Abstract
Sarcopenia, or senile muscle atrophy, is the slow and progressive loss of muscle mass with advancing age that constitutes the most prevalent form of muscle atrophy. The effects of ageing on skeletal muscle have been extensively studied in humans and laboratory animals (mice), while the few reports on wild animals are based on short-lived mammals. The present study describes the age-related changes in cetacean muscles regarding the three factors that determine muscle mass: fibre size, fibre number, and fibre type. We show that the skeletal muscle fibres in cetaceans change with advancing age, evolving towards a slower muscle phenotype. We suggest that this physiological evolution constitutes an adaptation that allows these marine mammals to perform prolonged, deep dives.
Over the past few decades, the elderly human population has dramatically increased in the developed countries and is expected to reach 2 billion by 20501. Elderly humans undergo many physiological changes (i.e., immune senescence and the decrease of bone density and muscle mass), which can be subclinical or can manifest as muscle weakness, vulnerability to infections and fatigue and have severe implications for several conditions, including type 2 diabetes and heart disease2. Among the age-associated conditions, the decrease of muscle mass is one of the most serious consequences of ageing3.
Sarcopenia, which is also called senile muscle atrophy, is the slow, inevitable, progressive loss of muscle mass with advancing age that constitutes the most prevalent form of muscle atrophy. Sarcopenia contributes to the disability of older people by increasing their risk of falls and their vulnerability to injury, consequently leading to their functional dependence and incapacity2. Previous studies have shown that the pattern of muscle atrophy with ageing is non-linear, beginning in the fourth and fifth decades of life and markedly accelerating at the age beyond the 80% survival rate in both humans and the rodent models of ageing4,5,6.
A pronounced muscle atrophy and weaking, and an apparent slowing appear to be inevitable followers of increasing age7. However, aging appears to impact fiber types in different manners. While an age-related declined in the size and number of type-specific muscle fibres have been described by some authors in relation to the ‘age-related motor-unit remodelling', which involves muscle fibre denervation and subsequent reinnervation8,9, a no predominant effect of aging on any fiber type, have been observed in the very elderly human skeletal muscle. A higher fibre co-expression of both myosin heavy chain (MHC) I and IIA have been observed related to the last description4,7,10. This apparent divergence reflects the complexity mechanisms involving the age-related changes affecting the muscle mass.
Skeletal muscles are composed of slow and fast types of muscle fibres with different contractile and metabolic properties which depend significantly on subclass of the type II fibre isoform as well on the concerned specie11. In cetaceans, a recently study on the Kogia breviceps and Tursiops truncatus species, showed that type I fibres posses a higher mitochondrial density than type II fibres in the both analyzed species, based on the succinate dehydrogenase stain intensity12.
It has been reported that the skeletal muscles of humans exhibit age-related changes in fibre type composition and in the structure of the myosin heavy chain7. Histological and morphometric analysis of aged muscles has revealed a significant reduction in the cross-sectional area (CSA) of the type II fibres, whereas the type I fibres were relatively unaffected13,14. Moreover, it is mostly the type II fibres that are lost with ageing, leaving a higher proportion of type I fibres in the muscle14,15. However, more recent data suggest that specific fibre type affection depends upon on the age of the specimen and on the distribution of fibre types in the adult muscles10,16.
The deleterious effects of ageing on skeletal muscle have been a topic of study since the early part of the 20th century, yielding extensive descriptions of muscular ageing in humans and laboratory animals (mice)5,6,17, while the few such reports on domestic18 and wild animals concerned short-lived mammals19. Recently, interest in the muscular senescence of marine mammals has arisen19,20,21,22.
Cetaceans are long-lived, large-brained mammals, so they have many physiological and pathological parallels with humans that enable them to be useful and unique comparative models23. However, little is known about muscular senility in marine mammals. A decreasing muscle mass, as indicated by a linearly decreasing serum creatinine with increasing age, has been observed in some geriatric dolphins20, while studies of pinnipeds have shown a decrease in the myocyte density and an increase in the extracellular space with advancing age22.
In this study, we used histological and morphometric approaches to evaluate the evolution of cetacean muscle fibres with age in stranded cetaceans. The age and the cause of death of the stranded cetaceans in this study were obtained from routine necropsies and were used to determine the aetiological cause of their muscular atrophy. The different species of cetaceans exhibit a wide range of diving behaviours based on their capabilities for deep diving and for remaining submerged, which may be related to the muscle characteristics of each species. Accordingly, we used one model of muscular senescence, the Atlantic spotted dolphin (Stenella frontalis), for comparison. The present study describes age-related changes in the three factors that determine muscle mass: fibre size, relative fibre number and fibre type. We found that skeletal muscle fibres in cetaceans change with advancing age, evolving towards a slower muscle phenotype. We suggest that this physiological adaptation constitutes an adaptation to the prolonged and deep dives of these marine mammals.
Results
Gross findings
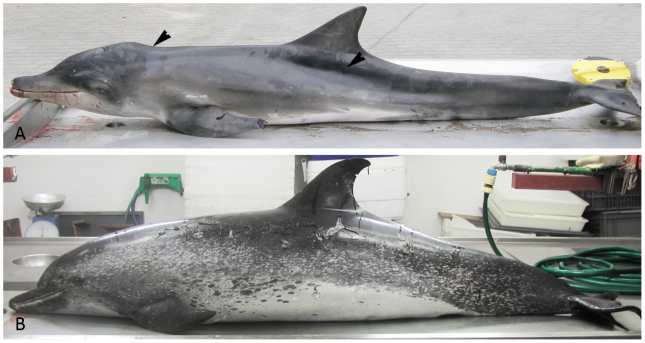
A remarkable reduction in the muscle mass parallel to the long sides of the axis was observed in animals with an aetiological diagnosis of cachexia/malnutrition, but not in the senile cetaceans that lacked consumptive pathologies (Fig. 1A–1B).
Figure 1. Gross appearance of senile and cachectic specimens.
(A): Female juvenile specimen of Steno bredanensis that had been undergoing a consumptive process, showing severe emaciation characterised by the protuberance of the axial bones (arrowheads). (B): Female senile specimen of Stenella frontalis (Case N. 10) that had not been undergoing consumptive process, showing a moderate to good external body condition. Older Atlantic spotted dolphins were identified mainly by their spot pattern (as the animals age, spots develop on both the ventral and dorsal surfaces).
Histopathological study
Twenty of the 155 animals (12.9%) exhibited skeletal muscle changes related to age, based on the overall data concerning the sample (n) and the histopathological criteria for skeletal muscle atrophy and senile tissue changes. The animals with a pathology condition of cachexia and/or malnutrition, that showed histopathological features consistent with generalised atrophy (unpublished data), were not included in this study.
Senile muscles exhibited a variety of abnormal features (Figure 2A–2B), consisting of wide variations in the sizes of the fibres; morphological alterations of the myofibres (angular atrophy) ranging from small to large fibre group atrophy; longitudinal splitting (caused by focal ingrowth of the sarcolemma that creates the appearance of more than 1 myofibre within the same basal lamina); increased internal nuclear migration, which is readily detected in PAS-stained transverse sections; the sporadic presence of cytoarchitectural alterations, such as ring fibres (peripheral rims of maloriented myofilaments) and hypertrophic fibres; a slight endomysial fibrosis, which is better visualised by PTAH staining; and the presence of large amount of juxtanuclear brownish pigment, which PAS staining showed to be lipofuscinosis (Figure 2B1). The use of the aforementioned histochemical stain also allowed the visualisation of significant vascularisation within the fibres. The vascularisation feature entailed vessels predominantly running parallel to the longitudinal fibre axis, with a few capillaries running transversely. The EM study revealed the presence of large membrane-bounded osmiophilic granules of lipofuscin, which were located mostly in the subsarcolemmal region (Figure 2B2).
Figure 2. Histopathological study.
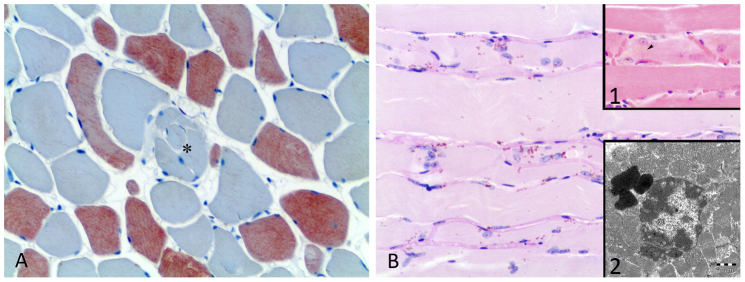
(A): Cross section of a muscle from a senile spotted dolphin specimen. The muscle exhibits a variety of abnormal features consisted of an increased variations in the fibre sizes and longitudinal splitting (*). Immunostaining for the fast MHC isoform. 20×. (B): Longitudinal section of a muscle from a senile spotted dolphin specimen. The muscle exhibits a large amount of juxtanuclear lipofuscin and significant vascularisation within the fibres. PAS. 20×. 1: Lipofuscin appears as a faint yellow pigment, mostly adjacent to the nucleus. HE. 20×. 2: An EM image showing the lipofuscin accumulation was located mostly in the mitochondria-rich subsarcolemmal area. Bar = 1 μm.
The skeletal muscle samples selected for morphometry (from the adult and the senile group of Atlantic spotted dolphins) displayed tightly packed, well-preserved fascicles. The shape and size of the fibres observed in cross-sections of muscles from the adult specimens appeared normal, with almost all of the fibres having an angular shape, whereas fibres with structural irregularities, including variations in size and morphology, predominantly “flattened”, “crushed” or “banana-shaped” fibres, were observed in the cross-sections of muscles from the older specimens.
Inmunohistochemical study
Immunostaining with slow and fast myosin heavy chain (MHC) antibodies, which recognise type I and II fibres, respectively, revealed differences within a type of fibre and alterations in distribution of the fibre type between the adult and senile cetaceans.
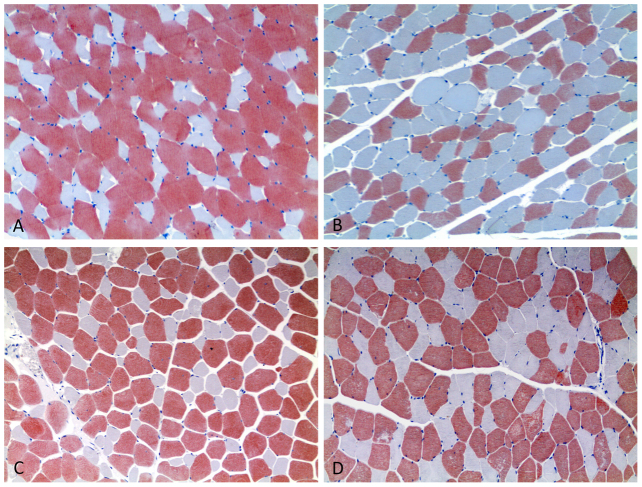
Our results showed two clearly different fibre types in the adult animals of all the species studied (19), with the type II fibres being much larger and more numerous than the type I fibres. In contrast, in the skeletal muscles from senile animals, the two types of fibres had similar sizes, and more type I fibres were observed in the cross sections. In addition, a tendency of the type I or II fibres to be in clustered was noticed in the aged animals. This clustering was a deviation from the normal mosaic-like pattern of fibres observed in the muscle fascicles of the younger animals (Figure 3A–3D).
Figure 3. Immunohistochemical stain for the fast MHC antibody showed differences within fibre type involved and alterations in fibre type distribution between adult and senile cetaceans.
10×. (A): Juvenile specimen of striped dolphin. Muscle cross section. The muscle contains two clearly different fibre type populations, forming a mosaic-like pattern, in which type II (red-stained fibres) are much larger and more numerous than are the type I fibres (blue-stained fibres). The shape and size of the fibres appear normal, with almost all of fibres having an angular shape. (B): Senile specimen of striped dolphin. Muscle cross-section. A larger population of type I fibres is observed, which tend to appear in clusters. The muscle contains fibres with different structural irregularities, including the “flattened” fibre. (C): Adult specimen of Atlantic spotted dolphin. Muscle cross section. The muscle exhibits the above-described features for juvenile specimens of the striped dolphin. (D): Adult-senile specimen of Atlantic spotted dolphin. Muscle cross-section. In senile muscles, both fibre types have a similar fibre size, and a higher proportion of type I fibres is present. Fibres tend to form clusters of one type of fibre, which leads to a deviation from the normal mosaic arrangement of fibres within the muscle fascicle than is observed in the younger animals.
Immunohistochemical fibre type identification also showed that the large amounts of juxtanuclear lipofuscin pigment observed in the histological study occurred mainly in the type I fibres and that the flattening process undergone by the fibres in the senile muscles was more pronounced among the type II fibres than the type I fibres.
The use of a mioglobin antibody showed a generalised diffuse pattern of this protein within both the type I and type II fibres, regardless of the species, sex and age of the animals examined.
Morphometric and Statistical studies
Statistical analysis of the data on the three factors that determine muscle mass, the fibre size (CSA and lesser diameter), the relative fibre number and the fibre type, was performed on the adult and senile groups of Atlantic spotted dolphins.
Student's t test could have been applied to compare the differences between the groups because all of the data, except the CSA values for the type I fibres of aged dolphins, showed a normal and homogeneous distribution; however, the small size of the sample (<25 per group) and the different sizes of groups (6 animals in the adult group and 5 in the senile group) required the use of the Mann-Whitney U non-parametric test.
Morphometry and statistics performed on selected skeletal muscle samples from both adult and senile specimens of Atlantic spotted dolphin showed a slight decrease of both CSA (2,272.34. μm2 versus 1,889.18 μm2) and lesser diameter (18.10 μm versus 16.19 μm) for the type II fibres in the aged compared with adult spotted dolphins. The differences showed a trend but were not statistically significant.
In contrast, the type I fibres displayed a larger measurements of CSA (1,349.39 μm2 versus 819.17 μm2) and lesser diameter (14.11 μm versus 11.35 μm) in the senile group (Figure 4–5). The Mann-Whitney U test for two independent-samples showed that there was a highly significant difference in the mean CSA (P < 0.004) and lesser diameter (P < 0.001) of the type I fibres of the adult and senile dolphins, i.e., the hypothesis of the homogeneity of the mean values for the adult and senile groups can be rejected at the 0.1% significance level.
Figure 4. Mean cross sectional area (μm2) (±SEM) of Type I and Type II fibres in the adult and senile groups.
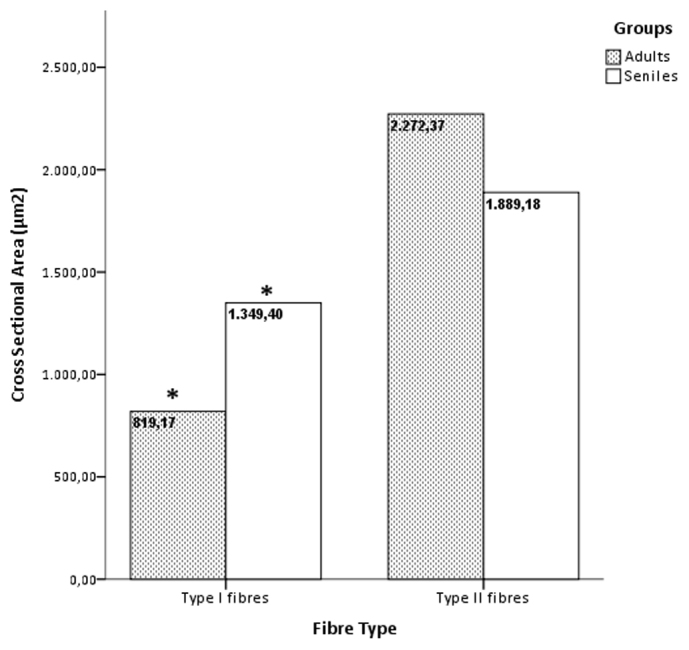
Asterisks denote significant differences across age groups of spotted dolphins for type I fibres (P < 0.05, the Mann-Whitney U test).
Figure 5. Lesser diametre (μm) (±SEM) of Type I and Type II fibres in the adult and senile groups.
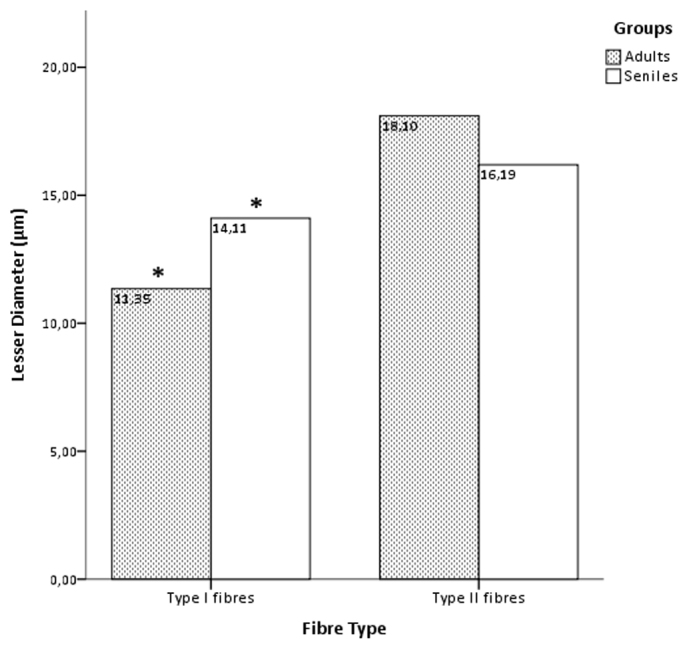
Asterisks denote significant differences across age groups of spotted dolphins for type I fibres (P < 0.05, the Mann-Whitney U test).
The occupied area of the type II fibres in the adult dolphins was 73.5% versus 58.3% in the senile dolphins, while the filled area space for type I fibres in the younger group was 26.5% versus 41.7% in the senile group.
A statistically significant (P < 0.05) difference of the sizes of the two fibre types was observed between the age groups. This size difference was greater in the adult group, revealing a similar sized type I and type II fibre population in the older animals.
The percentages of type I and type II fibres in the longissimus dorsi muscle differed significantly between the adult and senile groups of dolphins. The dolphins in the senile group had a significantly higher percentage of type I fibres (48.18 ± 11 versus. 35.18 ± 3%; P < 0.01) and a significantly lower percentage of type II fibres (51.82 ± 5 versus. 64.82 ± 1%; P < 0.01) compared with the younger group, by the statistical tests used in this study (Figure 6).
Figure 6. Fiber type proportion (%) (±SEM) of Type I and Type II fibres in the adult and senile groups.
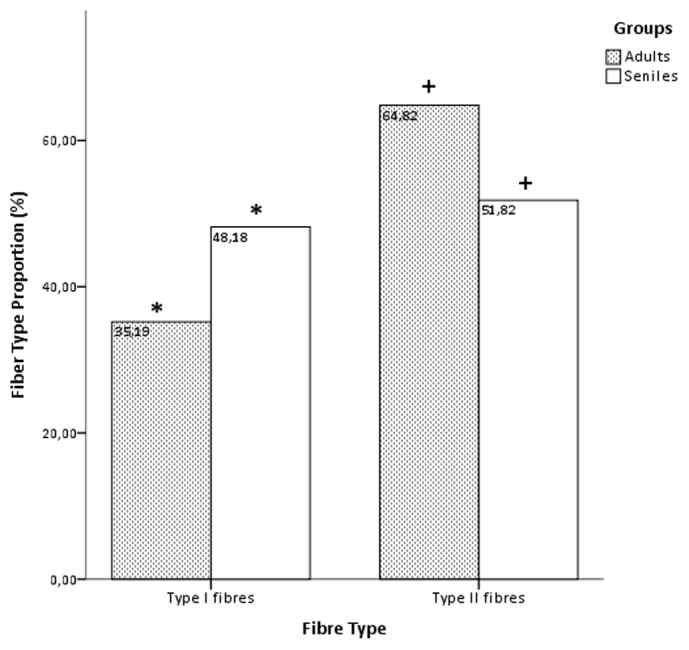
Asterisks and cross denote significant differences across age groups of spotted dolphins for type I fibres (P < 0.05, the Mann-Whitney U test).
If we defined a null hypothesis of not only that there would be a difference in the sizes of fibres in the two groups of dolphins but also that a larger or smaller size is expected, a one-tailed significance level could be applied, making our results even more statistically significant.
Discussion
The present study examined the ageing-related changes in the skeletal muscle fibres of free-ranging cetaceans and presented morphometric and statistical results for the Atlantic spotted dolphin.
While no gross evidence of muscular atrophy was observed in the senile cetacean specimens, several histopathological changes were found that are associated with the age of the older animals in our study. Our main findings consisted of the following: 1) with increasing age, there was a slight progressive decrease in the CSA of the fast type II fibres, whereas the CSA of the slow type I fibres significantly increased. 2) There was a slight progressive decrease in the lesser diameter of the fast type II fibres, whereas the lesser diameter of the slow type I fibres significantly increased with age. 3) The proportion of type I fibres was significantly higher in the senile dolphins in our study. 4) The differences between the CSAs and lesser diameters of type II and type I fibres significantly decreased with ageing, resulting in similar sized fibre types in older animals.
The age-related modifications in the skeletal muscle observed in our study suggests that older cetaceans display adaptations that characterise both humans' and animals' prolonged endurance training and sarcopenia (i.e., high proportion of type I fibres and modest type I fibre hypertrophy and selective atrophy of type II fibres)11,24,25.
Our results showed significant changes in the size and relative number of type I fibres in the older cetaceans group. In our study, type II fibres in the adult Atlantic spotted dolphins were slightly larger (1.2 times) than in the older animals, whereas the type I fibres were significantly larger (1.65 times) in the senile animals. We also observed that the relative number of each fibre type is significantly altered with age (13% of more type I fibres in the senile versus the adult animals).
Similar, but not identical, muscle age-related effects have been described in elderly humans and rodents7,8,26,27, although opposite results have also been reported, in which either no significant difference in the fibre type composition or increase of type II fibres in the aged muscle were observed28,29. The explanation for these controversial results is uncertain. Some authors suggested that the number of type I fibres might not increase with age as a general rule but rather provisionally increase due to the age-related fibre-type motor-unit degeneration, which is observed in the early elderly and is followed by a compensatory reestablishment of the ratio of the type I and II fibres in the very elderly7. Other theories are related to long-term strength training, suggesting that strength training can counteract the age-related changes in function and morphology of ageing human skeletal muscle30.
It is known that an increase in the relative area of type I fibres with age proceeds in parallel with the increase in slow MHC I30,31, consistent with the age-related effects observed in the longissimus dorsi of the stranded cetaceans in this study, especially the increase in the size and the relative number of type I fibres (slow contracting) in the older specimens, suggesting that the muscle function evolved toward a slow phenotype during senescence in these marine mammals.
Other histopathological muscles changes observed in old cetaceans in our study mainly consisted on: myofibre size variation, including angular atrophy and hypertrophic fibres; longitudinal splitting; internal nuclei migration; ring fibres; endomysial fibrosis; fibre type grouping; increased myofibre capillarisation; and the presence of juxtanuclear lipofuscinosis. Some of these changes, in particular, increased fibre size variation and internal nuclei, have been traditionally considered as chronic myopathic changes, which are defined as nonspecific findings associated with a variety of neuromuscular disorders32. However, they have been previously associated with increasing age in equids33 and humans13. Fibre type grouping, as was observed in the muscles of the older cetaceans in our study, is the phenomenon of clustering of a specific fibre type being rather than the random distribution observed in younger muscles. The most likely explanation seems to be that the fibre type grouping arises from a continuous process of denervation and partial reinnervation that is believed to accelerate with advancing age9,15. The same process is the responsible of the morphologically altered myofibres (i.e. flattened, crushed or angular banana shaped fibres), according to recent publications10,34, in which these fibres are described to be largely denervated based on the expression of a denervation-specific sodium channel isoform in the adult muscle. Enhanced myofibre capillarisation, as observed in the senile animals in our study, has been described in the skeletal muscles of both younger and older endurance-trained humans in association with an increased oxidative capacity11,31, although it has been also appreciated in sedentary aged rodents35. Our results showed a higher accumulation of juxtanuclear lipofuscin in the skeletal muscles of senile cetaceans, primarily in the type I fibres. According to Hutter et al. (2007)36, the intramuscular accumulation of lipofuscin is a robust marker of ageing in human skeletal muscle. In our study, EM showed that lipofuscin accumulation was predominantly located in the subsarcolemmal areas, which are mitochondria-rich regions and an important source of reactive oxygen species (ROS). The degradation of damaged mitochondria contributes to a progressive lipofuscin accumulation because it cannot be eliminated effectively. Accordingly, lipofuscin accumulation has been proposed as marker of cumulative oxidative damage37,38,39. The intracellular location and the distribution of lipofuscin according to the fibre-type are also consistent with the relationship between oxidative stress and lipofuscin37,38 because it accumulates more fibres with a high (type I) compared to low (type II) oxidative potential in humans with chronic obstructive pulmonary disease38. Cetaceans perform dives with a limited quantity of oxygen and because hypoxemia increases oxidative stress during exercise40 and in the reoxygenation phase following a period of hypoxia41, we speculated that a correlation exists between the repetitive hypoxic dives of cetaceans and the larger amount of lipofuscin pigment in the type I fibres of the longissimus muscle in these animals. We also propose that the chronic oxidative stress that cetaceans experience as a consequence of their repetitive hypoxic dives during their daily activities could only partially account for the age-related muscle changes in these animals.
The many potential cellular and molecular mechanisms that underlie sarcopenia are unclear. Oxidative stress, inflammation, endocrine disorders, malnutrition, and inactivity have been proposed as responsible cofactors for muscular senescence in humans and animals42. While an increasing state of chronic inflammation with age has been previously described in dolphins20, other mechanisms underlying the senility of marine mammals have not been yet elucidated, despite the potentially profound impact of the ageing phenomena on their foraging capacity and predator avoidance.
The slowing (fast-to-slow-fibre-type switch) of muscle function and the loss of anaerobic muscle fibres during the normal ageing process, as has been observed in our study, could be regarded as a possible “preservation mechanism” and an adaptive response to low muscle mass and muscle weakness, as previously proposed43. Wild cetaceans swim almost continuously with a wide range of speeds because the cetaceans' daily activities, including foraging, migration, territory defence, and predator avoidance, are all critically dependent on skeletal muscle function. Considering the established function of type I slow muscle fibres in the resistance to fatigue44, maintenance of the slow type of fibres would be beneficial for the quality of life of the elderly45. Therefore, a higher proportion of type I fibres in one of the main swimming muscle of cetaceans (longissimus dorsi)46 could be an adaptation to support the large amount of activity of the elderly.
Cetaceans are marine vertebrate breath-holding divers and consequently perform their immersions with a limited quantity of oxygen. Elevated muscle myoglobin concentrations, which enhance the oxygen storage capacity of muscle and increase the aerobic dive limit (ADL) of animals, are one of the most important adaptations of diving vertebrates47. However, energy-conserving modes of locomotion appear to slow the rate of oxygen utilisation and, consequently, can extend the duration of dives48. Previous studies on dolphins have reported that as the speed of locomotion increases, the period of sustainability decreases49,50, and therefore, the overall swim performance is constrained by the aerobic and anaerobic capacities of the locomotor muscles51. Several adaptive mechanisms to reduce the cost of the immersion in marine mammals have been proposed recently. Williams and Noren (2009)52 reported that Killer whales (Orcinus orca) expend less total energy swimming at slower speeds, while the behavioural adjustments of ageing pinnipeds, consisting of decreased mean swimming speeds and dive depths, may compensate for the age-related muscle effects22.
The age-related shift in fibre-type composition contributes to the slowing of muscle contractile properties, causing a leftward shift in the force frequency relationship. Several studies reported that a higher proportion of type I fibres in older muscles, as was observed in our study, significantly reduce the cost of locomotion in the elderly because the economy of force production is 3- to 4-fold higher for type I fibres than for type II fibres, based on the differences in both the myosin ATPase and the cost of calcium handling4,13. Thus, a fast-to slow muscle phenotype evolution in old cetaceans could reduce the cost of muscle contraction, reducing the energetic expense of locomotion and, consequently, prolonging muscular activities in the elderly cetaceans.
Type I fibres also have higher concentrations of Mb and higher oxidative metabolism than do type II fibres (fast-twitch)12,53. Accordingly, due to the higher proportion of type I fibres observed in the skeletal muscles of the older cetaceans in our study, the senile muscles shifted toward a more oxidative metabolism and enhanced the oxygen storage capacity, which would increase the ADL47.
In summary, the high proportion of type I fibres observed in the ageing cetaceans in our study, reflects their slow, oxidative, prolonged, and economical contractible movements, as has been reported in studies of ageing humans and animals7,26,27,43. The switch to more slow fibres could be an adaptive response with the functional benefits of greater resistance to fatigue and better recovery from fatigue.
Methods
Skeletal muscles from small and large stranded odontocetes and misticetes (n = 155) of 19 different species were examined. The following species and specimens of cetaceans were included in this study: Fin whale (Balaenoptera physalus) (3); Short-beaked common dolphin (Delphinus delphis) (10); Risso's dolphin (Grampus griseus) (3); Short-finned pilot whale (Globicephala macrorhynchus) (12); North Atlantic bottlenose whale (Hyperoodon ampullatus) (1); Pygmy sperm whale (Kogia breviceps) (11); Fraser's dolphin (Lagenodelphis hosei) (2); Sowerby's beaked whale (Mesoplodon bidens) (1); Blainville's beaked whale (Mesoplodon densirostris) (3); Gervais' beaked whale (Mesoplodon europaeus) (6); Harbour porpoise (Phocoena phocoena) (1); Sperm whale (Physeter macrocephalus) (9); False killer whale (Pseudorca crassidens) (1); Striped dolphin (Stenella coeruleoalba) (25); Atlantic spotted dolphin (Stenella frontalis) (30); Spinner dolphin (Stenella longirostris) (2); Rough-toothed dolphin (Steno bredanensis) (3); Bottlenose dolphin (Tursiops truncatus) (13); and Cuvier's beaked whale (Ziphius cavirostris) (19). The animals were of both sexes and ranged in age from neonatal to senile according to biological and morphometric parameters54.
The skeletal muscle samples were collecting during the necropsy, following standard protocols, over a 14-year period from 1996 to 2010. Necropsies were performed either in situ (beach or coast), in installations designed for that purpose or, when possible, in the necropsy room at the Veterinary Faculty in Las Palmas de Gran Canaria. I confirm that all experiments were not performed on live animals due to our work is based on stranded cetaceans.
All of the skeletal muscle samples were taken from the middle portion of the longissimus dorsi muscle lying immediately lateral to the dorsal fin, as previously described55. The longissimus dorsi is part of the epaxial musculature, which lies along both sides of the vertebral column and is involved primarily in the cetacean's locomotion; it is one of two muscles that power the dolphin's upstroke46.
The samples were mounted on a tongue depressor (fixed at both ends by pins) with the myofibres oriented lengthwise and immersed in 4% neutral-buffered formalin for 24–48 hours. Transverse and longitudinal muscle samples were then carved and were routinely processed, embedded in paraffin, serially sectioned and stained with haematoxylin and eosin (HE), periodic acid-Schiff (PAS) with and without amylase digestion, phosphotungstic acid haematoxylin (PTAH), and Von Kossa method for special stainings, as previously described56,57,58,59. In addition, the skeletal muscle samples were immunostained to detect the fast and slow MHC isoforms, type II and type I, respectively, (Sigma Co., St. Louis, MO, USA), and myoglobin (Mb) (Dako, Glostrup, Denmark), using the avidin-biotin-peroxidase method (Vector Laboratories, Burlingame, California, USA). Tissue sections in which the primary antibodies were replaced by phosphate-buffered saline or nonimmune serum (rabbit or mouse) were used as negative controls. Slow MHC recognises type I fibres (slow-twitch fibers) and fast MHC recognises type IIa, IIb and IId (IIx) (in fast-twitch fibers).
The muscle examinations included a macroscopic evaluation of the muscle mass and a histologic examination of the longissimus dorsi muscle sample obtained from all cases. Grossly, a moderate to severe emaciation was characterised by the protuberance of the axial bones (muscular atrophy). Histological diagnosis of the age-related effects on muscle included generalised atrophy, based on decreased myofibre diameter, increased fibre size variation, morphological myofibre alterations (angular atrophy), endomysial fibrosis, the fibre type involved and alterations in the distribution of the fibre types and the presence of yuxtanuclear lipofuscin. The degree of fibre size variation , fibre splitting, the number of internal nuclei, and the presence of ring fibres and lipofuscin accumulations were scored from 0 (normal) to 3+ (severe) to determine a chronic myopathic score as previously described33. Specifically, fiber size variation, fibre splitting, ring fibres and lipofuscin accumulations were subjectively judged to be absent (0), mild (1+), moderate (2+), or severe (3+). Internal nuclei were judged to be absent (0), present but with an average of less than 1 per 200× microscopic field (1+), present with an average of 1–2 per 200× microscopic field or occurring as 2 or more within myofibers (2+), and present with an average of 3 or more per 200× microscopic field (3+). All The scores were added to obtain a chronic myopathic score for each animal. The mean chronic myopathic score (±SEM) was compared between senile and non senile cetaceans. A significant difference (P < 0.05) was observed between groups with the non parametric Mann-Whitney U test (SPSS 21). The combination of the histopathological findings and data concerning the age and the cause of death60 was used to establish an aetiological diagnosis of muscular atrophy in some animals.
Because of the heterogeneity of the species included in this study, only skeletal muscles samples of adult and senile animals of Stenella frontalis were used for the morphometric and statistical studies. Two groups comprising adult (sexually mature age) and senile cetaceans were created. Animals with concomitant pathologies that cause muscle wasting were excluded. The older animals were identified by their skin spot pattern (as the animals age, spots on both ventral and dorsal surfaces develop) and their skull and body sizes61,62. Based on these features, an estimated relative age of 9–16 years for young adults and more than 16 years for old adults from the Atlanctic spotted dolphins has been published63. In addition, the senile dolphins showed most of the bones sutures fused, a pronounced tooth wear and age-related histological findings (i.e. siderofibrotic plaques in the spleen, lipofuscinosis in neurons and the myocardium, and calcifications in the vascular walls). The senile group was composed of 5 animals (3 males and 2 females), and the adult group was composed of 6 animals (3 males and 3 females).
For morphometry, 10 images per slide (200× magnification) of a muscle section that was immunohistochemically stained for anti-fast myosin were captured for each of the 11 Atlantic spotted dolphins (110 total images), using a digital Altra20 camera (2 MegaPixel CMOS colour camera for light microscopy, Olympus Soft Imaging Solutions GmbH, Münster, Germany). The CSA and the lesser diameter (the greatest distance between the opposite sides of the narrowest aspect of the fibre) of all the fibres in a field, with red stained fast-twitch muscle fibres (type II) and blue stained slow-twitch muscle fibres (type I) were measured. The relative number of type I and type II fibres was assessed by counting each fibre type from the 10 selected random fields of each animal. The percentage of each specific fibre type was calculated as the specific fibre count divided by total fibre count. A variable number of 872-2099 fibres were counted for each specimen. The muscle morphology data (CSA, lesser diameter and number of fibre types) was calculated using the Digital software Imaging Solutions, CellA. The mean values were obtained using the Soft Imaging System for Life Science Microscopy (Table 1).
Table 1. Morphometric data for the 11 Atlantic spotted dolphins in the study of adult and senile specimens. The mean values of CSA, LD and N° of fibres are given.
| Type I fibres | Type II fibres | ||||||
|---|---|---|---|---|---|---|---|
| Case N° | Group | CSA | LD | N° | CSA | LD | N° |
| 1 | Senile | 1,307.81 | 13.96 | 967 | 1,458.53 | 13.40 | 1132 |
| 2 | Adult | 564.11 | 12.10 | 403 | 1,893.12 | 17.09 | 810 |
| 3 | Adult | 730.14 | 10.99 | 722 | 1,959.43 | 17.94 | 1090 |
| 4 | Senile | 1,587.69 | 14.88 | 626 | 2,320.86 | 18.61 | 761 |
| 5 | Senile | 1,304.96 | 14.31 | 826 | 1,429.63 | 14.19 | 1122 |
| 6 | Adult | 727.20 | 10.60 | 676 | 1,663.54 | 16.19 | 1195 |
| 7 | Senile | 1,254.89 | 13.03 | 789 | 2,230.36 | 17.02 | 390 |
| 8 | Adult | 765.99 | 10.03 | 422 | 2,223.77 | 17.79 | 749 |
| 9 | Adult | 1,069.25 | 11.65 | 267 | 3,573.29 | 20.32 | 605 |
| 10 | Senile | 1,291.64 | 14.35 | 545 | 2,046.54 | 17.73 | 805 |
| 11 | Adult | 1,058.34 | 12.75 | 438 | 2,321.06 | 19.27 | 805 |
An average of 607 type I fibres and 860 type II fibres for each animal were analysed. Cross sectional area (CSA) (μm2), Lesser diameter (LD) (μm), Relative number of each fibre type per 10 random fields (N°).
Statistical analysis
The data for each age group (adult versus senile) are expressed as the means ± standard error of the mean (SEM) (Table 2) and were tested for normality using the Shapiro–Wilkes test. The homogeneity of variance was confirmed using a modified Levene test. The Mann Whitney U test (SPSS v.21) was used to test the statistical significance of differences in the mean values for CSA, the lesser diameters of the type I and type II fibres and the number of fibre types of the adults and old (senile) individuals. The significance level was set at P < 0.05.
Table 2. Data for each age group (adult and senile) are expressed as the means ± SEM. The Mann-Whitney U test (SPSS v.21) test was used to analyse the statistical significance of differences in the mean values of the CSA, the lesser diameters of the type I and type II fibres and the number of type I and type II fibres of adult and old (senile) individuals. The significance level was set at P < 0.05.
| Type I fibres | Type II fibres | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | CSA (μm2) | LD (μm) | N° | % | CSA (μm2) | LD (μm) | N° | % |
| Adult | 819.17 ± 201.99 | 11.35 ± 1 | 488 ± 174.94 | 35.18 ± 3.1 | 2,272.34 ± 679.64 | 18.10 ± 1.49 | 875.67 ± 222.04 | 64.82 ± 1.27 |
| Senile | 1,349.39 ± 134.86 | 14.11 ± 0.69 | 750.60 ± 167.17 | 48.18 ± 10.72 | 1,889.18 ± 435.95 | 16.19 ± 2.27 | 842 ± 306.07 | 51.82 ± 4.79 |
| Age effect (P) | 0.004 | 0.001 | 0.045 | 0.004 | NS | NS | NS | 0.004 |
The values are the means ± SEM for the 6 subjects in the adult group and the 5 subjects in the senile group. NS (Not significant). Cross sectional area [CSA (μm2)], lesser diameter [LD (μm)] and number (N°) of Type I fibres in the adult and senile specimens are significantly different (P < 0.05). P values refer to the significant significance of the age effects determined by one-way (age) analysis of variance.
Ultrastructural study
In addition, selected skeletal muscle samples from adult and senile animals were submitted for an electronic microscopic (EM) study in order to confirm the nature and location of lipofuscin granules. For the ultrastructural studies, the selected skeletal muscle samples were fixed in 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer (pH 7.2). The specimens were post-fixed in 1% osmium tetroxide in a 0.2% Veronal buffer, gradually dehydrated in an alcohol series and embedded in Epon 812 epoxy resin. Thin sections were stained with uranyl acetate and lead citrate and examined with a ZEISS EM-912 transmission electron microscope.
Author Contributions
E.S., P.H. and A.E. designed the research; E.S., M.A. and Y.B.de Q. conducted the research, E.S., P.H., A.E. and A.F. analysed the data, E.S. wrote the manuscript and E.S. and P.H edited the paper.
Acknowledgments
This work was supported by a project from Ministerio de Ciencia, Investigación e Innovación (CGL2009-13052), Spain.
References
- WHO. What are the public health implications of global ageing? Online Q&A 29 September 2011 Retrieved from http://www.who.int/features/qa/42/en/index.html (2011).
- Walston J. et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54, 991–1001 (2006). [DOI] [PubMed] [Google Scholar]
- Rogers M. A. & Evans W. J. Changes in skeletal muscle with aging: effects of exercise training. Exerc Sport Sci Rev 21, 65–102 (1993). [PubMed] [Google Scholar]
- Lexell J., Taylor C. C. & Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84, 275–294 (1988). [DOI] [PubMed] [Google Scholar]
- Hagen J. L. et al. Skeletal muscle aging in F344BN F1-hybrid rats: I. Mitochondrial dysfunction contributes to the age-associated reduction in VO2max. J Gerontol A Biol Sci Med Sci 59, 1099–1110 (2004). [DOI] [PubMed] [Google Scholar]
- Lushaj E. B., Johnson J. K., McKenzie D. & Aiken J. M. Sarcopenia accelerates at advanced ages in Fisher 344xBrown Norway rats. J Gerontol A Biol Sci Med Sci 63, 921–927 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. L. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13, 40–47 (2003). [DOI] [PubMed] [Google Scholar]
- Larsson L., Sjodin B. & Karlsson J. Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand 103, 31–39 (1978). [DOI] [PubMed] [Google Scholar]
- Vandervoort A. A. Aging of the human neuromuscular system. Muscle Nerve 25, 17–25 (2002). [DOI] [PubMed] [Google Scholar]
- Purves-Smith F. M., Solbak N. M., Rowan S. L. & Hepple R. T. Severe atrophy of slow myofibers in aging muscle is concealed by myosin heavy chain co-expression. Exp Gerontol 47, 913–918 (2012). [DOI] [PubMed] [Google Scholar]
- Proctor D. N., Sinning W. E., Walro J. M., Sieck G. C. & Lemon P. W. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol 78, 2033–2038 (1995). [DOI] [PubMed] [Google Scholar]
- Kielhorn C. E. et al. Locomotor muscle profile of a deep (Kogia breviceps) versus shallow (Tursiops truncatus) diving cetacean. J Morphol 28, 20124 (2013). [DOI] [PubMed] [Google Scholar]
- Jakobsson F., Borg K. & Edstrom L. Fibre-type composition, structure and cytoskeletal protein location of fibres in anterior tibial muscle. Comparison between young adults and physically active aged humans. Acta Neuropathol 80, 459–468 (1990). [DOI] [PubMed] [Google Scholar]
- Larsson L. & Edstrom L. Effects of age on enzyme-histochemical fibre spectra and contractile properties of fast- and slow-twitch skeletal muscles in the rat. J Neurol Sci 76, 69–89 (1986). [DOI] [PubMed] [Google Scholar]
- Luff A. R. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci 854, 92–101 (1998). [DOI] [PubMed] [Google Scholar]
- Carter E. E. et al. Slow twitch soleus muscle is not protected from sarcopenia in senescent rats. Exp Gerontol 45, 662–670 (2010). [DOI] [PubMed] [Google Scholar]
- Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr 127, 1011S–1013S (1997). [DOI] [PubMed] [Google Scholar]
- Braund K. G., McGuire J. A. & Lincoln C. E. Observations on normal skeletal muscle of mature dogs: a cytochemical, histochemical, and morphometric study. Vet Pathol 19, 577–595 (1982). [DOI] [PubMed] [Google Scholar]
- Hindle A. G., Lawler J. M., Campbell K. L. & Horning M. Muscle senescence in short-lived wild mammals, the soricine shrews Blarina brevicauda and Sorex palustris. J Exp Zool A Ecol Genet Physiol 311, 358–367 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn-Watson S., Smith C. R., Gomez F. & Jensen E. D. Physiology of aging among healthy, older bottlenose dolphins (Tursiops truncatus): comparisons with aging humans. J Comp Physiol B 181, 667–680 (2011). [DOI] [PubMed] [Google Scholar]
- Kanatous S. B. et al. The ontogeny of aerobic and diving capacity in the skeletal muscles of Weddell seals. J Exp Biol 211, 2559–2565 (2008). [DOI] [PubMed] [Google Scholar]
- Hindle A. G., Horning M., Mellish J. A. & Lawler J. M. Diving into old age: muscular senescence in a large-bodied, long-lived mammal, the Weddell seal (Leptonychotes weddellii). J Exp Biol 212, 790–796 (2009). [DOI] [PubMed] [Google Scholar]
- Venn-Watson S., Carlin K. & Ridgway S. Dolphins as animal models for type 2 diabetes: sustained, post-prandial hyperglycemia and hyperinsulinemia. Gen Comp Endocrinol 170, 193–199 (2011). [DOI] [PubMed] [Google Scholar]
- Freeman L. M. Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats. J Vet Intern Med 26, 3–17 (2012). [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Green H. J. & Cobb F. R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation 81, 518–527 (1990). [DOI] [PubMed] [Google Scholar]
- Caccia M. R., Harris J. B. & Johnson M. A. Morphology and physiology of skeletal muscle in aging rodents. Muscle Nerve 2, 202–212 (1979). [DOI] [PubMed] [Google Scholar]
- Kent-Braun J. A., Ng A. V., Doyle J. W. & Towse T. F. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93, 1813–1823 (2002). [DOI] [PubMed] [Google Scholar]
- Porter M. M., Vandervoort A. A. & Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 5, 129–142 (1995). [DOI] [PubMed] [Google Scholar]
- Frontera W. R. et al. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88, 1321–1326 (2000). [DOI] [PubMed] [Google Scholar]
- Klitgaard H. et al. Function, morphology and protein expression of ageing skeletal muscle: a cross-sectional study of elderly men with different training backgrounds. Acta Physiol Scand 140, 41–54 (1990). [DOI] [PubMed] [Google Scholar]
- Hameed M., Orrell R. W., Cobbold M., Goldspink G. & Harridge S. D. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol 547, 247–254 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V. Definition of pathological changes seen in muscle biopsies. In: Dubowitz, V. (Eds,), Muscle biopsy a practical approach. 2nd ed. Baillière Tindall, London, UK, 82–128 (1985). [Google Scholar]
- Valentine B. A. Pathologic findings in equine muscle (excluding polysaccharide storage): a necropsy study. J Vet Diagn Invest 20, 572–579 (2008). [DOI] [PubMed] [Google Scholar]
- Rowan S. L., Purves-Smith F. M., Solbak N. M. & Hepple R. T. Accumulation of severely atrophic myofibers marks the acceleration of sarcopenia in slow and fast twitch muscles. Exp Gerontol 46, 660–669 (2011). [DOI] [PubMed] [Google Scholar]
- Hepple R. T. & Vogell J. E. Anatomic capillarization is maintained in relative excess of fiber oxidative capacity in some skeletal muscles of late middle-aged rats. J Appl Physiol 96, 2257–2264 (2004). [DOI] [PubMed] [Google Scholar]
- Hutter E. et al. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell 6, 245–256 (2007). [DOI] [PubMed] [Google Scholar]
- Terman A. & Brunk U. T. Oxidative stress, accumulation of biological ‘garbage', and aging. Antioxid Redox Signal 8, 197–204 (2006). [DOI] [PubMed] [Google Scholar]
- Allaire J. et al. Lipofuscin accumulation in the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Muscle Nerve 25, 383–389 (2002). [DOI] [PubMed] [Google Scholar]
- Brunk U. T. & Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem 269, 1996–2002 (2002). [DOI] [PubMed] [Google Scholar]
- Vina J. et al. Exercise causes blood glutathione oxidation in chronic obstructive pulmonary disease: prevention by O2 therapy. J Appl Physiol 81, 2198–2202 (1996). [PubMed] [Google Scholar]
- Smith D. R., Stone D. & Darley-Usmar V. M. Stimulation of mitochondrial oxygen consumption in isolated cardiomyocytes after hypoxia-reoxygenation. Free Radic Res 24, 159–166 (1996). [DOI] [PubMed] [Google Scholar]
- Meng S. J. & Yu L. J. Oxidative stress, molecular inflammation and sarcopenia. Int J Mol Sci 11, 1509–1526 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici M. V., Bordini M. & Cerretelli P. Effect of aging on human adductor pollicis muscle function. J Appl Physiol 71, 1277–1281 (1991). [DOI] [PubMed] [Google Scholar]
- Linssen W. H. et al. Fatigue in type I fiber predominance: a muscle force and surface EMG study on the relative role of type I and type II muscle fibers. Muscle Nerve 14, 829–837 (1991). [DOI] [PubMed] [Google Scholar]
- Feng H. Z., Chen M., Weinstein L. S. & Jin J. P. Improved fatigue resistance in Gsalpha-deficient and aging mouse skeletal muscles due to adaptive increases in slow fibers. J Appl Physiol 111, 834–843 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst D. A. Intramuscular morphology and tendon geometry of the epaxial swimming muscles of dolphins. Journal of Zoology, London 230, 176 (1993). [Google Scholar]
- Kooyman G. L. & Ponganis P. J. The physiological basis of diving to depth: birds and mammals. Annu Rev Physiol 60, 19–32 (1998). [DOI] [PubMed] [Google Scholar]
- Williams T. M. et al. Sink or swim: strategies for cost-efficient diving by marine mammals. Science 288, 133–136 (2000). [DOI] [PubMed] [Google Scholar]
- Skrovan R. C., Williams T. M., Berry P. S., Moore P. W. & Davis R. W. The diving physiology of bottlenose dolphins (Tursiops truncatus). II. Biomechanics and changes in buoyancy at depth. J Exp Biol 202, 2749–2761 (1999). [DOI] [PubMed] [Google Scholar]
- Fish F. E. Power output and propulsive efficiency of swimming bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 185, 179–193 (1993). [Google Scholar]
- Hochachka P. W. & Storey K. B. Metabolic consequences of diving in animals and man. Science 187, 613–621 (1975). [DOI] [PubMed] [Google Scholar]
- Williams R. N. D. P. Swimming speed, respiration rate, and estimated cost of transport in adult killer whales. Marine Mammal Science 25, 327–350 (2009). [Google Scholar]
- Grefte S., Kuijpers-Jagtman A. M., Torensma R. & Von den Hoff J. W. Skeletal muscle development and regeneration. Stem Cells Dev 16, 857–868 (2007). [DOI] [PubMed] [Google Scholar]
- Boyd I. L., Lockyer C. &. Marsh H. D. Reproduction in marine mammals. In: Reynolds, J. E., Rommel, S. A., III (Eds.), Biology of Marine Mammals. Smithsonian Institution Press, Washington, DC, 239–241, 246–249 (1999). [Google Scholar]
- Noren S. R. & Williams T. M. Body size and skeletal muscle myoglobin of cetaceans: adaptations for maximizing dive duration. Comp Biochem Physiol A Mol Integr Physiol 126, 181–191 (2000). [DOI] [PubMed] [Google Scholar]
- Allen T. C. Hematoxylin and eosin. In: Laboratory methods in Histotechnology. Prophet, E. B., Mills, B., Arrington, J. B. and Sobin, L. H. (eds.). Armed Forces Institute of Pathology. American Registry of Pathology, Washington DC, 53–58 (1992). [Google Scholar]
- Arnicia E. D. A. E. Neuropathological histotechnology. In: Laboratory methods in Histotechnology. Prophet, E. B., Mills, B., Arrington, J. B. and Sobin, L. H. (eds.). Armed Forces Institute of Pathology. American Registry of Pathology, Washington DC, 98–100 (1992). [Google Scholar]
- Gaffney E. Carbohydrates. In: Laboratory methods in Histotechnology. Prophet, E. B., Mills, B., Arrington, J. B. and Sobin, L. H. (eds.). Armed Forces Institute of Pathology. American Registry of Pathology, Washington DC, 149–160 (1992). [Google Scholar]
- Johnson F. B. Pigments and minerals. In: Laboratory methods in Histotechnology. Prophet, E. B., Mills, B., Arrington, J. B. and Sobin, L. H. (eds.). Armed Forces Institute of Pathology. American Registry of Pathology, Washington DC, 197–198 (1992). [Google Scholar]
- Arbelo M. et al. Pathology and causes of death of stranded cetaceans in the Canary Islands (1999–2005). Dis Aquat Organ 103, 87–99 (2013). [DOI] [PubMed] [Google Scholar]
- Jefferson T. A., Webber M. A. & Pitman R. L. Marine mammals of the world. Elsevier, Amsterdam (2008). [Google Scholar]
- Perrin W. F. Atlantic spotted dolphin - Stenella frontalis. In: Encyclopedia of marine mammals. 2nd Ed. (Perrin, W. F., Würsig, B., Thewissen, J. G. M., eds.). Academic Press, Amsterdam (2009). [Google Scholar]
- Herzing D. L. The life history of free-ranging Atlantic spotted dolphins (Stenella frontalis): age classes, color phases, and female reproduction. Marine Mammal Science 13, 576–595 (1997). [Google Scholar]