Abstract
Purpose
Low-grade neuroepithelial tumors are frequent neuropathological findings in patients with pharmacoresistant epilepsies. Little is known regarding epileptogenic mechanisms in this group of neoplasms with gangliogliomas (GG) as the most common entity. Presence of hemosiderin deposits in GG points to impairment of the blood-brain barrier (BBB). Therefore, we hypothesized a potential role of BBB dysfunction and astrocytic albumin uptake as potential epileptogenic factor in GG.
Methods
Prussian blue staining and fluorescent double-immunohistochemistry with antibodies against albumin, GFAP, CD34 and GLUT-1 were used to analyze hemosiderin deposits and astroglial albumin accumulation in tumor and adjacent pre-existing brain tissue of GG (n = 10) and several control groups, i.e. dysembryoplastic neuroepithelial tumors (DNT; n = 5), focal cortical dysplasia with balloon cells (FCD IIb; n = 10), astrocytomas WHO grade II (n = 5) and clear renal cell carcinoma brain metastases (RCCM, n = 6).
Results
Our results revealed strong hemosiderin deposits in GG. Intriguingly, we noted substantial albumin uptake exclusively in neoplastic glial cell components of GG and DNT, whereas no significant albumin was present in perilesional reactive astrocytes. Strikingly, we did not observe substantial albumin uptake in further controls.
Conclusion
Glial albumin uptake was restricted to long-term epilepsy associated, vasculature-containing tumors. Intratumoural BBB dysfunction in concert with subsequent accumulation of albumin by neoplastic glial cell elements represent a new putatively epileptogenic mechanism for long-term epilepsy-associated tumors.
Keywords: Epilepsy, Ganglioglioma, DNT, Focal cortical dysplasia, Albumin, Hemosiderin, Blood-brain barrier
1. Introduction
Gangliogliomas (GG) represent the most frequent tumor entity in young patients undergoing surgery for drug-refractory epilepsy.1 GG commonly correspond to WHO grade I tumors and are histopathologically characterized by dysplastic neurons and neoplastic astroglial cells. Typically, contrast enhancement is present on magnetic resonance imaging (MRI) pointing to blood-brain barrier (BBB) dysfunction. Mechanisms underlying the high epileptogenicity of GG are still enigmatic.
Factors such as impaired neurochemical homeostasis, genetic and peritumoural changes have been suggested to underlie increased neuronal excitability.2,3 Recent data emphasized a contribution of BBB leakage to the progression of epilepsy,4–6 with astroglial albumin uptake as a key pathogenic factor.4,7,8 Frequent deposits of hemosiderin indicate an impairment of the BBB in this tumor entity.
Accordingly, we analyzed hemosiderin deposits and astroglial albumin accumulation in tumor and adjacent brain tissue of GG and in several ‘control’ pathologies.
2. Materials and methods
2.1. Patients and surgical specimens
Biopsy specimens were obtained from patients who underwent neurosurgery at the University of Bonn using controls as follows. Dysembryoplastic neuroepithelial tumors (DNT; WHO grade I) represent a further highly epilepsy-associated glioneuronal tumor entity. Diffuse astrocytomas (DA; WHO grade II) largely share morphological features of the astroglial component in GG but lack contrast enhancement and the strong association with drug-refractory epilepsy. Focal cortical dysplasias with balloon cells (FCD IIb) represent non-neoplastic epilepsy-associated lesions without a vascular component.9 Finally, clear renal cell carcinoma brain metastases (RCCM) often encounter neuropathological hemosiderin deposits and contrast enhancement on MRI. Here, seizures are rather acute than chronic (GG: n = 10; DNT: n = 5; FCD IIb: n = 10; DA: n = 5; RCCM: n = 6). All procedures were carried out in accordance with the declaration of Helsinki. The clinical parameters of the patients are summarized in Table 1A.
Table 1.
| A Clinical and neuropathological parameters of patients. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Sex | Therapy-refractory epilepsy |
Epilepsy duration (yrs) |
Age at surgery (yrs) |
Site of specimen collection |
Astrocytic albumin uptakea |
Hemosiderin deposits |
||
| Lesion | Perilesion | Lesion | Perilesion | ||||||
| Ganglioglioma (GG, WHO grade I) | |||||||||
| 1 | f | Yes | 26 | 30 | Temporal left | ++ | 0 | ++ | 0 |
| 2 | f | Yes | 15 | 31 | Temporal left | ++ | 0 | ++ | 0 |
| 3 | f | Yes | 15 | 39 | Frontal right | + | 0 | + | 0 |
| 4 | m | Yes | 3 | 6 | Frontal left | ++ | 0 | ++ | 0 |
| 5 | m | Yes | 38 | 51 | Temporal left | ++ | 0 | ++ | + |
| 6 | m | Yes | 2 | 17 | Temporal right | ++ | 0 | + | 0 |
| 7 | m | Yes | 2 | 12 | Temporal left | ++ | 0 | + | 0 |
| 8 | f | Yes | 13 | 36 | Temporal right | +++ | 0 | 0 | 0 |
| 9 | m | Yes | 3 | 39 | Temporal left | ++ | + | + | 0 |
| 10 | m | Yes | 1 | 20 | Temporal right | + | 0 | 0 | 0 |
| Dysembryoplastic neuroepethelial tumor (DNT, WHO grade I) | |||||||||
| 11 | f | Yes | 5 | 20 | Frontal left | + | 0 | 0 | 0 |
| 12 | m | Yes | 7 | 54 | Temporal left | + | 0 | + | 0 |
| 13 | m | Yes | 50 | 59 | Frontal left | ++ | 0 | ++ | + |
| 14 | m | Yes | 10 | 35 | Temporal right | + | 0 | + | 0 |
| 15 | f | Yes | 1 | 28 | Frontal left | + | 0 | 0 | 0 |
| Focal cortical dysplasia type IIb (FCD IIb) | |||||||||
| 16 | f | Yes | 8 | 9 | Parietal left | 0 | 0 | 0 | 0 |
| 17 | f | Yes | 2 | 9 | Temporal left | 0 | 0 | 0 | 0 |
| 18 | m | Yes | 10 | 10 | Insula left | 0 | 0 | 0 | 0 |
| 19 | m | Yes | 11 | 13 | Parietal left | + | 0 | 0 | 0 |
| 20 | f | Yes | 13 | 32 | Temporal right | + | 0 | 0 | + |
| 21 | m | Yes | 2 | 2 | Temporal left | 0 | 0 | 0 | 0 |
| 22 | m | Yes | 6 | 7 | Parietal left | 0 | 0 | + | 0 |
| 23 | f | Yes | 15 | 17 | Frontal left | 0 | 0 | 0 | 0 |
| 24 | f | Yes | 9 | 23 | Frontal right | 0 | 0 | 0 | 0 |
| 25 | m | Yes | 14 | 22 | Parietal left | + | 0 | 0 | 0 |
| Astrocytoma (WHO grade II) | |||||||||
| 26 | f | No | 45 | Temporal left | 0 | 0 | 0 | 0 | |
| 27 | f | No | 20 | Temporal right | 0 | 0 | 0 | 0 | |
| 28 | m | No | 36 | Insula right | 0 | 0 | 0 | 0 | |
| 29 | m | No | 33 | Temporal left | 0 | 0 | 0 | 0 | |
| 30 | f | No | 50 | Frontal left | 0 | 0 | 0 | 0 | |
| Renal cell carcinoma metastasis (RCCM) | |||||||||
| 31 | f | No | 61 | Frontal left | / | + | + | 0 | |
| 32 | m | No | 65 | Temporal left | / | 0 | ++ | + | |
| 33 | m | No | 57 | Frontal left | / | 0 | +++ | +++ | |
| 34 | f | No | 69 | Temporal left | / | + | ++ | 0 | |
| 35 | f | No | 43 | Frontal left | / | 0 | + | + | |
| 36 | m | No | 44 | Temporal left | / | 0 | + | 0 | |
| B Summarized lesional characteristics. | ||||
|---|---|---|---|---|
| GGa/DNTb | FCD IIbc | Astrocytomad | RCCMe | |
| Impaired BBBf | Yes | No | No | Yes |
| Glial tumor cell component | Yes | No | Yes | No |
| Chronic epilepsy | Yes | Yes | No | No |
| Astroglial albumin uptake | Yes | No | No | No |
Astrocytic albumin storage as observed in lesional and perilesional areas of gangliogliomas, DNT, FCD IIb and astrocytomas. Due to mainly absent astrocytes in lesional regions of carcinoma metastasis, only the perilesional brain tissue was considered for examination.
Ganglioglioma WHO grade I.
Dysembryoplastic neuroepethelial tumor complex variant.
Focal cortical dysplasia type IIb.
Astrocytoma WHO grade II.
Cerebral metastases of clear renal cell cancer.
As indicated by hemosiderin deposits and contrast enhancement.
2.2. Tissue processing, immunohistochemistry, double-immunofluorescence and image analysis
These procedures were performed as described before,10 see Appendix A for more details. Prussian blue staining and GFAP/albumin double-immunofluorescence labeling was used in all cases to analyze semiquantitatively (none, few, intermediate, abundant) hemosiderin deposits and astrocytic albumin uptake in lesional and perilesional regions (Tables 1A and 1B). Representative neuropathological findings for the different groups are shown in Figs. A1–A3.
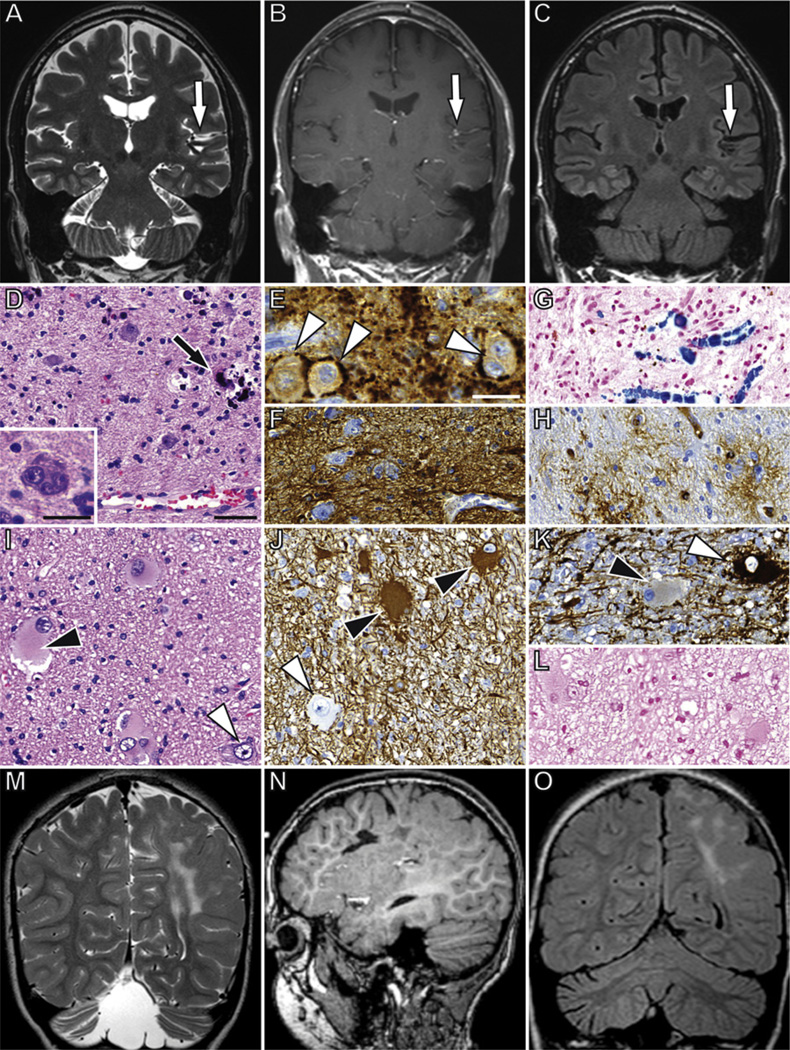
Fig. A1.
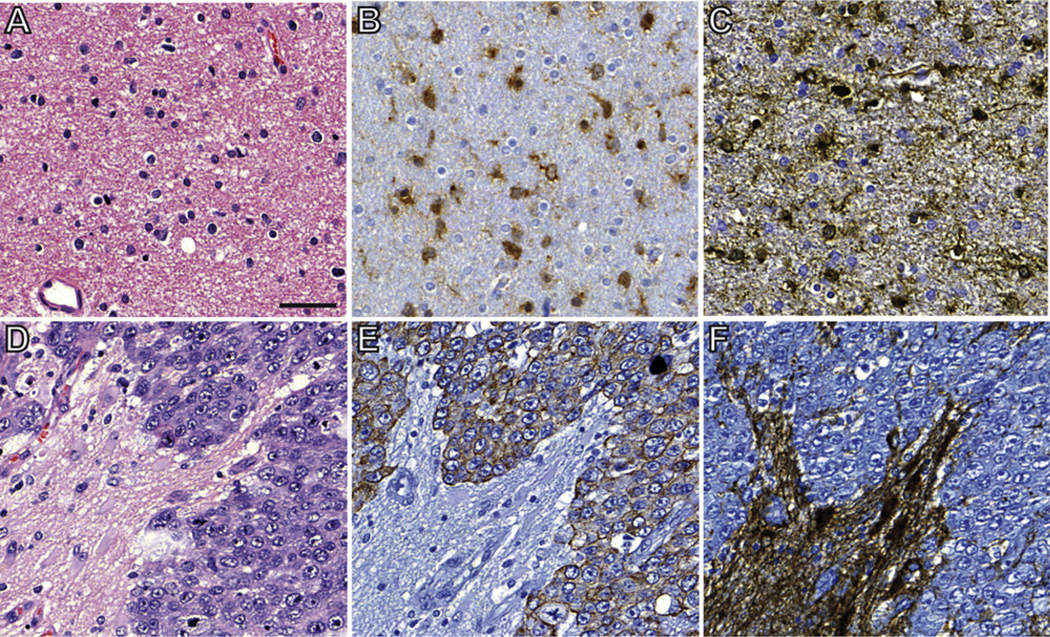
Neuroradiological and histopathological findings in gangliogliomas and FCD IIb. In a representative case of GG, coronal T2-weighted (A), T1-weighted contrast enhanced (B), and FLAIR fast spin echo (C) magnetic resonance images show a superior temporal gyrus and insular lesion (white arrows) with calcification (hypointense areas in A and C, proven by CT (not shown)) and contrast enhancement. Microscopically a mixture of glial and neuronal cells with focal calcifications (black arrow) can be identified (D, Hematoxylin/Eosin (H&E) staining). The neuronal component is represented by dysplastic neurons, which show prominent Nissl substance and lack uniform orientation. Bi- or multi-nucleate neurons may also be observed (Insert in D). A perisomatic expression of synaptophysin is a frequent observed feature of dysplastic neurons in GG (E, white arrowheads) The glial component is represented by astrocytic differentiated cells with strong expression of glial fibrillary acidic protein (GFAP, F). Multifocal hemosiderin deposits demonstrate blood-brain barrier (BBB) dysfunction within the tumor tissue (G, Prussian blue staining). CD34-immunoreactive cells with intense ramification of processes (satellite cells) represent a further feature of GG (H). H and E staining of a representative FCD IIb (I) shows characteristic histopathological features as the presence of dysmorphic neurons (white arrowheads in I–K) and balloon cells (black arrowheads in I–K). Immunohistochemical analysis reveals strong expression of vimentin (J), but absence of MAP2 immunoreactivity (K) in balloon cells, whereas dysmorphic neurons are negative for vimentin and positive for MAP2. Hemosiderin deposits are usually absent in FCD IIb (L). Coronal T2-weighted fast spin echo (M), sagittal 3D T1-weighted gradient echo (N), and coronal FLAIR fast spin echo (O) images show a large insular and parietal dysplasia with subcortical FLAIR and T2 hyperintensity suggestive of a FCD type 2B. Scale bars: D = 50 µm (applies also to F, G–L); Insert in D and E = 25 µm.
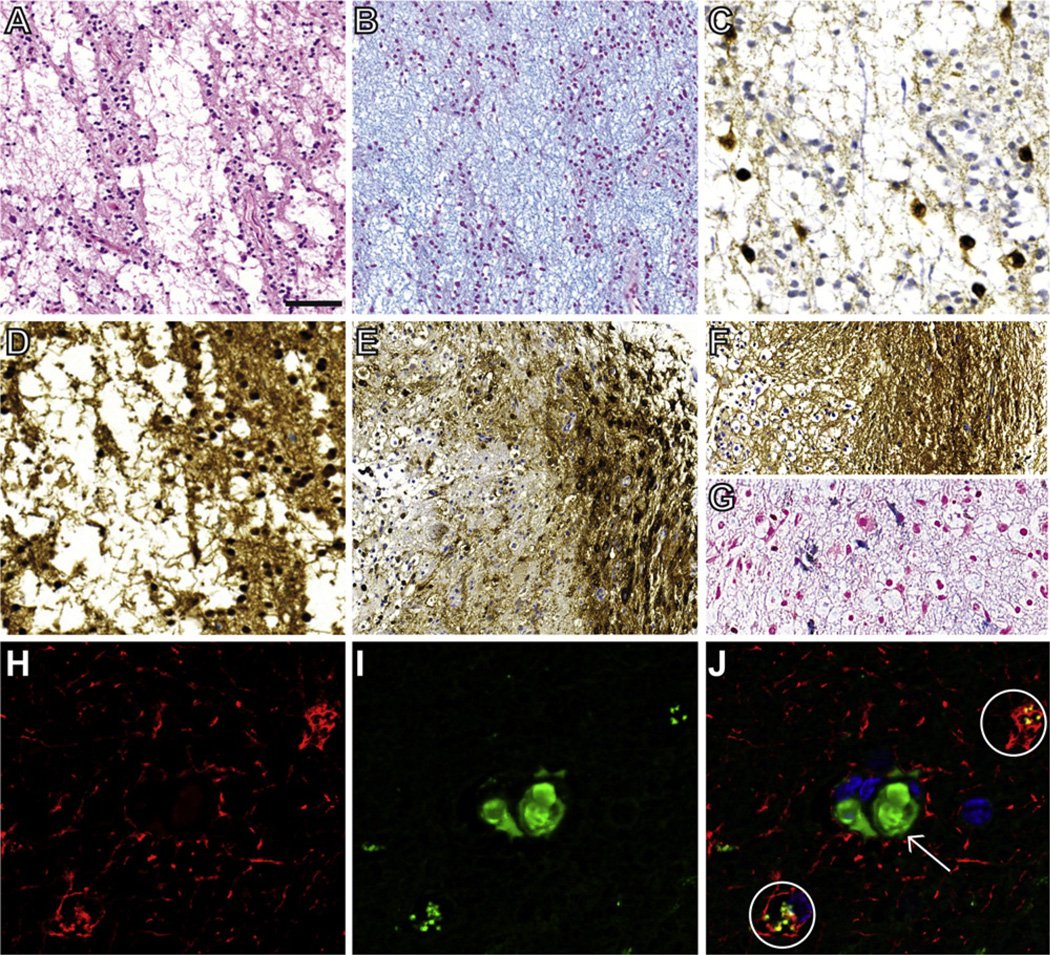
Fig. A3.
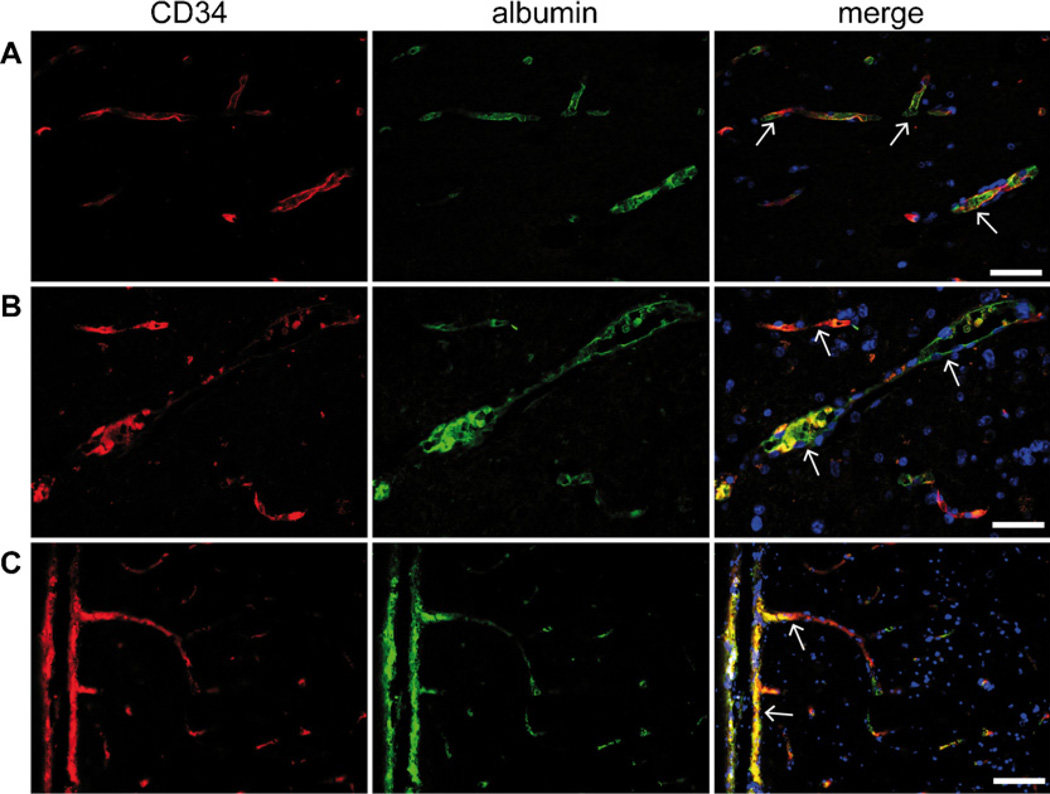
Neuropathological findings and GFAP/albumin double-immunohistochemistry in dysembryoplastic neuroepithelial tumors (DNT). The ‘specific glioneuronal element’, characterized by columnar arranged oligodendroglia-like cells and ‘floating neurons’ in a pale alcianophilic matrix, represents the hallmark of DNT (A, H and E-staining; B, alcianblue-staining). The non-dysplastic neurons are immunoreactive for NeuN (C) and the oligodendroglia-like cells show strong expression of S100-antigen (D). An association of glial nodules with the ‘specific glioneuronal element’ has been named complex DNT variant, in which hemorrhages and contrast enhancement are more frequent. Such glial nodules were present in all analyzed DNT samples. The glial components of the complex form often mimic low grade glioma with MAP2- (E) and GFAP-immunoreactivity (F). Prussian blue staining highlighted hemosiderin deposits in three DNT samples (G). In lesional region of DNT astrocytes (circles in J) show a colocalisation of GFAP (H, GFAP-staining, red) and albumin (I, albumin-staining, green) in double-immunohistochemistry (J, merged image). Intravascular albumin signals (white arrow) can be also detected. Scale bar: A, B, E, F = 50 µm; C, D, G–J = 25 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article).
3. Results
Lesional regions of GG were mostly positive for hemosiderin with a variation from none (n = 2) over few (n = 4) to intermediate (n = 4) deposits. In perilesional regions few (n = 1) to none (n = 9) hemosiderin amount were detected (Tables 1A and 1B). We observed astrocytic deposits of albumin in all GG varying from abundant (n = 1) over intermediate (n = 7) to few (n = 2; Fig. 1A). Linear regression analysis showed no correlation between albumin accumulation and duration of epilepsy (R2 = 0.019; p = 0.7). In perilesional regions we found less (n = 1) to none (n = 9) albumin uptake (Fig. 1B). Glucose transporter-1 (GLUT-1) is highly co-localized with CD-34 in vascular endothelial cells, but lacks differences in lesional versus perilesional tissue of GG or in comparison to other entities (Fig. A4).
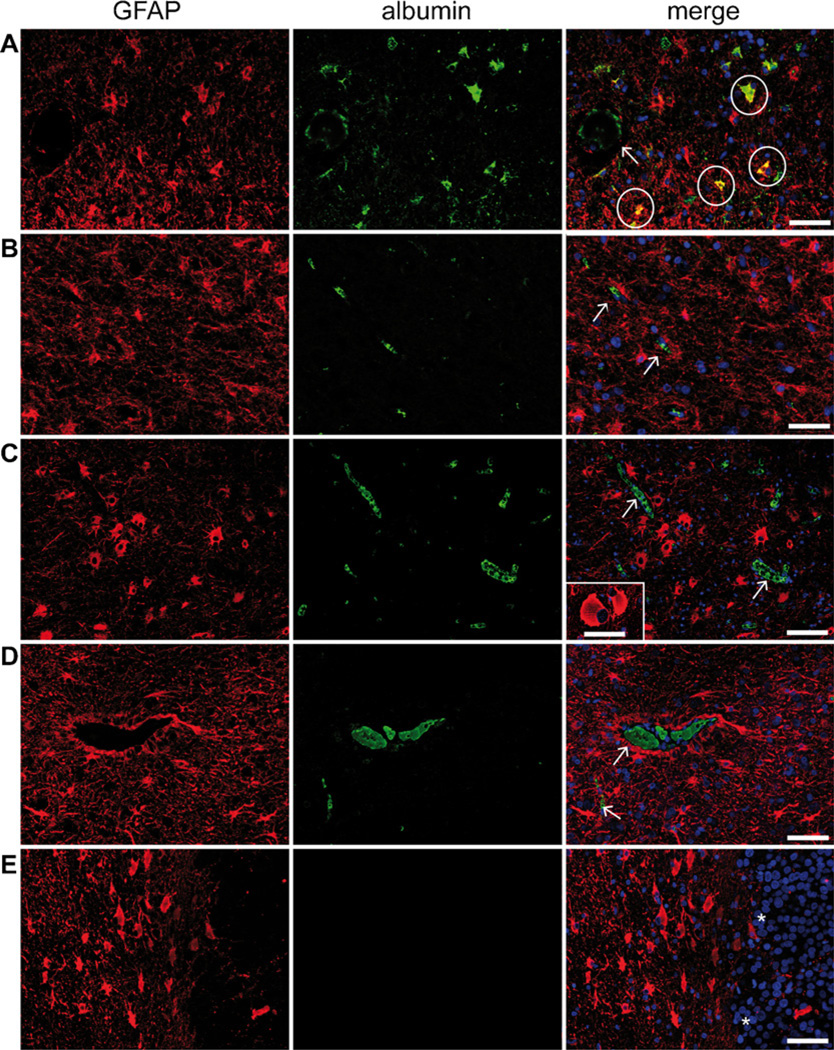
Fig. 1.
Representative GFAP/albumin double-immunohistochemistry in gangliogliomas and specific controls. (A) Neoplastic astrocytes in GG show co-localization of GFAP (red) and albumin (green) as visualized in the merge image (see circles in A). In contrast, other astrocytic differentiated cells, as they are (B) perilesional reactive astrocytes adjacent to GG, (C) reactive astrocytes in FCD IIb, (D) neoplastic astrocytes in DA and (E) reactive astrocytes adjacent to RCCM express GFAP (red) but are generally not immunoreactive with antibodies against albumin (merged images, B–E). However, multifocal intravascular albumin enhancement can be observed in most cases (arrows in A–D). Characteristic balloon cells in FCD IIb show partly GFAP expression, but no albumin uptake is detectable (insert in C). Transition region to the carcinoma metastasis can be clearly identified by high cell density in the metastasis (asterisks in E). Scale bars: A–E = 100 µm; Insert in C = 50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article).
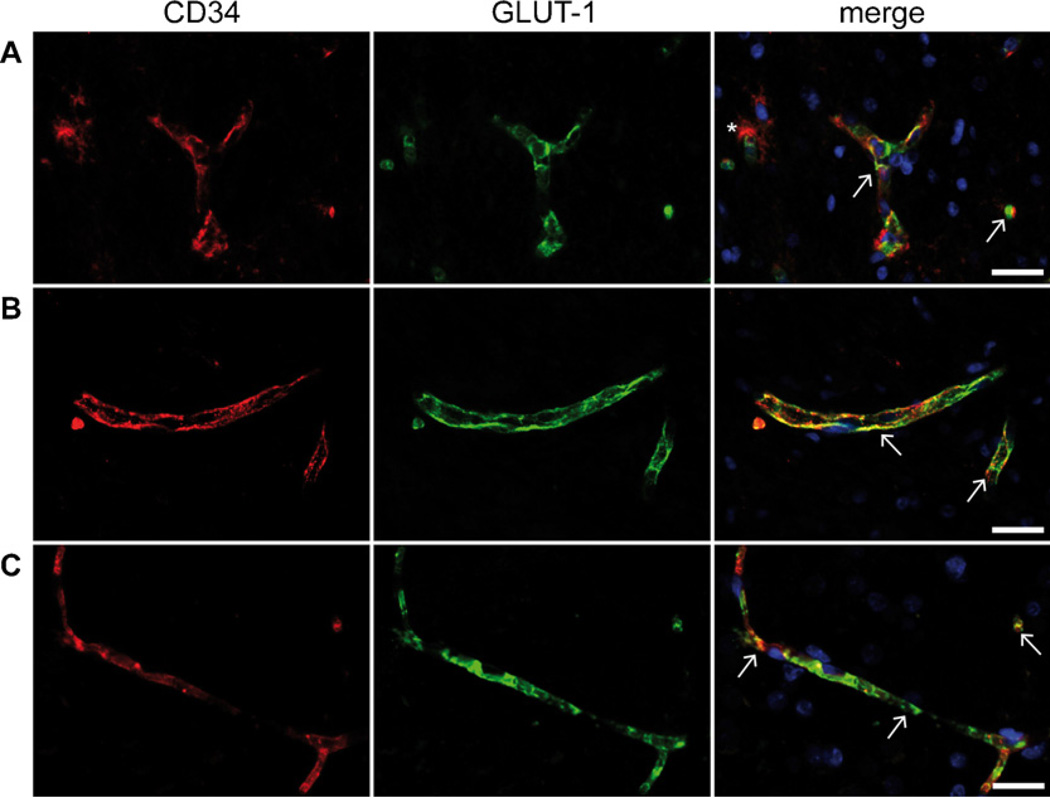
Fig. A4.
Fluorescent double-immunohistochemistry of GLUT-1 and CD34. Combined images reveal a co-localization of GLUT-1 (green) immunoreactivity and CD34 (red) positive vascular endothelial cells (white arrows) in lesional (A) and perilesional (B) region of ganglioglioma WHO grade I and lesional region of diffuse astrocytoma WHO grade II (C). Characteristic satellite cells in the tumor region of gangliogliomas are strongly CD34 positive (asterisk in A). Microscopically no differences of GLUT-1 expression between the analyzed entities were observed. Scale bar = 50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article).
In FCD IIb, hemosiderin deposits were exceptional, both in lesional (n = 1) and perilesional (n = 1) tissue. GFAP/albumin double-immunohistochemistry showed no substantial albumin uptake in the lesional and perilesional areas (n = 7, Fig. 1C). Only in three cases (n = 3), we observed few albumin in astrocytes. CD34/albumin co-stainings confirmed mainly intravascular localization of albumin in FCD IIb (Fig. A5A). In DA, neither hemosiderin nor astrocytic albumin deposits were present in lesional or perilesional regions (n = 5; Figs. 1D and A5B).
Fig. A5.
CD34/albumin double-immunohistochemistry control groups. Vascular endothelial cells show strong expression of CD34 (red). Combined images confirm mainly intravascular localization of albumin signals (green) in FCD IIb (A), DA (B) and RCCM (C) as indicated by white arrows. Scale bars = 50 µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article).
Intralesional hemosiderin deposits varied in RCCM between few (n = 3), intermediate (n = 2) and abundant (n = 1). In perilesional tissue none (n = 3), few (n = 2) or abundant (n = 1) accumulation of hemosiderin was present. Analysis of GFAP/albumin co-staining revealed none (n = 4) or few (n = 2) astrocytic albumin uptake in adjacent CNS (Tables 1A and 1B, Figs. 1E and A5C). Obviously, no relevant number of intratumoural asctrocytes is present in RCCMs.
In three DNT samples intralesional hemosiderin deposits were observed. Relevant albumin accumulation in perivascular glial elements were present in all cases, but mostly low (Fig. A3). Perilesional astrocytes showed no definite albumin uptake. Neither DNT nor GG were associated with perilesional focal cortical dysplasia.
4. Discussion
Although composed of highly similar cellular components, the epileptogenicity of individual low grade brain neoplasms is strikingly different. In contrast to what may be anticipated due to the diffusely infiltrating growth pattern of DAs, these tumors are not highly epileptogenic. In fact, circumscribed primary brain tumors, particularly GG are most frequently associated with chronic epilepsy. This has been primarily claimed to rely on the presence of dysmorphic neurons within the tumor resulting in imbalances of neurotransmitters, receptors and ion channels.3 Although these factors may indeed contribute to epileptogenicity, some aspects argue for additional relevant factors. Firstly, removal of the tumor does not always result in seizure relief. Secondly, also pure vascular lesions such as cavernomas are highly epileptogenic. Only recently BBB-dysfunction and albumin storage by adjacent reactive astroglia have been described in human epileptic tissue and claimed as pathogenetic factors.10
Here, we observed uptake of albumin by intratumoural astroglial cells in GG that from their cellular shape and distribution pattern have to be categorized as neoplastic astroglia. As summarized in Table 1B neither BBB impairment (indicated by hemosiderin deposits and contrast enhancement), nor a glial tumor component nor chronic epilepsy as single parameter were paralleled by astroglial albumin accumulation. However, in GG and DNT, which harbor all three features, glial albumin accumulation was also detectable.
Do our data argue for albumin uptake by astroglial GG components as an epileptogenic mechanism? Our control experiments revealed that low grade astroglial tumors, i.e. DA (WHO grade II), not associated with chronic seizures, do not show albumin uptake by tumor cells. This result may argue for albumin uptake in GG astroglia to represent an epilepsy-associated mechanism. However, the lack of albumin uptake in FCD IIb glial elements suggests that astroglial albumin uptake is not necessarily associated with epileptogenesis. Furthermore, the absence of albumin accumulation in astrocytes within FCD IIb strongly argues against albumin uptake by GG astroglia as an unspecific consequence of epileptic seizures.
In RCCM, as an example of fast growing neoplasms, that frequently demonstrate hemosiderin deposits as neuropathological correlate of BBB dysfunction, we did not observe significant uptake of albumin by adjacent reactive astroglia. However, patients with RCCM included in the present study showed acute sporadic epileptic seizures but did not suffer from chronic epilepsy.
In rats the application of albumin on the surface of the neocortex has been shown to generate an epileptic focus.4,11 Furthermore, artificial opening of BBB by mannitol in chronic epileptic rats results in increased seizure frequency.5 Regarding a potential molecular mechanism of BBB leakage and subsequent epileptogenesis or aggravation of epilepsy, recent studies indicated that albumin underlies the transformation of astrocytes from a “resting” to a “reactive” state through transforming growth factor β (TGF-β) signaling.4,8 Recent data on albumin uptake by reactive astroglia adjacent to malformed blood vessels argues for a phenomenon associated with long term chronic epilepsy.10 In contrast to data from experimental animal models, where albumin leakage is based on a transient functional BBB disruption, in human vascular malformations10 and GG, blood vessels are of aberrant architecture.12 These considerations may argue for a structural BBB dysfunction in such human chronic epilepsy associated lesions.
However, we were not able to resolve the molecular mechanisms that may cause BBB dysfunction in GG. We excluded reduced levels of GLUT-1 in GG endothelia as potential mechanism (Fig. A4). Others, we will address in the future.
Finally, our results have certain clinical aspects. Whereas gross total resection of GG may not always be indicated under neurooncologic perspectives, albumin-containing astroglia as potential epileptogenic cell population of GG may be neurosurgically be minimized based on careful MRI- and EEG based mapping of the seizure generating area prior to epilepsy-surgery. In future attempts, anti-angiogenic treatment may provide epilepsy-therapy perspectives in patients with GG in functional locations.
Supplementary Material
Fig. A2.
Neuropathological findings in diffuse astrocytoma (WHO grade II) and metastasis of clear renal cell carcinoma. H and E staining of a diffuse astrocytoma reveals a mildly cellular tumor with isomorph astrocytic cells (A). Strong expression of mutant IDH1 is present (B). Cytoplasm and processes show GFAP immunoreactivity (C). In contrast, high cellularity is present in RCCM (D, H and E staining). Immunohistochemistry shows strong expression of cytokeratin in tumor cells, but not in pre-existing brain tissue (E). GFAP immunoreactivity is restricted to non-neoplastic astrocytes (F). Scale bar = 50 µm.
Acknowledgements
Our work is supported by the Deutsche Forschungsgemeinschaft (SFB TR3, C6, B8, AJB; KForG “Innate Immunity” TP2, AJB), Bundesministerium für Bildung und Forschung (NGFNplus; AJB), European Union EPICURE (AJB), Euroepinomics Network of the European Science Foundation (AJB), Else-Kröner Fresenius Foundation (AJB), German Israeli Foundation (AJB) and the BONFOR program of the University of Bonn Medical Center (PN, AJB).
Appendix B
Footnotes
Conflict of interest
None of the authors has any conflict of interest to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.seizure.2012.10.014.
References
- 1.Blumcke I, Wiestler OD. Gangliogliomas: an intriguing tumor entity associated with focal epilepsies. Journal of Neuropathology and Experimental Neurology. 2002;61:575–584. doi: 10.1093/jnen/61.7.575. [DOI] [PubMed] [Google Scholar]
- 2.Shamji MF, Fric-Shamji EC, Benoit BG. Brain tumors and epilepsy: pathophysiology of peritumoral changes. Neurosurgical Review. 2009;32:275–285. doi: 10.1007/s10143-009-0191-7. [DOI] [PubMed] [Google Scholar]
- 3.Wolf HK, Birkholz T, Wellmer J, Blumcke I, Pietsch T, Wiestler OD. Neurochemical profile of glioneuronal lesions from patients with pharmacoresistant focal epilepsies. Journal of Neuropathology and Experimental Neurology. 1995;54:689–697. doi: 10.1097/00005072-199509000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 5.van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 6.Friedman A. Blood-brain barrier dysfunction, status epilepticus, seizures, and epilepsy: a puzzle of a chicken and egg? Epilepsia. 2011;52(Suppl. 8):19–20. doi: 10.1111/j.1528-1167.2011.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralay Ranaivo H, Patel F, Wainwright MS. Albumin activates the canonical TGF receptor-smad signaling pathway but this is not required for activation of astrocytes. Experimental Neurology. 2010;226:310–319. doi: 10.1016/j.expneurol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. Journal of Neuroscience. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raabe A, Schmitz AK, Pernhorst K, Grote A, von der Brelie C, Urbach H, et al. Cliniconeuropathologic correlations show astroglial albumin storage as a common factor in epileptogenic vascular lesions. Epilepsia. 2012;53:539–548. doi: 10.1111/j.1528-1167.2012.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, et al. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. Journal of Neuroscience. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodozuka A, Hashizume K, Nakai H, Tanaka T. Vascular abnormalities in surgical specimens obtained from the resected focus of intractable epilepsy. Brain Tumor Pathology. 2000;17:121–131. doi: 10.1007/BF02484283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.