Abstract
Thymus transplantation is a promising investigational therapy for infants born with no thymus. Because of the athymia, these infants lack of T cell development and have a severe primary immunodeficiency. Although thymic hypoplasia or aplasia is characteristic of DiGeorge anomaly, in “complete” DiGeorge anomaly, there is no detectable thymus as determined by the absence of naïve (CD45RA+, CD62L+) T cells. Transplantation of postnatal allogeneic cultured thymus tissue was performed in sixty subjects with complete DiGeorge anomaly who were under the age of 2 years. Recipient survival was over 70%. Naïve T cells developed 3–5 months after transplantation. The graft recipients were able to discontinue antibiotic prophylaxis, and immunoglobulin replacement. Immunosuppression was used in a subset of subjects but was discontinued when naïve T cells developed. The adverse events have been acceptable with thyroid disease being the most common. Research continues on mechanisms underlying immune reconstitution after thymus transplantation.
Keywords: thymus transplantation, DiGeorge, 22q11.2, Athymia
INTRODUCTION
Thymus transplantation is an investigational treatment for pediatric patients with profound primary immune deficiency due to primary athymia and the resulting lack of functional T cells. [1–4] To achieve reconstitution of the T cells, cultured postnatal allogeneic thymus tissue slices are transplanted into the quadriceps muscles of the athymic recipient. [4] Recipient bone marrow stem cells migrate to the allograft where they develop into naïve T cells. Thymopoiesis is observed in biopsies of the transplanted thymus within 2 months of transplantation [5] and naïve T cells are detected in the peripheral blood approximately 3–5 months after transplantation. [6, 7] At the current time in the United States, thymus transplantation is conducted under an Investigational New Drug application with the Food and Drug Administration and all protocols are approved by the Duke Institutional Review Board. The purpose of this review is to provide an updated summary of the subject population that may benefit from thymus transplantation, the methods used, and the clinical and immune outcomes.
Children with congenital athymia are candidates for thymus transplantation. Athymia is a rare condition and occurs in infants with 1) complete DiGeorge anomaly [1, 8–11] and 2) Foxn1 deficiency [12–15]. This review focuses on thymus transplantation in children with complete DiGeorge anomaly.
DiGeorge anomaly is characterized by congenital heart disease, hypoparathyroidism and thymic hypoplasia or athymia. [10, 11, 16] Other findings that have been observed in patients with DiGeorge anomaly include cleft lip and/or palate, club feet, single kidney, esophageal atresia, butterfly vertebra, rib anomalies, and laryngomalacia. [10, 11] Most children with clinical findings of DiGeorge anomaly have a small thymus, low T cell numbers but relatively normal T cell function. [17–20] This condition is termed “partial” DiGeorge anomaly and these children do not require thymus transplantation.
In approximately 1% of children with DiGeorge anomaly, there is an absence of functional thymus. This condition is termed “complete” DiGeorge anomaly and is fatal with almost all children dying by age 2 years due to infections. [1, 8, 20] In children with the clinical findings of DiGeorge anomaly, the diagnosis of athymia is made by examination of the blood to assess the numbers of T cells and their phenotype. Complete DiGeorge anomaly is defined as either having fewer than 50 T cells/mm3 or having fewer than 50 naïve (CD45RA+ CD62L+) T cells/mm3. [1] Because of their profound immunodeficiency, children with complete DiGeorge anomaly are maintained on immunoglobulin replacement and antibiotic prophylaxis for pneumocystis until immunoreconstitution is achieved.
There are two phenotypes of complete DiGeorge anomaly, typical and atypical. [21] Likely, all children with complete DiGeorge anomaly are born with the “typical” phenotype, which is characterized by fewer than 50 T cells/mm3, no rash, and no lymphadenopathy. At some point after birth, children with complete DiGeorge anomaly may switch from the “typical” complete DiGeorge anomaly phenotype to an “atypical” complete DiGeorge anomaly phenotype, which is characterized by fewer than 50/mm3 naïve T cells, a rash associated with T cell infiltration of the skin, lymphadenopathy, and circulating oligoclonal T cells. [21] The atypical phenotype can be considered a subgroup of Omenn syndrome. [22, 23] The findings in typical and atypical complete DiGeorge anomaly are contrasted in Table 1. [21, 24, 25] The peripheral blood T cells found in atypical DiGeorge anomaly are characterized by i) oligoclonality in which up to 75% or higher of the T cells may represent one clone, ii) lack of expression of the naïve T cell marker CD45RA, iii) expression of the αβ T cell receptor (TCR), and iv) lack of maternal T cells. The oligoclonal T cells can be predominantly CD4 single positive, CD8 single positive, or CD4−CD8− double negative cells; the numbers of these cells range from low to high for age. Functionally, these T cells may proliferate in response to mitogens, such as phytohemagglutinin, but they are not protective against opportunistic infections. Although the long term outcomes of thymus transplantation in typical and atypical complete DiGeorge subjects are similar, in this review some thymus transplantation outcomes are illustrated separately for those two groups of subjects because of differences in the use of immunosuppressive drugs during the peritransplantation period.
Table 1.
Characteristics of Typical and Atypical Complete DiGeorge anomaly
| Characteristic | Typical | Atypical | Reference |
|---|---|---|---|
| Number of naive (CD45RA+CD62L+) T cells | < 50/mm3 | < 50/mm3 a | [1] |
| Rash with T cells in skin biopsy | no | 100% | [21, 24 |
| Lymphadenopathy | no | >90% | [21] |
| Hepatosplenomegaly with T cell infiltration on biopsy | no | rare | [25] |
| Circulating oligoclonal T cells | usually none | 100% can be over 20,000/mm3 | [6], [7], [1] [21] |
For subjects with very high T cell numbers, the criterion used is “< 5% of total T cells”
METHODS
Thymus tissue procurement
Thymus tissue is frequently removed by pediatric cardiac surgeons to access the surgical field in infants with congenital heart disease. [1, 4] At Duke, this otherwise discarded tissue is retrieved from the operating room by our laboratory staff and processed promptly. The thymus tissue is used for transplantation only if the donor is under the age of 9 months and the parents of the donor give informed consent. Older infants are not used as donors for two reasons. First, thymuses become more fibrotic with age [26, 27], which makes the tissue more difficult to slice. Second, viral infections, such as cytomegalovirus (CMV), human herpes virus 6 (HHV6), and Epstein Barr virus (EBV), increase in frequency with age and are deleterious for immune deficient thymus recipients. [28–32]
Thymus donor screening
All thymus donors and thymus tissue undergo standard donor infectious disease screening as required by the Food and Drug Administration for cell and tissue based products. [33, 34] Screening includes assays for HIV, hepatitis B and C, Human T-lymphotropic virus, types I and II, and Treponema pallidum. Screening also covers exposure and lifestyle questionnaires and family history. If the donor is less than 1 month of age, screening assays are performed primarily on blood from the biologic mother. If the donor is over 1 month old, screening is performed on both the donor and the donor’s mother. Additional studies include testing the donor for EBV and CMV by PCR and antibody assays and testing the donor’s mother for Epstein Barr virus (EBV) antibodies. The donor tissue is rejected if the donor, donor tissue, or donor’s mother tests positive for any viral or infectious agent. Donor tissue is also rejected if the donor has EBV or CMV antibodies not present in the mother or if the antibody patterns in mother or donor suggest acute infection in either individual.
To confirm normal thymus function in the donor, the donor’s blood is examined by flow cytometry to insure that the donor has greater than 50% naïve (CD45RA+CD62L+) T cells. Other exclusions include 22q11.2 hemizygosity or Down syndrome in the thymus donor and autoimmune disease in primary relatives. HLA and ABO typing are done but matching is not required.
Thymus tissue processing
In the laboratory, thymus tissue is aseptically sliced into pieces approximately 15 by 15 mm and 0.5 mm thick and held in tissue culture (5% CO2, 37°C) floating on nitrocellulose filters which rest on surgical sponges in the presence of nutrient medium in tissue culture dishes. [34, 35] Every day during the 12 to 21 day culture period, the medium is aspirated from each plate and new media is added by dripping onto the tissue slices. The dripping facilitates the removal of donor thymocytes, which could theoretically cause graft versus host disease in the recipient.
The sterility of the thymus cultures is assured by aseptic handling and collection of multiple samples of the culture medium throughout the culture period. [34] These samples are tested for bacteria, mycoplasma and fungus. No more than 24 hours before transplantation, a sample of the culture medium must be shown to be free of endotoxin. A gram stain to test for microbial contamination is done immediately prior to the transplantation surgery.
To confirm the donor thymus tissue is normal in appearance and structure, a sample is collected on the day of harvest and 4 to 9 days before transplantation and evaluated by immunohistochemistry. The tissue must have the characteristics of normal thymus, such as lacy cytokeratin and Hassall bodies. Transplantation into the recipient occurs after 2–3 weeks of culture and after all evaluations are complete.
Thymus transplantation
A pediatric surgeon transplants the donor thymus tissue into the quadriceps muscles of the athymic recipient in the hospital operating room. [4] The surgeon creates individual pockets in the quadriceps muscle for each tissue slice, utilizing both quadriceps muscles. [4]
Use of immunosuppression in thymus allograft recipients
The use of immunosuppression is dictated by the phenotype of the subject. [1] Subjects with typical complete DiGeorge anomaly do not have T cells or T cell function [1, 6], thus they do not have the cells that can effect graft rejection and no immunosuppression is needed. Immunosuppression is used in subjects with atypical complete DiGeorge anomaly. [1] The oligoclonal T cells can destroy the thymus allograft before immune reconstitution occurs. [5]
Immunosuppression for atypical complete DiGeorge anomaly subjects is started when the diagnosis of atypical complete DiGeorge anomaly is first made and typically includes treatment with calcineurin inhibitors, cyclosporine or tacrolimus. On days -5, -4 and -3 prior to thymus transplantation, atypical patients are treated with rabbit anti thymocyte globulin (2 mg/kg/dose for 3 daily doses). [1] Steroids, diphenhydramine, and acetaminophen are also given to decrease the cytokine storm that can be associated with administration of rabbit anti thymocyte globulin. The calcineurin inhibitor is continued after thymus transplantation until the naïve T cells reach over 10% of total T cell numbers. The calcineurin inhibitor is then weaned over the next 8–10 weeks.
RESULTS
SUBJECT POPULATION
Seventy two infants with complete DiGeorge anomaly were consented for thymus transplantation. Each subject met criteria for athymia plus had one of the following: congenital heart disease, hypoparathyroidism, 22q11.2 or CHARGE association. [1] In this subject population, slightly less than half of subjects were hemizygous at 22q11.2. This percentage is consistent with a recent report of 55% 22q11.2 hemizygosity in which DiGeorge anomaly patients were defined based on clinical and immunologic findings. [36]
Twelve of the 72 consented subjects did not undergo transplantation. Typically the decision to not transplant was due to death of the subject or medical conditions that increased the risk of transplantation surgery. [1]
OUTCOMES
Survival
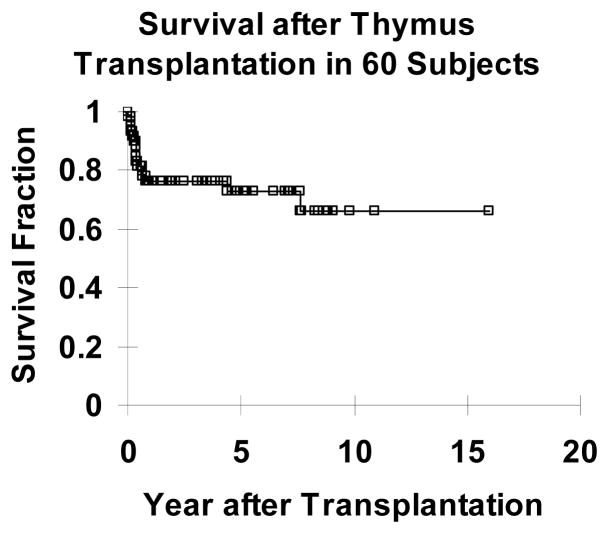
Because the profound immune deficiency of athymia leads to death from infection usually before the age of 2 years, survival was the primary end point of the clinical studies. Of 60 subjects who underwent thymus transplantation, 43 (72%) are alive at this time (Figure 1). The median survival of the 43 subjects alive at this time is 4.7 years. (One of the 43 is within 6 months of transplantation.) Of the 49 subjects who were transplanted over 2 years ago, the two year survival rate was 73%, 36 of 49. (Two subjects subsequently died after the 2 year time point.) The etiologies of early deaths (within one year of transplantation) are detailed in Table 2.
Figure 1.
Kaplan Meier survival curve of subjects with complete DiGeorge anomaly who underwent thymus transplantation. Forty three of sixty transplanted subjects survive.
Table 2.
Cause of death within one year of thymus transplantation.
| Cause of death | Number | |
|---|---|---|
| Infection n=11 | CMV, preexisting in 3, CMV with GVHD1 from unirradiated blood given prior to transfer for thymus transplantation in 1 of the 3 | 4 |
| RSV (preexisting in 1) | 2 | |
| Adenovirus (preexisting) | 1 | |
| Aspergillus pneumonia, related to immunosuppression given for adverse event of enteritis/colitis | 1 | |
| Unspecified infection | 3 | |
| Bleeding problems n=3 | Hemorrhage during unrelated surgery, associated with calcium accretion in vessel associated with previous intravenous calcium therapy | 1 |
| Intracerebral hemorrhage associated with an unspecified infection | 1 | |
| Intracerebral hemorrhage (patient on enoxaparin because of preexisting clotting problem, also had preexisting bleeding problem; patient with palliated congenital heart disease) | 1 | |
| Respiratory failure n=1 | Respiratory failure secondary to chronic ventilation required because of congenital rib anomalies | 1 |
Abbreviations: GVHD, graft versus host disease; RSV, respiratory syncytial virus
Two subjects died more than 1 year after thymus transplantation. One subject had an excellent immune outcome in terms of reconstitution of T cell numbers and function but also had congenital heart disease. The subject died suddenly of a presumed cardiac arrhythmia at 4.4 years after thymus transplantation. The second subject met the criteria for complete DiGeorge anomaly (congenital heart disease with absence of T cells) but also had ectodermal dysplasia with an ill-defined, recurrent coagulopathy. After thymus transplantation, this subject had naïve T cells and a diverse T cell receptor repertoire. The T cells had proliferative responses to mitogens and anti-CD3 but the T cells did not proliferate in response to antigens. The inability of the T cells to respond to antigens likely contributed to the recurrent infections that this child had after thymus transplantation. The subject died of complications from infections and coagulopathy 7.6 years after transplantation.
Immune outcomes
Immune outcomes are assessed after thymus transplantation by examining biopsies of the allografts by immunohistochemistry; monitoring the peripheral blood for naïve T cells and other T cell phenotypic markers; assessing diversity of the T cell receptor beta gene variable (TCRβV) region diversity; assessing T cell proliferative responses and measuring B cell antibody responses to antigens. Summaries of each are provided below.
Allograft biopsies
Biopsies of the allografts are usually performed 2 months after thymus transplantation. Immunohistochemical evaluations of allograft biopsies have been published and confirm thymopoiesis with the presence of cortical CD1a+Ki-67+CD3+ thymocytes associated with thymic epithelium. [2, 5, 25, 37] Some biopsies reveal a distinct thymus medulla with Hassall bodies. [4, 5, 7, 38] Finding thymopoiesis on biopsy supports the conclusion that appearance of circulating T cells is related to thymopoiesis in the graft and is not spontaneous recovery of the native thymus. If the native thymus did recover and produce T cells, those new T cells would quickly reject the donor allograft. Data from the biopsy is the usually the first indication that the transplanted thymus tissue is functioning properly. In subjects with atypical complete DiGeorge anomaly, maternal engraftment, or viral infections such as HHV6 that could affect thymic development, the biopsy information is particularly important for the subject’s family and for making decisions regarding allowing the subject to return to the care of their local doctor.
T cell counts
The numbers of CD3+, CD3+CD4+, and CD3+CD8+ T cells reach a peak between 1 and 2 years after transplantation and then stabilize. [1] The numbers are usually below the 10th percentile for age. [39] These data have been published for typical and atypical complete DiGeorge anomaly subjects and are similar for the two groups. [1] Molecular and/or cytogenetic testing has shown that the T cells that develop after thymus transplantation are genetically recipient in all subjects tested to date (n=31).
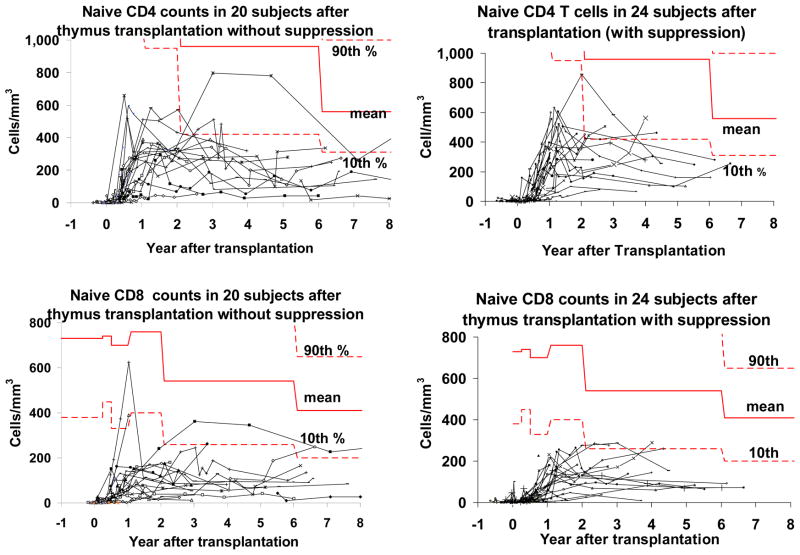
Circulating naïve (CD45RA+CD62L+) T cells are first detected 3–5 months after thymus transplantation (Figure 2). [3] As can be seen in Figure 2, naïve T cell numbers appear to reach their peak by 2 years after transplantation. Using general estimating equations (GEE) analysis, we showed that the naïve CD4+ numbers begin to decrease 2 years after transplantation (p=0.02). The numbers for naïve CD8+ T cells remain stable after year 2.
Figure 2.
Naïve CD4+ and CD8+ T cell counts after thymus transplantation. Each subject’s data are on a single line. The 10th and 90th percentiles for children of this age in years are indicated by the dashed bold lines, the mean is indicated by the solid bold line. [39] All subjects who survive over 1 year after transplantation are included. The left panels include the subjects who did not receive peritransplantation immunosuppression; the right panels include the subjects who did receive peritransplantation immunosuppression.
Although the naïve CD4+ T cell counts remain below the 10th percentile for age, the numbers are only slightly lower than the numbers of naïve CD4+ T cells in children with partial DiGeorge anomaly. [17] Naïve CD8+ T cell counts are well below the 10th percentile for age after thymus transplantation. The transplanted subjects’ naïve CD8 T cell counts are approximately half of the naïve CD8 counts of children with partial DiGeorge anomaly. The low level of the naïve T cells in both the naturally occurring partial DiGeorge anomaly patients and the complete DiGeorge anomaly subjects who undergo transplantation may be the result of low thymus output in both groups, possibly due to small thymus size.
Of note, only one of 43 subjects who survived to one year after transplantation did not develop naïve T cells by 15 months after transplantation. This subject is now lost to follow up. The subject had a cardiac arrest secondary to hypocalcemia 2 months after transplantation. The subject reportedly developed autoimmune hepatitis at 8 months after transplantation with elevated ALT and AST and was treated with azathiaprine and steroids. As of our last contact, the subject was not weaned off these medications.
T cell function
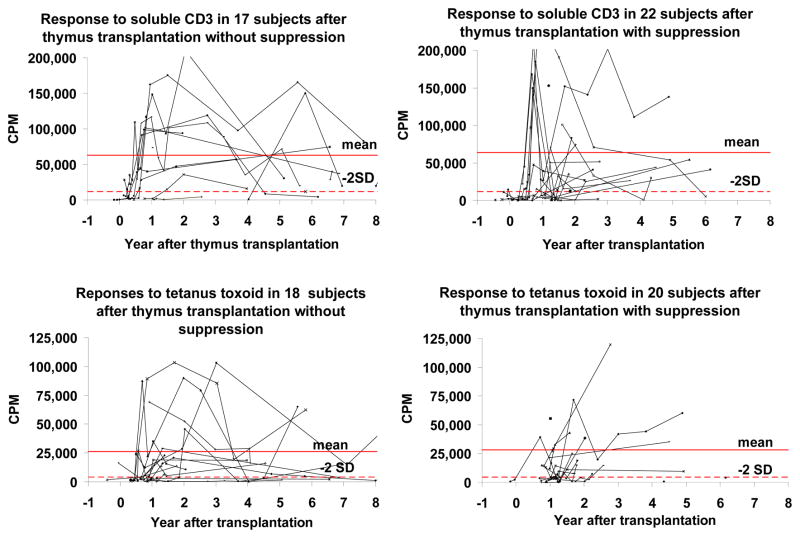
As previously reported, the T cell proliferative responses to the mitogen phytohemagglutinin (PHA) are normal in all thymus transplant recipients who develop naïve T cells. [1] Most subjects have normal responses to soluble CD3. The subgroup of subjects with low proliferative responses to CD3 has no obvious clinical problems (Figure 3). The tetanus toxoid proliferative response increased in all subjects after they were immunized between the 1st and 3rd year after transplantation (Figure 3).
Figure 3.
T cell proliferative responses to soluble CD3 and tetanus toxoid after thymus transplantation. The responses to soluble CD3 are in the upper panels and responses to tetanus toxoid are in the lower panels. The responses to tetanus toxoid include time points before and after immunization with tetanus toxoid. The subjects who received immunosuppression are on the right and those without immunosuppression are on the left. All subjects who have been tested are shown. The mean and 2 standard deviations (2SD) below the mean (calculated with log transformed data) are based on the healthy adult volunteers run in these assays.
T cell diversity
T cell diversity was assessed by spectra typing of the T cell receptor variable beta chain. Both typical and atypical subjects develop diverse repertoires after thymus transplantation [3, 6, 7] and the diverse repertoire persists for many years. [1]
B cell function
Immunoglobulin synthesis and antibody formation develops after thymus transplantation. Table 3 includes a summary of immunoglobulin levels and antibody formation in the 42 subjects who are alive past 6 months plus the 2 subjects who died after 1 year (total of 44).
Table 3.
B cell function
| Serum Immunoglobulin (Ig) levels (number of subjects evaluated)1 | High level | Normal level | Low level | On IVIG (cannot evaluate) |
|---|---|---|---|---|
| IgG (34) | 2 (6%) | 32 (94%) | 0 (0%) | 10 |
| IgA levels (44) | 10 (23%) | 30 (68%) | 4 (9%) | |
| IgM levels (44) | 4 (9%) | 30 (68%) | 10 (23%) | |
| IgE levels (44) | 9 (20%) | 32 (73%) | 3 (7%) | |
| Tetanus titers1 | Normal | Low | Not tested | On IVIG (cannot evaluate) |
| 24 (100%) | 0 (0%) | 10 | 10 | |
| Isohemagglutinins2 (number of subjects evaluated) | Normal | Low | absent | |
| Anti A (18) | 7 (39%) | 10 (56%) | 1 (6%) | |
| Anti B (25) | 13 (52%) | 6 (24%) | 6 (24%) |
Numbers tested are based on the most recent data point for all subjects who are alive past 6 months (n=42) plus the 2 subjects who died after 1 year (total = 44)
Numbers tested are based on all subjects who have data over 2 years after transplantation. Anti-A data are for all subjects who are not blood type A and did not receive an A thymus. Anti-B data are for all subjects who are not blood type B and did not receive a B thymus. A total of 30 subjects are included in these isohemagglutinin studies.
Until 2009, all subjects were maintained on immunoglobulin replacement for 2 years. At that time, if the T cell proliferative response to tetanus toxoid (after immunization) was over 10 fold, the replacement was stopped and antibody responses to tetanus toxoid and unconjugated pneumococcal immunizations were obtained. All subjects tested had a normal antibody response to tetanus and a normal response to one pneumococcal serotype and, thus, were not restarted on replacement. In 2009, the criteria for stopping immunoglobulin replacement were changed to i) greater than 9 months after transplantation, ii) normal trough IgG level for age, proliferative response to phytohemagglutinin of over 100,000 counts per minute, and iii) no ongoing immunosuppression. Two months after stopping the immunoglobulin replacement, an IgG trough has to be normal for age or the immunoglobulin replacement is restarted.
Ten subjects in Table 3 remain on immunoglobulin replacement. Six are within two years of transplantation and have not been weaned off immunosuppression or completed necessary immune function testing required prior to stopping the immunoglobulin replacement. Two are maintained on immunoglobulin replacement by the local physician although they meet criteria to stop replacement. One subject never developed T cell proliferative responses to antigens. That subject had ectodermal dysplasia and died at 7.6 years after transplantation. One subject was lost to follow up (as discussed above).
Although most subjects have come off immunoglobulin replacement and have normal levels of serum immunoglobulins and protective levels of tetanus toxoid antibodies, there appears to be a high percentage of subjects with low serum IgM (Table 3). The IgM isohemagglutinin responses were assessed. Of the 18 subjects tested for anti-A isohemagglutinins, only seven had normal levels of isohemagglutinins (Figure 4). Of the 25 subjects tested for anti-B isohemagglutinins, only 13 had normal levels of anti-B (Figure 4). We assessed whether there was a correlation of low isohemagglutinins with low serum IgM but the relationship was not significant (p=0.2 for both anti-A and anti-B, one tailed Fisher exact test).
Figure 4.
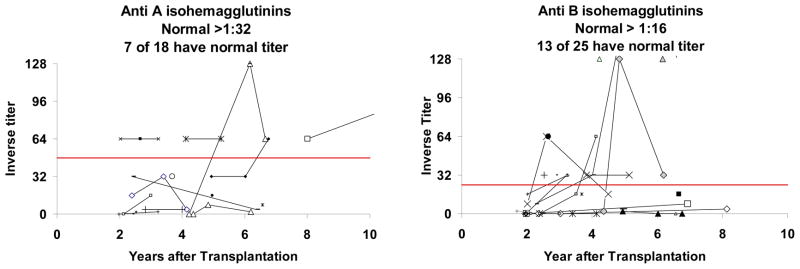
Production of anti-A and anti-B isohemagglutinins. The left panel includes subjects who are not blood group A and did not receive a blood group A thymus. The right panel includes subjects who are not blood group B and did not receive a blood group B thymus. For inclusion in this figure, subjects had to be tested past 2 years after transplantation. A total of 30 subjects were tested past 2 years after transplantation. Each subject is represented by a separate line or marker. Normal values are those above the solid bold line in each graph.
Although not included in Table 3, of the 20 subjects tested with an unconjugated pneumococcal vaccine (prior to conjugated pneumococcal vaccination) all made positive responses to some serotypes. [1] However, the post thymus transplantation subjects did not make antibodies to as many serotypes as normal children do. [40]
ABO compatibility
Thymus tissue was transplanted into recipients without regard to ABO compatibility. Retrospectively, we tested the impact on T cell counts of ABO incompatibility between the thymus donor and recipient. We previously reported that ABO incompatibility (e.g., an A thymus transplanted into an O recipient) results in lower CD4+ T cell counts at 1 and 2 years after thymus transplantation. [41] When these correlations were extended to 3 years after transplantation, we did not find an adverse effect of ABO incompatibility on total CD3, CD4, or CD8 counts (p=0.5, 0.17, and 0.44, respectively). Similarly, there was no effect of ABO incompatibility on naïve CD4 or naïve CD8 counts (p=0.48 and 0.14, respectively). These data are consistent with the ability to transplant hearts across an ABO barrier in infants less than 2 years of age. [42] We have previously published the lack of effect of HLA matching. [41]
Immune outcomes in special circumstances
Maternal engraftment
We have detected engraftment of maternal T cells in 4 subjects in the peritransplant period. In the first subject, the maternal T cells (3% of total T cells) were associated with a rash that developed at 6–9 days after transplantation. No thymus donor T cells were detected out of a total T cell count of approximately 60/mm3. The rash resolved with topical steroid therapy. For the second and third subjects, both of whom had atypical complete DiGeorge anomaly associated with rash and lymphadenopathy, less than 10% of total T cells were found to be maternal prior to transplantation. Per our standard protocol for atypical complete DiGeorge subjects, the subjects were treated with cyclosporine prior to transplantation and given 3 doses of rabbit anti thymocyte globulin immediately prior to transplantation. Cyclosporine therapy was continued after transplantation until naïve T cells increased to over 10% of the total T cells. These three subjects have excellent immune outcomes. When tested between 5 months and 3.2 years after transplantation, all T cells in these subjects were shown to be genetically recipient with no maternal cells detected. Based on the data on the first three subjects, who have developed 474–948 naïve T cells/mm3 by 1.9–2.5 years after transplantation, naïve T cells can develop in subjects with maternal engraftment when the standard immunosuppression regimen for atypical complete DiGeorge anomaly is used. The fourth subject had a significantly higher percentage of maternal T cells, 96% (of 110 T cells/mm3), but no rash at presentation. This subject was recently transplanted and remains on calcineurin inhibitor therapy while the immune parameters are monitored.
Human herpes virus 6 (HHV6) infection
Because HHV6 suppresses hematopoiesis, [43] we were concerned that HHV6 would adversely affect thymopoiesis during the reconstitution of the transplanted thymus tissue. Two subjects had HHV6 infections at the time of thymus transplantation. The first subject’s allograft biopsy, obtained 5 months after transplantation, showed thymopoiesis but this subject later died from adenoviral pneumonia. The second subject developed naïve T cells but has low total T cell numbers (336/mm3) at 2.6 years after transplantation. (No graft tissue was found on biopsy in this subject.) Thus, it appears that thymopoiesis can occur in the presence of HHV6 infection.
Adverse events
The adverse events seen most frequently after thymus transplantation are infections and autoimmune disease. Infections typically occur prior to the development of naïve T cells or are associated with central venous catheters as previously reported. [1]
All autoimmune diseases post thymus transplantation are considered to be adverse events related to transplantation. Thyroid disease is the most common autoimmune disease after thymus transplantation. It has occurred in 13 subjects from 0.5 to 14.4 years after transplantation (median time 1.8 years after transplantation). [1] Two subjects presented with elevated free thyroxine (T4) and low thyroid stimulating hormone (TSH); the remaining presented with high TSH and low T4. Eleven subjects are being treated with levothyroxine; one subject is being treated with methimazole. One subject was treated with levothyroxine but at autopsy had normal thyroid histology putting the original diagnosis in doubt.
Thyroid disease has been seen in nine complete DiGeorge anomaly subjects prior to thymus transplantation. [1] Because thyroid disease has been reported to occur in 20% of adults with partial DiGeorge anomaly, [44] it is possible that thyroid disease is not an adverse event caused by thymus transplantation but is component of DiGeorge anomaly. Irrespective of the etiology, we recommend thyroid function studies every 6 months after thymus transplantation.
Cytopenias occurred in 9 subjects, all within the first two years of thymus transplantation. [1] Four subjects had autoimmune thrombocytopenia, one of whom presented with autoimmune thrombocytopenia before thymus transplantation and had another episode after transplantation; 2 subjects had autoimmune hemolytic anemia; 2 subjects had autoimmune neutropenia; and one subject had all three cytopenias sequentially over a 2 month period. Some episodes were associated with HHV6 infection, adenovirus infection, or upper respiratory tract infections. Treatments included red cell transfusions, intravenous immunoglobulin, rituximab, and steroids. To date, cytopenias have not been observed beyond two years after thymus transplantation, which suggests that the cytopenias are related to low T cell numbers or limited T cell function early after transplantation. It is likely that cytopenias will occur more than 2 years after transplantation since autoimmune cytopenias occur in approximately 5% of children with partial DiGeorge anomaly. [45–48]
The most serious adverse event was enteritis/colitis that occurred 5 months after transplantation in a subject with atypical complete DiGeorge anomaly. [1] The resulting diarrhea led to inability to maintain cyclosporine levels. When the subject returned to the transplant center for evaluation, there was denudation of the entire intestinal tract associated with T cell and neutrophil infiltration of the intestine. Large doses of immunosuppression (tacrolimus plus 15 mg/kg methylprednisolone every 12 hours for 4 doses) reversed the diarrhea but the subject then died from fungal pneumonia. It is likely that this serious adverse event was related to thymus transplantation. Graft versus host disease from the donor thymus was ruled out because the T cells in the peripheral blood were recipient in origin.
Other non-life threatening autoimmune adverse events have occurred in one subject each and have been reported previously. [1]
Immunologic mechanisms underlying allogeneic thymus transplantation
Thymus transplantation is performed without matching the major histocompatibility complex (MHC) antigens of the thymus donor to the recipient. Allogeneic thymus transplantation results in the maturation of recipient T cells that protect the recipient from infection, yet the mismatched allogeneic thymus is the site of both negative and positive selection. Understanding the biology of this process is challenging and intriguing.
Negative selection is the process in which self reactive T cells are deleted to prevent development of autoimmunity. Normally, negative selection is effected by thymic dendritic cells. [49–51] CD83+ dendritic cells are located throughout the medulla and at the corticomedullary junction. These hematopoietic cells migrate to the thymus from the bone marrow. In the thymus transplant model, recipient dendritic cells likely migrate to the thymus. There, they cause clonal deletion of thymocytes with high affinity to recipient MHC and thus prevent a graft versus host like disease. The recipient dendritic cells also present tissue restricted antigens, such as those regulated by the gene autoimmune regulator (AIRE). [49, 52, 53] Clonal deletion of thymocytes reacting with tissue restricted antigens prevents multiple autoimmune diseases.
Positive selection is the process by which the thymus, in particular the cortical thymic epithelium, teaches the developing thymocytes what is “self” with respect to MHC molecules. [54–56] Developing thymocytes undergo apoptosis if they have low or no affinity to self MHC as presented on the cortical thymocytes. Thymocytes that have insufficient affinity to self MHC undergo apoptosis, which prevents the production of T cells that lack affinity for self MHC and, therefore, would not be able to respond to foreign pathogenic peptides in the context of self MHC. Thymocytes that have the appropriate affinity for self peptide:self MHC are signaled by the thymus to continue to proliferate and are thus, positively selected. When the donor thymus is mismatched to the MHC of the recipient, it is reasonable to predict that the positively selected thymocytes will be restricted to thymus-donor MHC. In that case, the T cells emigrating from the thymus will not be able to interact with antigen presenting cells carrying recipient MHC in the periphery and these T cells, because of MHC mismatching, will not be able to protect the recipient from infection. The successful reconstitution of recipient T cell function in the thymus recipients forces us to consider alternative sources of cells that provide positive selection in the allogeneic donor thymus.
In the thymus transplant model, recipient cells may contribute to positive selection in the transplanted thymus. A precedent for this can be found in murine studies in which thymocytes present self MHC to each other. [54, 57–59] This allows for positive selection to recipient MHC and, thus, enables new T cells to fight infection in the recipient. Another mechanism contributing to positive selection may involve recruitment of recipient epithelial cells to the allograft. In humans, recipient epithelial cells have been found in donor lungs after lung transplantation. [60, 61] Evidence for recipient epithelium in the thymus after thymus transplantation has not been published for animal models and has not been evaluated in humans. Another possibility is that cortical immature dendritic cells (CD209+) from the recipient may effect positive selection after unmatched thymus transplantation and result in recipient MHC restriction. [62] Immature dendritic cells typically do not express high levels of MHC molecules, which would make these cells unlikely candidates to effect positive selection. At the present, the mechanisms of positive selection in allogeneic thymus transplantation are not defined.
Summary
Thymus transplantation is currently performed in subjects with complete DiGeorge anomaly resulting in the survival of over 70% of treated subjects. This investigational therapy has reconstituted recipient T cells and T cell function. The adverse event profile is acceptable. These subjects provide opportunities to study mechanisms underlying the development of functional T cells in an allogeneic thymus.
Acknowledgments
We acknowledge the research nursing and technical contributions of Stephanie Gupton, Alice Jackson, Marilyn Alexieff, Jie Li, Chia-San Hsieh, Jennifer Lonon, Julie Smith, and Anita Croasmun and the statistician assistance of Yi-Ju Li, PhD. The expert assistant of the Duke Comprehensive Cancer Center flow cytometry resource under Dr. J. Michael Cook is appreciated. We also acknowledge the support of NIH grants R01 AI 47040, R01 AI 54843, R21 AI 60967 and the FDA Office of Orphan Product Development Grant FD-R-002606. The support of the staff of the NCRR GCRC M03 RR30 is appreciated. M.L.M. is a member of the Duke Comprehensive Cancer Center. Lastly we acknowledge the excellent clinical care of the DiGeorge anomaly subjects by the faculty and fellows of the Division of Pediatric Allergy and Immunology.
References
- 1.Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: outcome of 44 consecutive transplants. Blood. 2007 May 15;109(10):4539–47. doi: 10.1182/blood-2006-10-048652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markert ML, Devlin BD. Thymic reconstitution. In: Rich RR, Fleischer SW, Schroeder T, Weyand HW, Frew CM, editors. A Clinical Immunology. 3. Edinburgh: Elsevier; 2008. pp. 1253–62. [Google Scholar]
- 3.Markert MLDB, McCarthy EA, Chinn IK, Hale LP. Thymus Transplantation. In: Lavinin CMC, Morandi U, Schoenhuber R, editors. Thymus Gland Pathology: Clinical, Diagnostic, and Therapeutic Features. Milan, Springer-Verlag; Italia: 2008. pp. 255–67. [Google Scholar]
- 4.Rice HE, Skinner MA, Mahaffey SM, Oldham KT, Ing RJ, Hale LP, et al. Thymic transplantation for complete DiGeorge syndrome: medical and surgical considerations. J Pediatr Surg. 2004 Nov;39(11):1607–15. doi: 10.1016/j.jpedsurg.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Markert ML, Li J, Devlin BH, Hoehner JC, Rice HE, Skinner MA, et al. Use of allograft biopsies to assess thymopoiesis after thymus transplantation. J Immunol. 2008 May 1;180(9):6354–64. doi: 10.4049/jimmunol.180.9.6354. [DOI] [PubMed] [Google Scholar]
- 6.Markert ML, Sarzotti M, Ozaki DA, Sempowski GD, Rhein ME, Hale LP, et al. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. Blood. 2003 Aug 1;102(3):1121–30. doi: 10.1182/blood-2002-08-2545. [DOI] [PubMed] [Google Scholar]
- 7.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, et al. Postnatal thymus transplantation with immunosuppression as treatment for DiGeorge syndrome. Blood. 2004 Oct 15;104(8):2574–81. doi: 10.1182/blood-2003-08-2984. [DOI] [PubMed] [Google Scholar]
- 8.Markert ML, Hummell DS, Rosenblatt HM, Schiff SE, Harville TO, Williams LW, et al. Complete DiGeorge syndrome: persistence of profound immunodeficiency. J Pediatr. 1998 Jan;132(1):15–21. doi: 10.1016/s0022-3476(98)70478-0. [DOI] [PubMed] [Google Scholar]
- 9.Hong R. The DiGeorge anomaly. Clin Rev Allergy Immunol. 2001 Feb;20(1):43–60. doi: 10.1385/CRIAI:20:1:43. [DOI] [PubMed] [Google Scholar]
- 10.Conley ME, Beckwith JB, Mancer JF, Tenckhoff L. The spectrum of the DiGeorge syndrome. J Pediatr. 1979 Jun;94(6):883–90. doi: 10.1016/s0022-3476(79)80207-3. [DOI] [PubMed] [Google Scholar]
- 11.Barrett DJ, Ammann AJ, Wara DW, Cowan MJ, Fisher TJ, Stiehm ER. Clinical and immunologic spectrum of the DiGeorge syndrome. Journal of clinical & laboratory immunology. 1981 Jul;6(1):1–6. [PubMed] [Google Scholar]
- 12.Flanagan SP. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genetical research. 1966 Dec;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 13.Pignata C, Gaetaniello L, Masci AM, Frank J, Christiano A, Matrecano E, et al. Human equivalent of the mouse Nude/SCID phenotype: long-term evaluation of immunologic reconstitution after bone marrow transplantation. Blood. 2001 Feb 15;97(4):880–5. doi: 10.1182/blood.v97.4.880. [DOI] [PubMed] [Google Scholar]
- 14.Adriani M, Martinez-Mir A, Fusco F, Busiello R, Frank J, Telese S, et al. Ancestral founder mutation of the nude (FOXN1) gene in congenital severe combined immunodeficiency associated with alopecia in southern Italy population. Annals of human genetics. 2004 May;68(Pt 3):265–8. doi: 10.1046/j.1529-8817.2004.00091.x. [DOI] [PubMed] [Google Scholar]
- 15.Frank J, Pignata C, Panteleyev AA, Prowse DM, Baden H, Weiner L, et al. Exposing the human nude phenotype. Nature. 1999 Apr 8;398(6727):473–4. doi: 10.1038/18997. [DOI] [PubMed] [Google Scholar]
- 16.DiGeorge AM. Maldescent of the thymus. Pediatr Pathol. 1994 Jan-Feb;14(1):178. author reply 9–80. [PubMed] [Google Scholar]
- 17.Chinen J, Rosenblatt HM, Smith EO, Shearer WT, Noroski LM. Long-term assessment of T-cell populations in DiGeorge syndrome. The Journal of allergy and clinical immunology. 2003 Mar;111(3):573–9. doi: 10.1067/mai.2003.165. [DOI] [PubMed] [Google Scholar]
- 18.Piliero LM, Sanford AN, McDonald-McGinn DM, Zackai EH, Sullivan KE. T-cell homeostasis in humans with thymic hypoplasia due to chromosome 22q11.2 deletion syndrome. Blood. 2004 Feb 1;103(3):1020–5. doi: 10.1182/blood-2003-08-2824. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan KE, Jawad AF, Randall P, Driscoll DA, Emanuel BS, McDonald-McGinn DM, et al. Lack of correlation between impaired T cell production, immunodeficiency, and other phenotypic features in chromosome 22q11.2 deletion syndromes. Clin Immunol Immunopathol. 1998 Feb;86(2):141–6. doi: 10.1006/clin.1997.4463. [DOI] [PubMed] [Google Scholar]
- 20.Bastian J, Law S, Vogler L, Lawton A, Herrod H, Anderson S, et al. Prediction of persistent immunodeficiency in the DiGeorge anomaly. J Pediatr. 1989 Sep;115(3):391–6. doi: 10.1016/s0022-3476(89)80837-6. [DOI] [PubMed] [Google Scholar]
- 21.Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, et al. Complete DiGeorge syndrome: development of rash, lymphadenopathy, and oligoclonal T cells in 5 cases. The Journal of allergy and clinical immunology. 2004 Apr;113(4):734–41. doi: 10.1016/j.jaci.2004.01.766. [DOI] [PubMed] [Google Scholar]
- 22.Junker AK, Chan KW, Massing BG. Clinical and immune recovery from Omenn syndrome after bone marrow transplantation. J Pediatr. 1989 Apr;114(4 Pt 1):596–600. doi: 10.1016/s0022-3476(89)80702-4. [DOI] [PubMed] [Google Scholar]
- 23.Omenn GS. Familial Reticuloendotheliosis with Eosinophilia. The New England journal of medicine. 1965 Aug 19;273:427–32. doi: 10.1056/NEJM196508192730806. [DOI] [PubMed] [Google Scholar]
- 24.Selim MA, Markert ML, Burchette JL, Herman CM, Turner JW. The cutaneous manifestations of atypical complete DiGeorge syndrome: a histopathologic and immunohistochemical study. J Cutan Pathol. 2008 Apr;35(4):380–5. doi: 10.1111/j.1600-0560.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 25.Markert ML, Devlin BH, Chinn IK, McCarthy EA. Thymus transplantation in complete DiGeorge anomaly. Immunologic research. 2009;44(1–3):61–70. doi: 10.1007/s12026-008-8082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 27.Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP. Analysis of the human thymic perivascular space during aging. The Journal of clinical investigation. 1999 Oct;104(8):1031–9. doi: 10.1172/JCI7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yow MD, White NH, Taber LH, Frank AL, Gruber WC, May RA, et al. Acquisition of cytomegalovirus infection from birth to 10 years: a longitudinal serologic study. J Pediatr. 1987 Jan;110(1):37–42. doi: 10.1016/s0022-3476(87)80284-6. [DOI] [PubMed] [Google Scholar]
- 29.Chan KH, Tam JS, Peiris JS, Seto WH, Ng MH. Epstein-Barr virus (EBV) infection in infancy. J Clin Virol. 2001 Apr;21(1):57–62. doi: 10.1016/s1386-6532(01)00149-4. [DOI] [PubMed] [Google Scholar]
- 30.Savolainen H, Lautenschlager I, Piiparinen H, Saarinen-Pihkala U, Hovi L, Vettenranta K. Human herpesvirus-6 and -7 in pediatric stem cell transplantation. Pediatric blood & cancer. 2005 Nov;45(6):820–5. doi: 10.1002/pbc.20337. [DOI] [PubMed] [Google Scholar]
- 31.Reece ER, Gartner JG, Seemayer TA, Joncas JH, Pagano JS. Epstein-Barr virus in a malignant lymphoproliferative disorder of B-cells occurring after thymic epithelial transplantation for combined immunodeficiency. Cancer research. 1981 Nov;41(11 Pt 1):4243–7. [PubMed] [Google Scholar]
- 32.Dictor M, Fasth A, Olling S. Abnormal B-cell proliferation associated with combined immunodeficiency, cytomegalovirus, and cultured thymus grafts. American journal of clinical pathology. 1984 Oct;82(4):487–90. doi: 10.1093/ajcp/82.4.487. [DOI] [PubMed] [Google Scholar]
- 33.21CFR1271 F. Guidance for Industry. Eligibility etermination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) Aug, 2007. [Google Scholar]
- 34.Markert ML, Devlin BH, McCarthy EA, Chinn IK, Hale LP. Thymus Transplantation. In: Lavinin CMC, Morandi U, Schoenhuber R, editors. Thymus Gland Pathology: Clinical, Diagnostic, and Therapeutic Features. Milan: Springer-Verlag Italia; 2008. pp. 255–67. [Google Scholar]
- 35.Hong R, Moore AL. Organ culture for thymus transplantation. Transplantation. 1996 Feb 15;61(3):444–8. doi: 10.1097/00007890-199602150-00023. [DOI] [PubMed] [Google Scholar]
- 36.Rope AF, Cragun DL, Saal HM, Hopkin RJ. DiGeorge anomaly in the absence of chromosome 22q11.2 deletion. J Pediatr. 2009 Oct;155(4):560–5. doi: 10.1016/j.jpeds.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 37.Markert ML, Kostyu DD, Ward FE, McLaughlin TM, Watson TJ, Buckley RH, et al. Successful formation of a chimeric human thymus allograft following transplantation of cultured postnatal human thymus. J Immunol. 1997 Jan 15;158(2):998–1005. [PubMed] [Google Scholar]
- 38.Markert ML, Boeck A, Hale LP, Kloster AL, McLaughlin TM, Batchvarova MN, et al. Transplantation of thymus tissue in complete DiGeorge syndrome. The New England journal of medicine. 1999 Oct 14;341(16):1180–9. doi: 10.1056/NEJM199910143411603. [DOI] [PubMed] [Google Scholar]
- 39.Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. The Journal of allergy and clinical immunology. 2003 Nov;112(5):973–80. doi: 10.1016/j.jaci.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 40.Kamchaisatian W, Wanwatsuntikul W, Sleasman JW, Tangsinmankong N. Validation of current joint American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology guidelines for antibody response to the 23-valent pneumococcal vaccine using a population of HIV-infected children. The Journal of allergy and clinical immunology. 2006 Dec;118(6):1336–41. doi: 10.1016/j.jaci.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 41.Markert ML, Devlin BH, Chinn IK, McCarthy EA, Li YJ. Factors affecting success of thymus transplantation for complete DiGeorge anomaly. Am J Transplant. 2008 Aug;8(8):1729–36. doi: 10.1111/j.1600-6143.2008.02301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan X, Ang A, Pollock-Barziv SM, Dipchand AI, Ruiz P, Wilson G, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med. 2004 Nov;10(11):1227–33. doi: 10.1038/nm1126. [DOI] [PubMed] [Google Scholar]
- 43.Isomura H, Yamada M, Yoshida M, Tanaka H, Kitamura T, Oda M, et al. Suppressive effects of human herpesvirus 6 on in vitro colony formation of hematopoietic progenitor cells. Journal of medical virology. 1997 Aug;52(4):406–12. doi: 10.1002/(sici)1096-9071(199708)52:4<406::aid-jmv11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 44.Bassett AS, Chow EW, Husted J, Weksberg R, Caluseriu O, Webb GD, et al. Clinical features of 78 adults with 22q11 Deletion Syndrome. American journal of medical genetics. 2005 Nov 1;138(4):307–13. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jawad AF, McDonald-Mcginn DM, Zackai E, Sullivan KE. Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) J Pediatr. 2001 Nov;139(5):715–23. doi: 10.1067/mpd.2001.118534. [DOI] [PubMed] [Google Scholar]
- 46.DePiero AD, Lourie EM, Berman BW, Robin NH, Zinn AB, Hostoffer RW. Recurrent immune cytopenias in two patients with DiGeorge/velocardiofacial syndrome. J Pediatr. 1997 Sep;131(3):484–6. doi: 10.1016/s0022-3476(97)80085-6. [DOI] [PubMed] [Google Scholar]
- 47.Duke SG, McGuirt WF, Jr, Jewett T, Fasano MB. Velocardiofacial syndrome: incidence of immune cytopenias. Arch Otolaryngol Head Neck Surg. 2000 Sep;126(9):1141–5. doi: 10.1001/archotol.126.9.1141. [DOI] [PubMed] [Google Scholar]
- 48.Davies JK, Telfer P, Cavenagh JD, Foot N, Neat M. Autoimmune cytopenias in the 22q11.2 deletion syndrome. Clinical and laboratory haematology. 2003 Jun;25(3):195–7. doi: 10.1046/j.1365-2257.2003.00508.x. [DOI] [PubMed] [Google Scholar]
- 49.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003 Apr;4(4):350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 50.Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nature reviews. 2003 May;3(5):383–91. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 51.Siggs OM, Makaroff LE, Liston A. The why and how of thymocyte negative selection. Curr Opin Immunol. 2006 Apr;18(2):175–83. doi: 10.1016/j.coi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The Cellular Mechanism of Aire Control of T Cell Tolerance. Immunity. 2005 Aug;23(2):227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002 Nov 15;298(5597):1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 54.Chidgey AP, Boyd RL. Thymic stromal cells and positive selection. Apmis. 2001 Jul-Aug;109(7–8):481–92. doi: 10.1111/j.1600-0463.2001.apm090701.x. [DOI] [PubMed] [Google Scholar]
- 55.von Boehmer H. Positive selection of lymphocytes. Cell. 1994 Jan 28;76(2):219–28. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 56.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 57.Choi EY, Jung KC, Park HJ, Chung DH, Song JS, Yang SD, et al. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005 Oct;23(4):387–96. doi: 10.1016/j.immuni.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Li W, Kim MG, Gourley TS, McCarthy BP, Sant’Angelo DB, Chang CH. An alternate pathway for CD4 T cell development: thymocyte-expressed MHC class II selects a distinct T cell population. Immunity. 2005 Oct;23(4):375–86. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Bix M, Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992 Sep 24;359(6393):330–3. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 60.Spencer H, Rampling D, Aurora P, Bonnet D, Hart SL, Jaffe A. Transbronchial biopsies provide longitudinal evidence for epithelial chimerism in children following sex mismatched lung transplantation. Thorax. 2005 Jan;60(1):60–2. doi: 10.1136/thx.2004.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, et al. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. The American journal of pathology. 2003 May;162(5):1487–94. doi: 10.1016/S0002-9440(10)64281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paessens LC, Fluitsma DM, van Kooyk Y. Haematopoietic antigen-presenting cells in the human thymic cortex: evidence for a role in selection and removal of apoptotic thymocytes. The Journal of pathology. 2008 Jan;214(1):96–103. doi: 10.1002/path.2260. [DOI] [PubMed] [Google Scholar]