Abstract
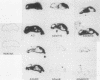
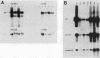
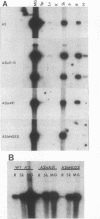
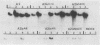
Viral replication in mice infected with murine polyomavirus strains with novel enhancer rearrangements was analyzed by direct in situ hybridization of whole mouse sections and by hybridization of nucleic acids extracted from a specific set of organs. The enhancer rearrangements included a deletion of the B domain as well as duplications within the A domain. Comparisons between enhancer variants demonstrate that the B domain plays an important role in replication in most organs, in particular in the kidney, at the neonatal stage (days 0 to 7 postbirth). In contrast, the B domain is not required in those organs which can sustain replication in the adult, i.e. mammary gland, skin, and bone (class I organs [J. J. Wirth, A. Amalfitano, R. Gross, M. B. A. Oldstone, and M. M. Fluck, J. Virol. 66:3278-3286, 1992]). Altogether, the results suggest that the B and A domains mediate very different functions in infection of mice, controlling the acute and persistent phases of infection, respectively. A model of mouse infection based on the crucial role of differentially expressed host transcription factors is presented.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P. Polyoma regulatory region: a potential probe for mouse cell differentiation. Cell. 1985 Dec;43(3 Pt 2):561–562. doi: 10.1016/0092-8674(85)90225-9. [DOI] [PubMed] [Google Scholar]
- BUFFETT R. F., LEVINTHAL J. D. Polyoma virus infection in mice. Pathogenesis. Arch Pathol. 1962 Dec;74:513–526. [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berebbi M., Dandolo L., Hassoun J., Bernard A. M., Blangy D. Specific tissue targeting of polyoma virus oncogenicity in athymic nude mice. Oncogene. 1988 Feb;2(2):149–156. [PubMed] [Google Scholar]
- Bolwig G. M., Hearing P. Interaction of nuclear factor EF-1A with the polyomavirus enhancer region. J Virol. 1991 Apr;65(4):1884–1892. doi: 10.1128/jvi.65.4.1884-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhnlein E., Gruss P. Interaction of distinct nuclear proteins with sequences controlling the expression of polyomavirus early genes. Mol Cell Biol. 1986 May;6(5):1401–1411. doi: 10.1128/mcb.6.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso M., Iacobini C., Passananti C., Felsani A., Amati P. Protein recognition sites in polyomavirus enhancer: formation of a novel site for NF-1 factor in an enhancer mutant and characterization of a site in the enhancer D domain. EMBO J. 1990 Mar;9(3):947–955. doi: 10.1002/j.1460-2075.1990.tb08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe C. J., Freund R., Mandel G., Ballmer-Hofer K., Talmage D. A., Benjamin T. L. Variations in polyoma virus genotype in relation to tumor induction in mice. Characterization of wild type strains with widely differing tumor profiles. Am J Pathol. 1987 May;127(2):243–261. [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Murphy F. A., Villarreal L. P. Detection of DNA and RNA virus genomes in organ systems of whole mice: patterns of mouse organ infection by polyomavirus. J Virol. 1984 Jun;50(3):779–783. doi: 10.1128/jvi.50.3.779-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Villarreal L. P. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol. 1984 May;50(2):541–546. doi: 10.1128/jvi.50.2.541-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunteun J., Sompayrac L., Fluck M., Benjamin T. Localization of gene functions in polyoma virus DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4169–4173. doi: 10.1073/pnas.73.11.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura F. K. Nuclear activity from F9 embryonal carcinoma cells binding specifically to the enhancers of wild-type polyoma virus and PyEC mutant DNAs. Nucleic Acids Res. 1986 Apr 11;14(7):2845–2861. doi: 10.1093/nar/14.7.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS L. A filterable agent, recovered from Ak leukemic extracts, causing salivary gland carcinomas in C3H mice. Proc Soc Exp Biol Med. 1953 Jun;83(2):414–421. doi: 10.3181/00379727-83-20376. [DOI] [PubMed] [Google Scholar]
- Graves B. J., Johnson P. F., McKnight S. L. Homologous recognition of a promoter domain common to the MSV LTR and the HSV tk gene. Cell. 1986 Feb 28;44(4):565–576. doi: 10.1016/0092-8674(86)90266-7. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Fried M., Cowie A. Polyoma DNA: a physical map. Proc Natl Acad Sci U S A. 1974 May;71(5):2077–2081. doi: 10.1073/pnas.71.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Kamachi Y., Ogawa E., Asano M., Ishida S., Murakami Y., Satake M., Ito Y., Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990 Oct;64(10):4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Firak T. A., Schroll C. T., Subramanian K. N. Activation of gene expression is adversely affected at high multiplicities of linked simian virus 40 enhancer. Proc Natl Acad Sci U S A. 1986 May;83(10):3199–3203. doi: 10.1073/pnas.83.10.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL J. D., JAKOBOVITS M., EATON M. D. Polyoma disease and tumors in mice: the distribution of viral antigen detected by immunofluorescence. Virology. 1962 Mar;16:314–319. doi: 10.1016/0042-6822(62)90252-0. [DOI] [PubMed] [Google Scholar]
- Markowitz R. B., Tolbert S., Dynan W. S. Promoter evolution in BK virus: functional elements are created at sequence junctions. J Virol. 1990 May;64(5):2411–2415. doi: 10.1128/jvi.64.5.2411-2415.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. E., Piette J., Yaniv M., Tang W. J., Folk W. R. Activation of the polyomavirus enhancer by a murine activator protein 1 (AP1) homolog and two contiguous proteins. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5839–5843. doi: 10.1073/pnas.85.16.5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J. Growth and persistence of polyoma early region deletion mutants in mice. J Virol. 1981 Sep;39(3):958–962. doi: 10.1128/jvi.39.3.958-962.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C. R., Mes-Masson A. M., Bouvier M., Hassell J. A. Location of sequences in polyomavirus DNA that are required for early gene expression in vivo and in vitro. Mol Cell Biol. 1984 Dec;4(12):2594–2609. doi: 10.1128/mcb.4.12.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Dufort D., Hassell J. A. Multiple subelements within the polyomavirus enhancer function synergistically to activate DNA replication. Mol Cell Biol. 1988 Nov;8(11):5000–5015. doi: 10.1128/mcb.8.11.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W. J., Mueller C. R., Mes A. M., Hassell J. A. Polyomavirus origin for DNA replication comprises multiple genetic elements. J Virol. 1983 Sep;47(3):586–599. doi: 10.1128/jvi.47.3.586-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostapchuk P., Diffley J. F., Bruder J. T., Stillman B., Levine A. J., Hearing P. Interaction of a nuclear factor with the polyomavirus enhancer region. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8550–8554. doi: 10.1073/pnas.83.22.8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Kryszke M. H., Yaniv M. Specific interaction of cellular factors with the B enhancer of polyoma virus. EMBO J. 1985 Oct;4(10):2675–2685. doi: 10.1002/j.1460-2075.1985.tb03987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Yaniv M. Molecular analysis of the interaction between an enhancer binding factor and its DNA target. Nucleic Acids Res. 1986 Dec 22;14(24):9595–9611. doi: 10.1093/nar/14.24.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piette J., Yaniv M. Two different factors bind to the alpha-domain of the polyoma virus enhancer, one of which also interacts with the SV40 and c-fos enhancers. EMBO J. 1987 May;6(5):1331–1337. doi: 10.1002/j.1460-2075.1987.tb02372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., ESTES J. D., HUEBNER R. J. Studies of mouse polyoma virus infection. 1. Procedures for quantitation and detection of virus. J Exp Med. 1959 Apr 1;109(4):379–391. doi: 10.1084/jem.109.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford R., Campbell B. A., Villarreal L. P. Genetic analysis of the enhancer requirements for polyomavirus DNA replication in mice. J Virol. 1990 Feb;64(2):476–485. doi: 10.1128/jvi.64.2.476-485.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART S. E., EDDY B. E., BORGESE N. Neoplasms in mice inoculated with a tumor agent carried in tissue culture. J Natl Cancer Inst. 1958 Jun;20(6):1223–1243. doi: 10.1093/jnci/20.6.1223. [DOI] [PubMed] [Google Scholar]
- Tang W. J., Berger S. L., Triezenberg S. J., Folk W. R. Nucleotides in the polyomavirus enhancer that control viral transcription and DNA replication. Mol Cell Biol. 1987 May;7(5):1681–1690. doi: 10.1128/mcb.7.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndall C., La Mantia G., Thacker C. M., Favaloro J., Kamen R. A region of the polyoma virus genome between the replication origin and late protein coding sequences is required in cis for both early gene expression and viral DNA replication. Nucleic Acids Res. 1981 Dec 11;9(23):6231–6250. doi: 10.1093/nar/9.23.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte M., De Somer P. Runting syndrome in mice inoculated with polyoma virus. J Natl Cancer Inst. 1965 Aug;35(2):237–250. [PubMed] [Google Scholar]
- Veldman G. M., Lupton S., Kamen R. Polyomavirus enhancer contains multiple redundant sequence elements that activate both DNA replication and gene expression. Mol Cell Biol. 1985 Apr;5(4):649–658. doi: 10.1128/mcb.5.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Imler J. L., Chatton B., Schatz C., Wasylyk C. Negative and positive factors determine the activity of the polyoma virus enhancer alpha domain in undifferentiated and differentiated cell types. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7952–7956. doi: 10.1073/pnas.85.21.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Wasylyk C., Flores P., Begue A., Leprince D., Stehelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990 Jul 12;346(6280):191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Wasylyk C., Flores P., Gutman A., Wasylyk B. PEA3 is a nuclear target for transcription activation by non-nuclear oncogenes. EMBO J. 1989 Nov;8(11):3371–3378. doi: 10.1002/j.1460-2075.1989.tb08500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk C., Imler J. L., Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. EMBO J. 1988 Aug;7(8):2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Amalfitano A., Gross R., Oldstone M. B., Fluck M. M. Organ- and age-specific replication of polyomavirus in mice. J Virol. 1992 Jun;66(6):3278–3286. doi: 10.1128/jvi.66.6.3278-3286.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W. A small segment of polyoma virus DNA enhances the expression of a cloned beta-globin gene over a distance of 1400 base pairs. Nucleic Acids Res. 1981 Dec 11;9(23):6251–6264. doi: 10.1093/nar/9.23.6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers J., Schaffner W., Tyndall C., Lupton S., Kamen R. Polyoma virus DNA replication requires an enhancer. Nature. 1984 Nov 15;312(5991):242–246. doi: 10.1038/312242a0. [DOI] [PubMed] [Google Scholar]