Abstract
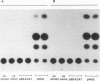
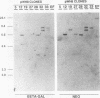
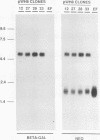
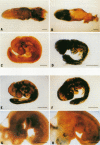
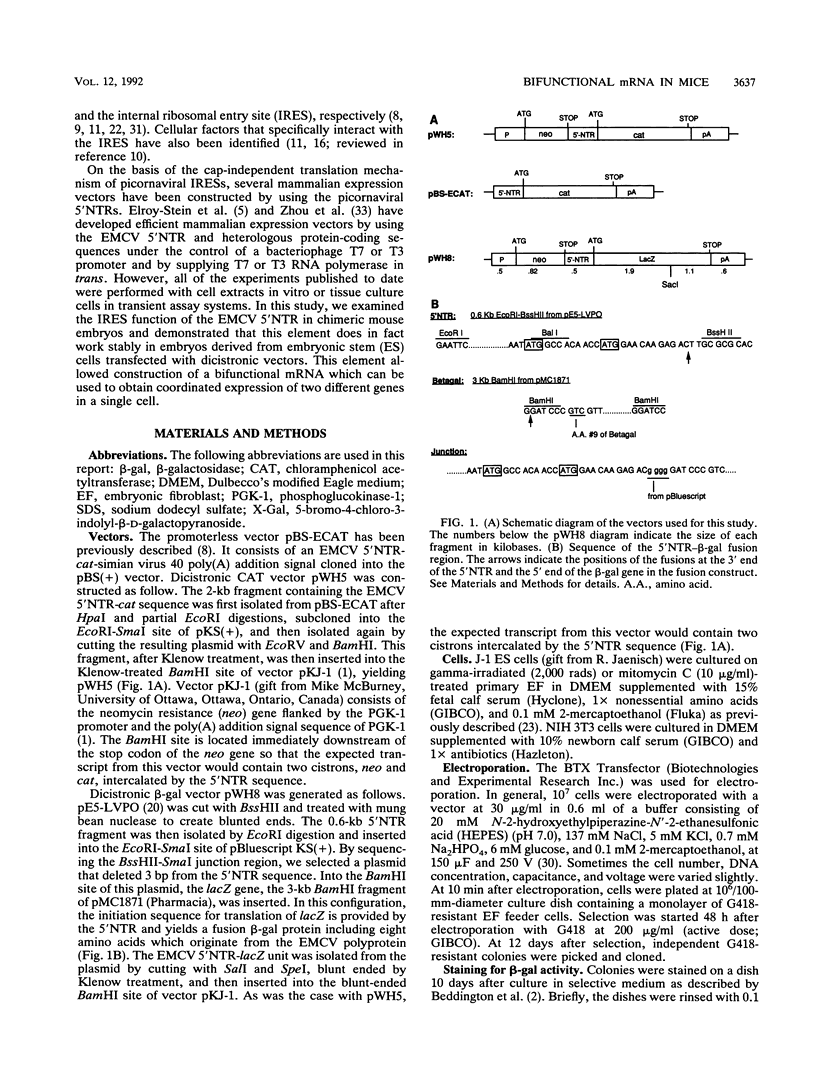
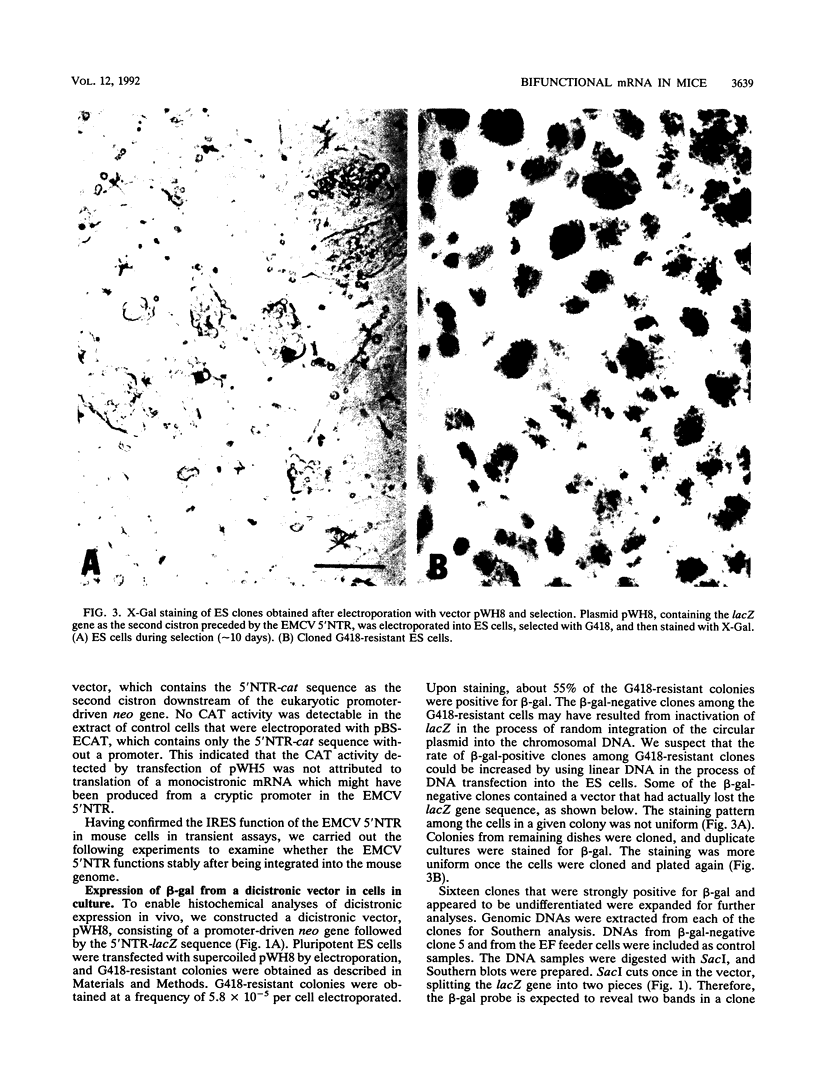
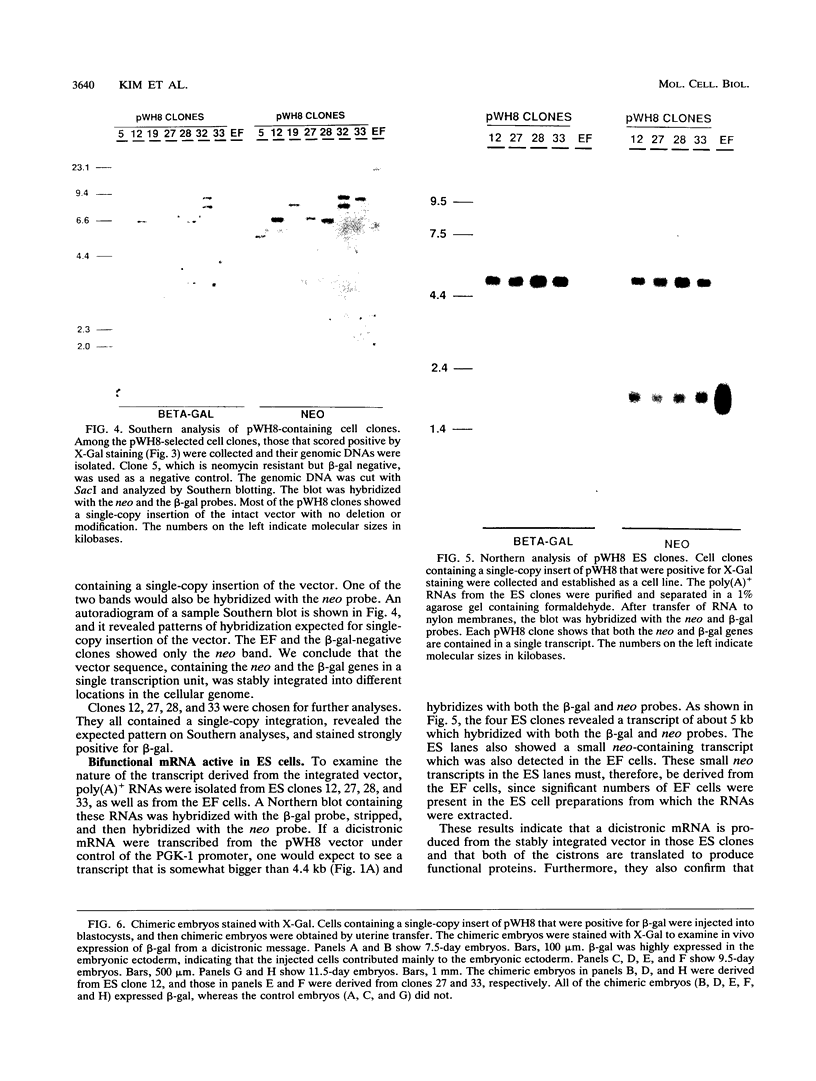
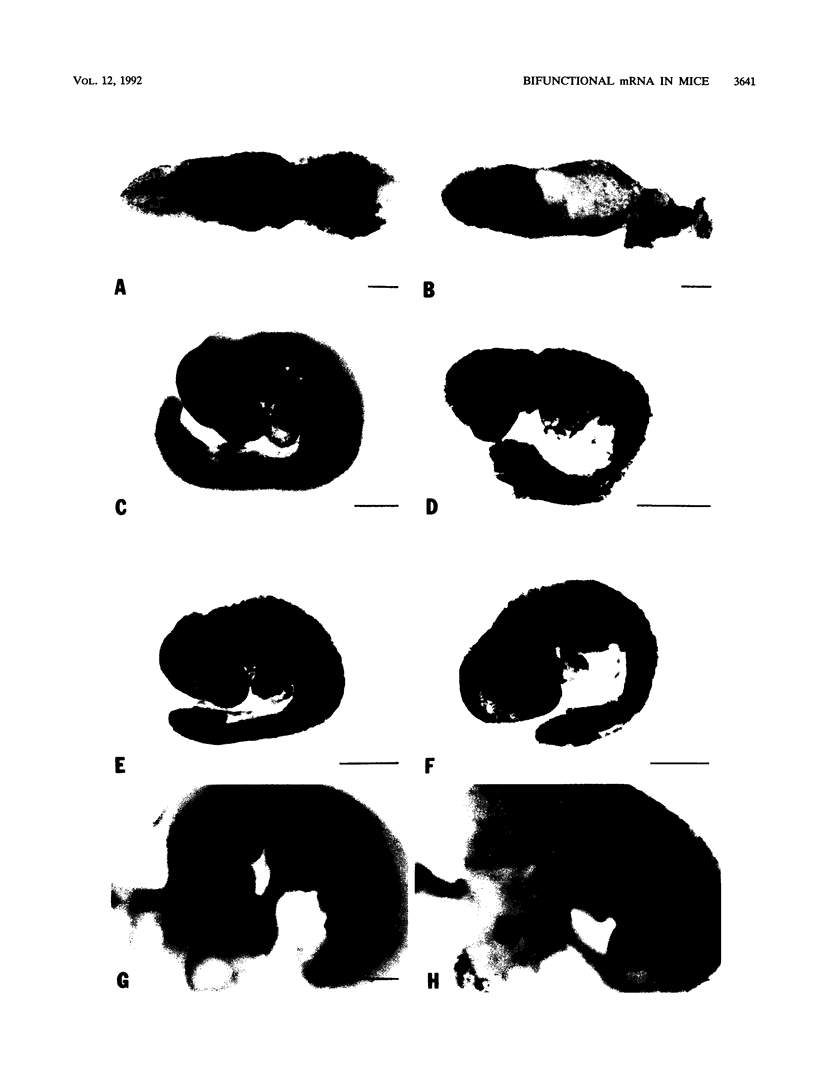
Picornaviral mRNAs have been shown to possess special structures in their 5' nontranslated regions (5'NTRs) that provide sites for internal binding of ribosomes and thus direct cap-independent translation. The translational cis-acting elements for ribosomal internal entry into the 5'NTR of encephalomyocarditis virus (EMCV), a member of family Picornaviridae, have been named the internal ribosomal entry site (IRES). All of the published experiments regarding the IRES function of the picornavirus 5'NTR, however, were performed with cell extracts in vitro or with tissue culture cells in transient assay systems. In this study, we examined the IRES function of the EMCV 5'NTR in chimeric mouse embryos and demonstrated that this element does in fact work stably in mouse embryos as well as in embryonic stem (ES) cells. By using a dicistronic vector, pWH8, consisting of a promoter-driven neomycin resistance gene (neo) followed by the EMCV 5'NTR-lacZ sequence, we showed that more than half of the ES cells made G418 resistant by the vector stained positive for beta-galactosidase (beta-gal). On Northern (RNA) blots, all of the clones analyzed revealed a transcript of the expected size containing both the beta-gal and the neo cistrons. These results indicate that dicistronic mRNAs are produced from the stably integrated vector in those ES clones and that both of the cistrons are translated to produce functional proteins. The chimeric embryos derived from these ES clones also stained positive for beta-gal, suggesting that the bifunctional mRNAs are active in the embryos. This dicistronic vector system provides a novel tool by which to obtain temporally and spatially coordinated expression of two different genes driven by a single promoter in a single cell in mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adra C. N., Boer P. H., McBurney M. W. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60(1):65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- Beddington R. S., Morgernstern J., Land H., Hogan A. An in situ transgenic enzyme marker for the midgestation mouse embryo and the visualization of inner cell mass clones during early organogenesis. Development. 1989 May;106(1):37–46. doi: 10.1242/dev.106.1.37. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5' sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghattas I. R., Sanes J. R., Majors J. E. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991 Dec;11(12):5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Pestova T. V., Hellen C. U., Witherell G. W., Wimmer E. Cap-independent translation of picornavirus RNAs: structure and function of the internal ribosomal entry site. Enzyme. 1990;44(1-4):292–309. doi: 10.1159/000468766. [DOI] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Kozak M. How do eucaryotic ribosomes select initiation regions in messenger RNA? Cell. 1978 Dec;15(4):1109–1123. doi: 10.1016/0092-8674(78)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Lee S. J. Expression of growth/differentiation factor 1 in the nervous system: conservation of a bicistronic structure. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4250–4254. doi: 10.1073/pnas.88.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macejak D. G., Sarnow P. Internal initiation of translation mediated by the 5' leader of a cellular mRNA. Nature. 1991 Sep 5;353(6339):90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- Meerovitch K., Pelletier J., Sonenberg N. A cellular protein that binds to the 5'-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989 Jul;3(7):1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- Mercola M., Goverman J., Mirell C., Calame K. Immunoglobulin heavy-chain enhancer requires one or more tissue-specific factors. Science. 1985 Jan 18;227(4684):266–270. doi: 10.1126/science.3917575. [DOI] [PubMed] [Google Scholar]
- Molla A., Jang S. K., Paul A. V., Reuer Q., Wimmer E. Cardioviral internal ribosomal entry site is functional in a genetically engineered dicistronic poliovirus. Nature. 1992 Mar 19;356(6366):255–257. doi: 10.1038/356255a0. [DOI] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Parks G. D., Duke G. M., Palmenberg A. C. Encephalomyocarditis virus 3C protease: efficient cell-free expression from clones which link viral 5' noncoding sequences to the P3 region. J Virol. 1986 Nov;60(2):376–384. doi: 10.1128/jvi.60.2.376-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Robertson E. J. Using embryonic stem cells to introduce mutations into the mouse germ line. Biol Reprod. 1991 Feb;44(2):238–245. doi: 10.1095/biolreprod44.2.238. [DOI] [PubMed] [Google Scholar]
- Rossant J., Joyner A. L. Towards a molecular-genetic analysis of mammalian development. Trends Genet. 1989 Aug;5(8):277–283. doi: 10.1016/0168-9525(89)90102-9. [DOI] [PubMed] [Google Scholar]
- Sarnow P. Translation of glucose-regulated protein 78/immunoglobulin heavy-chain binding protein mRNA is increased in poliovirus-infected cells at a time when cap-dependent translation of cellular mRNAs is inhibited. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5795–5799. doi: 10.1073/pnas.86.15.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Beer-Romero P., Brown L. G., Nussbaum R., Page D. C. ZFX has a gene structure similar to ZFY, the putative human sex determinant, and escapes X inactivation. Cell. 1989 Jun 30;57(7):1247–1258. doi: 10.1016/0092-8674(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Bargiello T. A., Clark B. T., Jackson F. R., Young M. W. An unusual coding sequence from a Drosophila clock gene is conserved in vertebrates. Nature. 1985 Oct 3;317(6036):445–448. doi: 10.1038/317445a0. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Cap-binding proteins of eukaryotic messenger RNA: functions in initiation and control of translation. Prog Nucleic Acid Res Mol Biol. 1988;35:173–207. doi: 10.1016/s0079-6603(08)60614-5. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Trono D., Andino R., Baltimore D. An RNA sequence of hundreds of nucleotides at the 5' end of poliovirus RNA is involved in allowing viral protein synthesis. J Virol. 1988 Jul;62(7):2291–2299. doi: 10.1128/jvi.62.7.2291-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmut I., Hooper M. L., Simons J. P. Genetic manipulation of mammals and its application in reproductive biology. J Reprod Fertil. 1991 Jul;92(2):245–279. doi: 10.1530/jrf.0.0920245. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Giordano T. J., Durbin R. K., McAllister W. T. Synthesis of functional mRNA in mammalian cells by bacteriophage T3 RNA polymerase. Mol Cell Biol. 1990 Sep;10(9):4529–4537. doi: 10.1128/mcb.10.9.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibert A., Selinka H. C., Elroy-Stein O., Moss B., Wimmer E. Vaccinia virus-mediated expression and identification of the human poliovirus receptor. Virology. 1991 May;182(1):250–259. doi: 10.1016/0042-6822(91)90668-2. [DOI] [PubMed] [Google Scholar]