012;5:62–68. doi: 10.1161/CIRCINTERVENTIONS.111.966937. doi:10.1161/CIRCINTERVENTIONS.111.966937. [DOI] [PubMed] [Google Scholar]
- 12.Muller O, Mangiacapra F, Ntalianis A, Verhamme KM, Trana C, Hamilos M, Bartunek J, Vanderheyden M, Wyffels E, Heyndrickx GR, van Rooij FJ, Witteman JC, Hofman A, Wijns W, Barbato E, De Bruyne B. Long-term follow-up after fractional flow reserve-guided treatment strategy in patients with an isolated proximal left anterior descending coronary artery stenosis. JACC Cardiovasc Interv. 2011;4:1175–1182. doi: 10.1016/j.jcin.2011.09.007. doi:10.1016/j.jcin.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Sels JW, Tonino PA, Siebert U, Fearon WF, Van't Veer M, De Bruyne B, Pijls NH. Fractional flow reserve in unstable angina and non-st-segment elevation myocardial infarction experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) Study. JACC Cardiovasc Interv. 2011;4:1183–1189. doi: 10.1016/j.jcin.2011.08.008. doi:10.1016/j.jcin.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Koo BK, Park KW, Kang HJ, Cho YS, Chung WY, Youn TJ, Chae IH, Choi DJ, Tahk SJ, Oh BH, Park YB, Kim HS. Physiological evaluation of the provisional side-branch intervention strategy for bifurcation lesions using fractional flow reserve. Eur Heart J. 2008;29:726–732. doi: 10.1093/eurheartj/ehn045. doi:10.1093/eurheartj/ehn045. [DOI] [PubMed] [Google Scholar]
- 15.Aqel R, Zoghbi GJ, Hage F, Dell'Italia L, Iskandrian AE. Hemodynamic evaluation of coronary artery bypass graft lesions using fractional flow reserve. Catheter Cardiovasc Interv. 2008;72:479–485. doi: 10.1002/ccd.21675. doi:10.1002/ccd.21675. [DOI] [PubMed] [Google Scholar]
- 16.Pijls NH. Fractional flow reserve after previous myocardial infarction. Eur Heart J. 2007;28:2301–2302. doi: 10.1093/eurheartj/ehm333. doi:10.1093/eurheartj/ehm333. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Palop R, Pinar E, Lozano I, Saura D, Pico F, Valdes M. Utility of the fractional flow reserve in the evaluation of angiographically moderate in-stent restenosis. Eur Heart J. 2004;25:2040–2047. doi: 10.1016/j.ehj.2004.07.016. doi:10.1016/j.ehj.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Jensen LO, Thayssen P, Lassen JF, Hansen HS, Kelbaek H, Junker A, Pedersen KE, Hansen KN, Krusell LR, Botker HE, Thuesen L. Recruitable collateral blood flow index predicts coronary instent restenosis after percutaneous coronary intervention. Eur Heart J. 2007;28:1820–1826. doi: 10.1093/eurheartj/ehm067. doi:10.1093/eurheartj/ehm067. [DOI] [PubMed] [Google Scholar]
- 19.Puymirat E, Muller O, Sharif F, Dupouy P, Cuisset T, de Bruyne B, Gilard M. Fractional flow reserve: concepts, applications and use in France in 2010. Arch Cardiovasc Dis. 2010;103:615–622. doi: 10.1016/j.acvd.2010.10.006. doi:10.1016/j.acvd.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Legalery P, Schiele F, Seronde MF, Meneveau N, Wei H, Didier K, Blonde MC, Caulfield F, Bassand JP. One-year outcome of patients submitted to routine fractional flow reserve assessment to determine the need for angioplasty. Eur Heart J. 2005;26:2623–2629. doi: 10.1093/eurheartj/ehi484. doi:10.1093/eurheartj/ehi484. [DOI] [PubMed] [Google Scholar]
- 21.Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, Boersma E, Regar E, van Geuns RJ, de Jaegere PJ, Serruys PW, Krestin GP, de Feyter PJ. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52:636–643. doi: 10.1016/j.jacc.2008.05.024. doi:10.1016/j.jacc.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. doi: 10.1161/01.cir.92.8.2333. doi:10.1161/01.CIR.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 23.Lavi S, Rihal CS, Yang EH, Fassa AA, Elesber A, Lennon RJ, Mathew V, David HR, Jr, Lerman A. The effect of drug eluting stents on cardiovascular events in patients with intermediate lesions and borderline fractional flow reserve. Catheter Cardiovasc Interv. 2007;70:525–531. doi: 10.1002/ccd.21154. doi:10.1002/ccd.21154. [DOI] [PubMed] [Google Scholar]
- 24.Brosh D, Higano ST, Lennon RJ, Holmes DR, Jr, Lerman A. Effect of lesion length on fractional flow reserve in intermediate coronary lesions. Am Heart J. 2005;150:338–343. doi: 10.1016/j.ahj.2004.09.007. doi:10.1016/j.ahj.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. doi:10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 26.Sant'Anna FM, Silva EE, Batista LA, Ventura FM, Barrozo CA, Pijls NH. Influence of routine assessment of fractional flow reserve on decision making during coronary interventions. Am J Cardiol. 2007;99:504–508. doi: 10.1016/j.amjcard.2006.09.097. doi:10.1016/j.amjcard.2006.09.097. [DOI] [PubMed] [Google Scholar]
- 27.Pijls NH, Sels JW. Functional measurement of coronary stenosis. J Am Coll Cardiol. 2012;59:1045–1057. doi: 10.1016/j.jacc.2011.09.077. doi:10.1016/j.jacc.2011.09.077. [DOI] [PubMed] [Google Scholar]
- 28.Magni V, Chieffo A, Colombo A. Evaluation of intermediate coronary stenosis with intravascular ultrasound and fractional flow reserve: its use and abuse. Catheter Cardiovasc Interv. 2009;73:441–448. doi: 10.1002/ccd.21812. doi:10.1002/ccd.21812. [DOI] [PubMed] [Google Scholar]
- 29.De Bruyne B, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter H, Van Crombrugge P, Heyndrickx GR, Wijns W. Fractional flow reserve in patients with prior myocardial infarction. Circulation. 2001;104:157–162. doi: 10.1161/01.cir.104.2.157. doi:10.1161/01.CIR.104.2.157. [DOI] [PubMed] [Google Scholar]
- 30.Melikian N, Cuisset T, Hamilos M, De Bruyne B. Fractional flow reserve–the influence of the collateral circulation. Int J Cardiol. 2009;132:e109–e110. doi: 10.1016/j.ijcard.2007.08.034. doi:10.1016/j.ijcard.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Koolen JJ, Pijls NH. Coronary pressure never lies. Catheter Cardiovasc Interv. 2008;72:248–256. doi: 10.1002/ccd.21528. doi:10.1002/ccd.21528. [DOI] [PubMed] [Google Scholar]
- 32.Misaka T, Kunii H, Mizukami H, Sakamoto N, Nakazato K, Takeishi Y. Long-term clinical outcomes after deferral of percutaneous coronary intervention of intermediate coronary stenoses based on coronary pressure-derived fractional flow reserve. J Cardiol. 2011;58:32–37. doi: 10.1016/j.jjcc.2011.03.007. doi:10.1016/j.jjcc.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Nam CW, Mangiacapra F, Entjes R, Chung IS, Sels JW, Tonino PA, De Bruyne B, Pijls NH, Fearon WF. Functional SYNTAX score for risk assessment in multivessel coronary artery disease. J Am Coll Cardiol. 2011;58:1211–1218. doi: 10.1016/j.jacc.2011.06.020. doi:10.1016/j.jacc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Fearon WF, Bornschein B, Tonino PA, Gothe RM, Bruyne BD, Pijls NH, Siebert U. Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) Study Investigators. Economic evaluation of fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. Circulation. 2010;122:2545–2550. doi: 10.1161/CIRCULATIONAHA.109.925396. doi:10.1161/CIRCULATIONAHA.109.925396. [DOI] [PubMed] [Google Scholar]
- 35.Murtagh B, Higano S, Lennon R, Mathew V, Holmes DR, Jr, Lerman A. Role of incremental doses of intracoronary adenosine for fractional flow reserve assessment. Am Heart J. 2003;146:99–105. doi: 10.1016/S0002-8703(03)00120-0. doi:10.1016/S0002-8703(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Palop R, Saura D, Pinar E, Lozano I, Pérez-Lorente F, Picó F, Valdez M. Adequate intracoronary adenosine doses to achieve maximum hyperaemia in coronary functional studies by pressure derived fractional flow reserve: a dose response study. Heart. 2004;90:95–96. doi: 10.1136/heart.90.1.95. doi:10.1136/heart.90.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leone AM, Porto I, De Caterina AR, Basile E, Aurelio A, Gardi A, Russo D, Laezza D, Niccoli G, Burzotta F, Trani C, Mazzari MA, Mongiardo R, Rebuzzi AG, Crea F. Maximal hyperemia in the assessment of fractional flow reserve: intracoronary adenosine versus intracoronary sodium nitroprusside versus intravenous adenosine: the NASCI (Nitroprussiato versus Adenosina nelle Stenosi Coronariche Intermedie) study. JACC Cardiovasc Interv. 2012;5:402–408. doi: 10.1016/j.jcin.2011.12.014. doi:10.1016/j.jcin.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Yong AS, Ng AC, Brieger D, Lowe HC, Ng MK, Kritharides L. Three-dimensional and two-dimensional quantitative coronary angiography, and their prediction of reduced fractional flow reserve. Eur Heart J. 2011;32:345–353. doi: 10.1093/eurheartj/ehq259. doi:10.1093/eurheartj/ehq259. [DOI] [PubMed] [Google Scholar]
- 39.Koo BK, Yang HM, Doh JH, Choe H, Lee SY, Yoon CH, Cho YK, Nam CW, Hur SH, Lim HS, Yoon MH, Park KW, Na SH, Youn TJ, Chung WY, Ma S, Park SK, Kim HS, Tahk SJ. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. JACC Cardiovasc Interv. 2011;4:803–811. doi: 10.1016/j.jcin.2011.03.013. doi:10.1016/j.jcin.2011.03.013. [DOI] [PubMed] [Google Scholar]