Abstract
Large artery stiffening and small artery inflammation are both well-known pathological features of pulmonary and systemic hypertension, but the relationship between them has been seldom explored. We previously demonstrated that stiffening-induced high pulsatility flow stimulated a pro-inflammatory response in distal pulmonary artery endothelial cells (PAEC). Herein, we hypothesized that high pulsatility flow activated PAEC pro-inflammatory responses are mediated through cell structural remodeling and cytoskeletal regulation of NF-κB translocation. To test this hypothesis, cells were exposed to low and high pulsatility flows with the same mean physiological flow shear stress. Results showed that unidirectional, high pulsatility flow led to continuous, high-level NF-κB activation, whereas low pulsatility flow induced only transient, minor NF-κB activation. Compared to cell shape under the static condition, low pulsatility flow induced cell elongation with a polarity index of 1.7, while high pulsatility flow further increased the cell polarity index to a value greater than 3. To explore the roles of cytoskeletal proteins in transducing high flow pulsatility into NF-κB activation, PAECs were treated with drugs that reduce the synthesis-breakdown dynamics of F-actin or microtubules (cytochalasin D, phalloidin, nocodazole, and taxol) prior to flow. Results showed that these pre-treatments suppressed NF-κB activation induced by high pulsatility flow, but drugs changing dynamics of F-actin enhanced NF-κB activation even under low pulsatility flow. Taxol was further circulated in the flow to examine its effect on cells. Results showed that circulating taxol (10nM) reduced PAEC polarity, NF-κB activation, gene expression of pro-inflammatory molecules (ICAM-1 and VCAM-1), and monocyte adhesion on the PAECs under high pulsatility flow. Therefore, taxol effectively reduced high pulsatility flow-induced PAEC overpolarization and pro-inflammatory responses via inhibiting cytoskeletal remodeling. This study suggests that stabilizing microtubule dynamics might bea potential therapeutic means of reducing endothelial inflammation caused by high pulsatility flow.
Keywords: vascular stiffening, pulmonary vascular hypertension, inflammation, endothelial cells, high pulsatility flow, NF-kB, cytoskeleton
Introduction
It is increasingly appreciated that arterial stiffening is an important factor that determines risks of cardiovascular diseases and guides pharmaceutical treatment 1–5. Several previous studies have associated arterial stiffening with microvascular damage in kidney, brain or other highly-perfused organs.6, 7 This association was attributed to the impact of arterial stiffening on increasing flow pulsatility in downstream small arteries or even in the microvasculature, which exerts detrimental hemodynamic effects on these less deformable vessels and ultimately leading to organ dysfunction6, 8–10. Recently, arterial stiffening effects have also been recognized in the pulmonary circulation, particularly in pulmonary arterial hypertension.11–13 We previously used a compliance-adjustment system which resembles the effect produced by proximal arterial stiffening to generate unidirectional, high pulsatility flow with physiological mean flow shear stress (10–20 dyne/cm2); the magnitude of high pulsatility flow shows rapid temporal acceleration (a pulsatility index of >1 at a frequency of 1Hz) and flow waves propagate along the flow direction with no disturbance. With that system, we showed that decreased upstream compliance stimulated expression of pro-inflammatory molecules in downstream distal pulmonary artery endothelial cells.14 These observations support the idea that arterial stiffening, through changes in pulse flow characteristics, contributes to small arterial inflammation which is a characteristic finding in pulmonary arterial diseases15, 16. At present, the cellular mechanisms involved in the effects of high pulsatility flow on pro-inflammatory responses in pulmonary arterial endothelial cells (PAECs) remain unknown.
Previous studies on endothelial inflammation induced by flow magnitude or pattern provide ample evidence that translocation or activation of nuclear factor-kappa B (NF-κB), a nuclear transcription factor, plays an important role in regulating flow-mediated inflammatory responses in vascular endothelial cells17–20. Flow shear stresses, such as those with turbulent or disturbed flow pattern or low flow magnitude, activated NF-κB and NF-κB-dependent genes, contributing to endothelial inflammation.21–23 NF-κB activation upregulates endothelial genes such as ICAM-1 which playa key role in recruiting inflammatory cells.17, 24 In contrast, endothelial cells exposed to physiologically-normal flows for an extended period exhibited reduced NF-κB binding activity and inactive p65 was retained in the cytoplasm by inhibitory I-kappa-B (IkB) proteins25. Normal fluid shear forces also reduced cytokine-induced activation of NF-κB in endothelial cells, maintaining endothelial cell homeostasis 26. Though molecular signaling mechanisms involved in the flow regulation of NF-κB have been partly revealed, previous studies mainly focused on examining the effects of low or oscillatory/turbulent flow, in light of the hypothesis regarding how flow conditions changed by vessel geometry in the case of atherosclerosis lead to vascular inflammation. It is yet to be explored whether NF-κB also mediates endothelial responses to unidirectional high pulsatility flows with the physiological mean flow shear force, which is associated with the medical conditions characterized by vessel mechanical stiffening.
The mechanisms underlying how cells sense and transduce the flow signal for gene regulation have been explored using different flow conditions. Though many possibilities exist, strong evidence supports the role of the cytoskeleton as a major mechano-tranduction mediator, capable of sensing mechanical forces, transmitting and modulating tension within the cell, and transducing tension changes into intracellular signaling cascades through binding directly or indirectly to receptors.27, 28 It is also known that endothelial cytoskeleton undergoes rapid changes in response to flow 29, 30. Laminar flow induced endothelial elongation and rapid alignment of actin filament and microtubule to the direction of flow, and pulsatile flow further expedited the structural reorganization, whereas oscillatory or turbulent flow failed to polarize endothelial cells or properly organize the cytoskeletal proteins.31 The structural reorganization of cytoskeletal proteins changes intracellular signaling events32–34. The effect of flow pulsatility on endothelial remodeling remains largely unexplored; little is known about how the cytoskeleton transduces the differences in flow pulsatility in to pro-inflammatory signals in endothelial cells. Understanding the cellular and molecular mechanisms underlying the high pulsatility flow-induced endothelial inflammation is important to identify new therapeutic targets for treating stiffening-associated disease conditions. To this end, we hypothesized that high pulsatility flow activates PAEC pro-inflammatory responses through cytoskeletal remodeling and regulation on the NF-κB activation. To test this hypothesis, cells were exposed to low and high pulsatility flow conditions with physiological mean flow rates. We sought to study the effects of high pulsatility flow on cell shape, cytoskeleton reorganization and NF-κB activation in endothelial cells and to identify the drugs that reverse those effects and thus reduce high pulsatility flow-induced pro-inflammatory responses.
Materials and Methods
Cell culture
Bovine pulmonary artery endothelial cells (PAEC) were isolated from neonatal calves as previously described 35. PAECs were cultured in a growth medium (D-Valine MEM medium; Mediatech, Inc.; Herndon, VA) containing 20% fetal bovine serum (FBS, Gemini Bio-products; West Sacramento, CA), 2% L-glutamine (Invitrogen; Carlsbad, CA) and 1% penicillin/streptomycin (Invitrogen). For flow experiments, medium with 1% FBS was used. Cells at passages 4–8 were used for all experiments. Human acute monocytic leukemia cell line (THP-1) was purchased from ATCC (Manassas, VA) and maintained in 10% FBS medium. Primary monocytes or macrophages isolated from same species (bovine) showed high variability due to infrequent availability. As THP-1 was previously used to study monocyte adhesion on bovine cells36, 37, it was used here for adhesion assay.
Flow set up
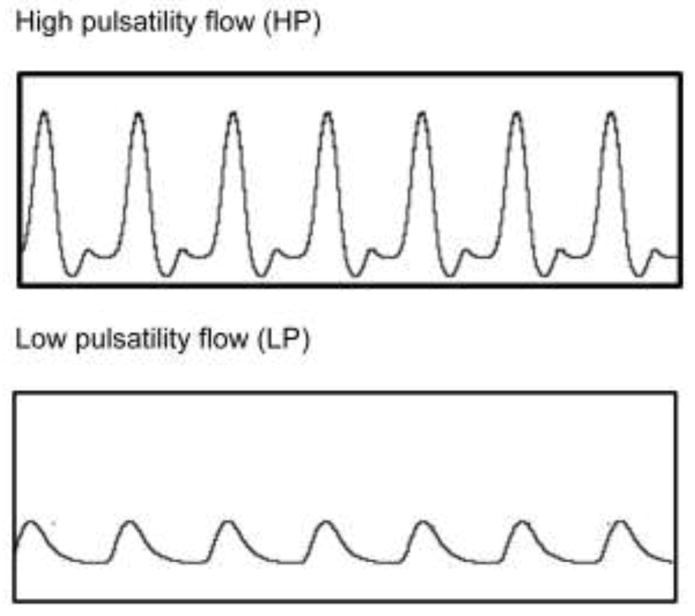
PAECs were seeded on the fibronectin-coated (25μg/ml) slides and grown to confluence. Fibronectin was chosen as a coating molecule due to its presence in the basement membrane of vascular matrix and its efficiency of retaining cells under flow. Monolayer of confluent PAECs was exposed to dampened flow (pulsatility index, PI=0.4) and high pulsatility flow (PI=1.7). Representative flow waveforms recorded are shown in Figure 1. The flow pulsatility index (PI) was defined as it is commonly used in the evaluation of vascular stiffening effects and the evaluation of vascular diseases 38, 39: PI = (Vmax − Vmin)/Vmean, where Vmax is the peak systolic velocity, Vmin is the minimum forward diastolic velocity and Vmean is the average velocity. For both flow conditions, the mean flow rate was the same with a mean shear stress of 12 dyne/cm2 applied for up to 24 hr. The mean flow shear used here is close to the mean resting flow shear stress measured in distal pulmonary artery in vivo (13 dyne/cm2).40 With a constant mean velocity and frequency, a flow condition with a higher pulsatility index denotes a higher energy level. Regarding the design of PI condition in this study, previous studies showed that the mean flow PI in the large elastic PAs ranged from 4.4 to 5.1 in vivo, while the PI in the pulmonary capillaries decreased to ~1 in vivo 41, 42. In addition, our previous study with flows of several PI conditions (1, 1.7 and 2.6) found that only flows with higher PI (1.7 and 2.6) exerted detrimental effects on endothelium while no elastic deformation existed.14 Thus, we have used a PI of 1.7 to represent high pulsatility flow condition in the distal pulmonary artery condition under the condition of pulmonary artery stiffening and a PI of 0.4 to represent normal, low pulsatility flow. For the NF-κB detection, cells were exposed to experimental flow conditions for 30 min, 1hr, 2hr, 4hr and 24hr.
Figure 1.
Representative flow waveforms of the experimental flow conditions
The flow system setup was elaborated in our previous study 14. Briefly, the flow system included a pulsatile blood pump (Harvard Apparatus, Holliston, MA) to generate mimetic flow after ventricular action, a compliance-adjustment chamber which acted as a hydraulic buffer to simulate changes in upstream arterial stiffness for creating flow with varied pulsatility, a flow chamber that held the cell culture under flow shear, and a medium reservoir for continuously recycling the medium. Flow rate was measured at the inlet and outlet of the compliance chamber with a digital flow meter (Alicat Scientific, Tuscon, AZ). The pulsatile flow waveforms for the experiments are different in their pulsatilities.
Pharmacological treatments
To explore whether cytoskeletal proteins are involved in transmitting mechanical stimulation of high pulsatility flow to activate NF-kB, we pre-incubated PAECs with four types of drugs purchased from Sigma Inc. (Saint Louis, CA): the actin-polymerization inhibitor (cytochalasin D, 0.1μM), the actin-depolymerization inhibitor (phallodin, 10nM), the microtubule-polymerization inhibitor (nocodazole, 1nM) and the microtubule-depolymerization inhibitor (taxol, 1nM) for 30min. The concentrations of these agents were pre-determined by identifying the lowest concentration that caused cytoskeletal changes under the static cell culture after drug treatment and did not induce significant cell detachment under low pulsatility flow. Cells were then exposed to high pulsatility flow for 30min and NF-κB translocation was measured at the end of flow exposure. For circulating taxol experiments, the cells were first preconditioned with dampened, low pulsatility flow for 4hr and then subjected to high pulsatility flow for 20hr. Taxol (1nM or 10nM) was dissolved in the circulating medium.
Immunofluorescent staining
After cells were exposed to flow for predetermined time intervals, slides were fixed with 4% paraformaldehyde, blocked with FBS, incubated with primary rabbit polyclonal anti-NF-κBp65 antibody (Novus Biologicals, LLC; Littleton, CO), followed by incubation with anti-rabbit IgG antibody conjugated with Cy3, and then mounted with DAPI SlowFade (Invitrogen) to detect cell nuclei. The activation of NF-κB was determined by measuring the fluorescent intensity of NF-κB signal in the nucleus versus that in the cytoplasm, because NF-κB in unstimulated cells is sequestered in the cytoplasm and activation is characterized by translocation of NF-κB from cytoplasm to the nucleus. To test the effect of different drugs on cytoskeleton structure, cells were incubated with rabbit anti-b-tubulin III (Sigma) followed by incubation with anti-rabbit IgG antibody conjugated with Cy3 to detect microtubule; or slides were incubated with phalloidin conjugated with Alexa Fluor 488 (Invitrogen) to detect F-actin stress fibers, and then mounted with DAPI. Fluorescently labeled cells were evaluated using an epifluorescent microscope.
Cell shape analysis
Cell shape or elongation under flow conditions was quantitatively determined by cell polarity. Cell polarity, defined by the polarity index, was calculated as the maximum cell length/maximum cell width ratio, and then averaged among a number of cell samples. The polarity index was determined for 20–25 cells for 3–4 independent experiments for each experimental condition.
Real-time RT-PCR
Total cellular RNA from each sample was extracted using RNeasy Mini Kit (Qiagen; Hilden, Germany). Complementary DNA was synthesized from 1μg of total cellular RNA using iScriptcDNA Synthesis Kit (Bio-Rad, Hercules, CA). PCR primers are designed using Primer 3 Software to target bovine inflammation-related adhesion molecules (ICAM-1, VCAM-1 and E-selectin) and chemokine (MCP-1). Quantitative real-time RT-PCR was performed in triplicate with iQ SYBRGreen Supermix (BioRad). Genes were normalized to the housekeeping gene hypoxanthine-xanthine phosphoribosyl transferase (HPRT). The sequences for primers were as follows: ICAM-1 (fwd 5′-GACTTCTTCAGCTCCCCAAG-3′, rev 5′-CCCACATGCTATTTGTCCTG-3′), VCAM-1 (fwd 5′-GAGCTTGGACGTGACCTTCT-3′, rev 5′-TGGGTGGAGAATCATCATCA-3′), E-selectin (fwd 5′-CTCCCCGTCCAAGAACTACA-3′, rev 5′-CGCCTCTACCTGTCCTTGAG-3′), MCP-1 (fwd 5′-CGCCTGCTGCTATACATTCA-3′, rev 5′-ACACTTGCTGCTGGTGACTC-3′), and HPRT (fwd 5′-CTGGCTCGAGATGTGATGAA-3′, rev 5′-CAACAGGTCGGCAAAGAACT-3′). The SYBR Green I assay and the iCycleriQ real-time PCR detection system (Bio-Rad MyiQ Real-Time PCR System; Hercules, CA) were used for detecting quantitative PCR products from 2 ng of reverse-transcribed cDNA. PCR thermal profile consisted of 95 °C for 10 min followed by 40 cycles of 95 °C for 15 sec, 60 °C for 30 sec and 95 °C for 1 min. Fold change relative to static condition was calculated using the ΔΔCT method 43.
Monocyte adhesion assay
To confirm pharmacological effects on PAEC expression of adhesion molecules, we examined monocyte adhesion to PAEC after PAECs were exposed to flow. Briefly, after PAECs were exposed to high pulsatility flow for 24 hr with the absence or presence of circulating taxol, monocytic THP-1 cells (5 × 105 cells/ml) were incubated with PAECs that were still attached to slides for 30 min. Non-adherent THP-1 cells were removed by gentle washing. The adherent THP-1 cells on the PAECs were visualized under microscope and pictures were taken for six randomly selected microscopic fields on each slide. The numbers of monocyte in each field were counted with the Image J software.
Data analysis
All data were expressed as means ± SEM, and n indicates the number of sample studied. Comparisons among means of the experimental groups were made using one-way ANOVA and/or two-way ANOVA (when more than 2 influencing factors were involved). If the difference was significant, then Tukey’s post-hoc test was used for comparison among several groups and a student’s t-test for one-to-one comparison. A P value < 0.05 was considered significantly different.
Results
High pulsatility flow induced early, continuous and high-level activation of NF-κB, whereas low pulsatility flow induced only minor and transient activation of NF-κB
It is known that NF-κB plays a significant role in regulating inflammatory gene expression. Since our previous study showed that high pulsatility flow stimulated proinflammatory gene expression in PAEC14, we asked whether high pulsatility flow induced NF-κB activation. Experiments were designed to examine NF-κB activation in PAECs exposed to low and high pulsatility flows, both of which had the same mean flow rates. The NF-κB activation was determined by measuring the translocation of NF-κB from the cytoplasm into the cell nucleus at varied time periods. NF-κB in unstimulated cells was sequestered in the cytoplasm. The results were compared with the cytoplasm-to-nucleus NF-κB translocation in PAECs under the static culture. Results showed that low pulsatility flow caused transient NF-κB activation in PAECs, which was then attenuated by extended flow exposure (Figure 2). The time-lapse analysis showed that after 30min of cell exposure to flow, the nucleus-to-cytoplasm ratio of NF-κB remained low, not significantly different from the static condition; after 1hr and 2hr of flow exposure, this ratio was increased by 78% and 63%, respectively; after 4hr, the NF-κB fluorescence was mainly cytoplasmic and the nucleus-to-cytoplasm ratio was not significantly different compared to the static condition. By 24hr, this ratio decreased to a level that was significantly lower than the static condition. On the other hand, high pulsatility flow with the same mean shear stress led to much higher, earlier and continuous nuclear translocation of NF-κB in PAECs. After 30min of cell exposure to high pulsatility flow, the nucleus-to-cytoplasm ratio of NF-κB in the cells increased by 400 percent compared to the static condition (P < 0.0001). This high-level activation of NF-κB was sustained and found after 1hr, 2hr and 4hr of cell exposure to high pulsatility flow. After 24hr exposure to this flow, the cells exhibited elevated nucleus-to-cytoplasm NF-κB ratios, which were more than 10 folds compared to those under 24hr of low pulsatility flow. Additionally, higher cell loss (~40% after 24hr) in high pulsatility flow condition was observed, which could trigger NF-kB activation independent of the flow condition.
Figure 2.
Low pulsatility (LP) flow induced small, transient activation of NF-kB in PAECs, while high pulsatility (HP) flow with the same mean flow rate induced much higher, constant activation of NF-kB. (A) Representative images demonstrate the cytoplasm-to-nucleus translocation (or activation) of NF-kB varies with the flow conditions over the time. NF-kB p65 is shown in red and nuclei are shown in blue fluorescence. Overlapping of both stains shows purple color. (B) Quantitative measurements of static and flow-induced NF-kB translocations over the time. “†”: Significantly different from the static condition (P<0.05); “‡”: Significantly different from the LP flow condition (P<0.05).
Flow pulsatility altered cell shape, polarity and structural organization
The above observations led to the questions regarding sensing mechanisms underlying how PAECs transduce high pulsatility flow signals to activate the nuclear factor NF-κB. As the cell structure plays an important role in transmitting mechanical forces throughout the cell and modulating assembly and activation of signaling molecule complexes, we asked whether the cell structure and shape polarity changed with the flow pulsatility level. Our results showed that cells under both flow conditions underwent significant morphological changes, exhibiting elongated shape and alignment with the flow direction, which are different from the cells in the static condition (Figure 3). Compared to those under low pulsatility flow, PAECs under high pulsatility flow were more elongated and polarized with a significantly higher polarity index. The polarity index of PAEC under low pulsatility flow is around 1.7, whereas the polarity index of PAEC under high pulsatility flow reaches a value greater than 3. Cells under high pulsatility flow also formed long projection of cytoplasmic pseudopodia.
Figure 3.
Changes of the endothelial structure and polarity with flow conditions: (A) Fluorescent images showing F-actin (green) and nuclei (blue) of the PAECs. (B) Quantitative analysis of the cell structure showing that LP flow increases the polarity index of cells when compared to the static condition and HP flow further elevates cell polarity. “†”: Significantly different from the static condition (P<0.05); “‡”: Significantly different from the LP condition (P<0.05).
Actin and microtubule dynamics mediate the transduction of high pulsatility flow into NF-κB activation in PAEC
Because the adaptation of cell structure to flow relies on major cytoskeletal protein networks including F-actin and microtubule, we further tested the hypothesis that the structural adaptation of cytoskeleton mediated the transduction of high pulsatility flow into the NF-κB activation. To delineate a possible link between NF-κB activation and cytoskeletal dynamics in response to varied flow pulsatility, PAECs were treated with drugs that interfere with the synthesis-breakdown dynamics of F-actin filaments (cytochalasin D and phalloidin) or microtubules (nocodazole and taxol) prior to flow exposure. Considering the potential effects of these drugs on cell adhesion, the drug concentrations were evaluated and set to a level at which microscopic changes in the PAEC shape started to show while drug influence on cell adhesion under the static condition and low pulsatility flow was minimized. Results on drug-treated cells showed that all the drug pre-treatments suppressed high pulsatility flow-induced NF-κB activation (Figure 4A). These results demonstrated that synthesis-breakdown dynamics of both actin and microtubule played important roles in transmitting high pulsatility flow to induce NF-κB activation. However, the drugs inhibiting F-actin dynamics increased NF-κB activation under low pulsatility flow whereas the drugs inhibiting microtubule dynamics did not. This observation showed that pretreatment of cells with ultralow concentrations of taxol or nocodazole, which were at least more than 3000 times lower than that previously used in flow studies 33,44,45, reduced NF-κB activation under high pulsatility flow without affecting that activation in cells under normal flow conditions. Gene expression results further showed that drug effects on pro-inflammatory ICAM-1 mRNA expression in PAECs under high pulsatility flow were similar to their effects on NF-κB activation (Figure 4B). Also, two-way ANOVA analysis of the data shows that flow conditions significantly influence both NF-kB and ICAM1, and there is significant interaction between the two factors, drug and flow. In addition to their effects on inflammatory signaling, cytochalasin D and nocodazole, the drugs that depolymerized F-actin and microtubule, reduced cell capability of resisting the high hemodynamic energy, eventually resulting in sloughing off cells after 24 hr, whereas the drugs that stabilized the cytoskeletal proteins, in particular taxol, did not cause significant cell loss at the experimental drug dose. Therefore, taxol was identified as a potential drug and further circulated to investigate its effects on endothelial inflammation induced by high pulsatility flow.
Figure 4.
Drugs that inhibit the synthesis-breakdown dynamics of cytoskeletal proteins, F-actin or microtubulin, reduce immediate NF-kB activation and pro-inflammatory response in PAECs under HP flow. Cells were pretreated with drugs for 30 min before exposure to flow conditions. CytoD here represents cytochalasin D. (A) Drug influences on flow-induced cytoplasm-to-nucleus translocation of NF-kB after 30min conditioning; (B) Drug influences on flow-induced cell expression of ICAM-1 mRNA. “*”: Significantly different from the untreated condition under the same flow condition (P<0.05).
Circulating taxol suppressed high pulsatility flow-induced NF-κB activation and pro-inflammatory responses via cytoskeletal and structural rearrangement
To mimic the delivery of taxol in the flow circulation when therapeutically used in vivo, taxol was added to the flow media to further evaluate its effects on NF-κB activation and pro-inflammatory responses. Taxol was circulated at a low concentration, 1nM or 10nM. We first explored the influence of circulating taxol on endothelial inflammatory responses. Results showed that circulating taxol at 10nM significantly inhibited PAEC mRNA expressions of pro-inflammatory molecules, ICAM-1 and VCAM, under high pulsatility flow, whereas circulating taxol at a lower concentration (1nM) did not significantly reduce mRNA expressions of pro-inflammatory molecules. Results also showed that taxol at 1 or 10nM did not significantly influence the pro-inflammatory gene expression in PAECs under static culture conditions (Figure 5). Two-way ANOVA analysis of the data shows that significant difference exists between static and high pulsatility flow in gene expressions of all these molecules, and there is significant interaction between two factors (i.e. taxol concentration and flow). These studies further demonstrated the role of microtubule dynamics in mechano-transduction of high pulsatility flow into pro-inflammatory signals into cells. Circulating a higher concentration (10nM) of taxol may be required to block the pro-inflammatory stimulation by high pulsatility flow.
Figure 5.
Effects of taxol circulating in the flow media on PAEC mRNA expression of pro-inflammatory molecules. The pro-inflammatory molecules include: VCAM-1 (A), ICAM-1 (B), MCP-1 (C) and E-selectin (D). Low taxol concentrations (1nM or 10nM) did not significantly affect these molecules in the static condition. Circulating 10nM of taxol significantly reduced PAEC mRNA expression of all the pro-inflammatory molecules under HP flow. “*”: Significantly different from untreated condition under the flow condition (P<0.05).
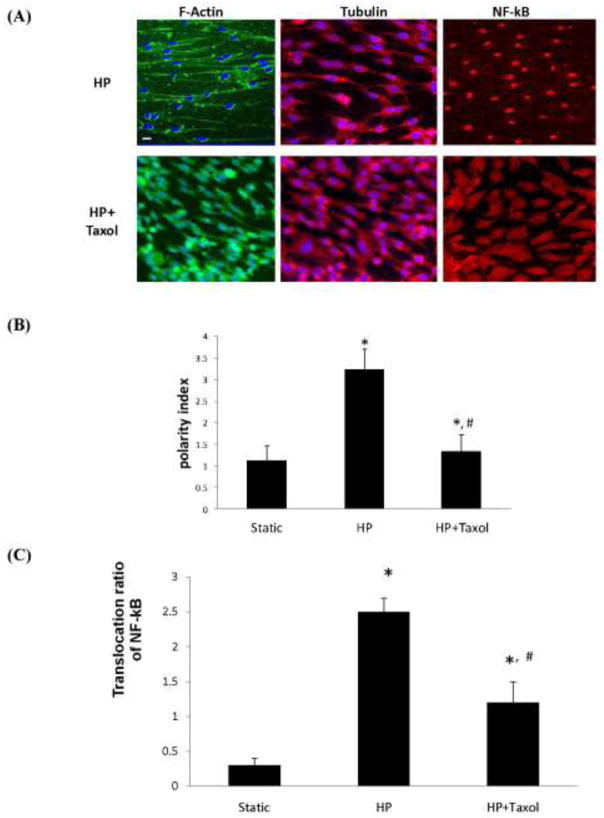
To study whether the inhibitory function of circulating taxol (10nM) on endothelial inflammation under high pulsatility flow is mediated by the effects of taxolon cell structure reorganization and NF-κB activation, immunofluorescent stains of F-actin, microtubulin and NF-κB were respectively performed on the cells under high pulsatility flow with or without the presence of taxol in the circulation media. Results showed that circulating taxol reduced the cell polarity, while cells still exhibited directional alignment with the flow direction (Figure 6A–B). Taxol also downregulated NF-κB activation under high pulsatility flow (Figure 6C).
Figure 6.
Effects of circulating taxol (10nM) on the cell structure, polarity and NF-kB translocation. (A) Fluorescent images showing F-actin (green) and nuclei (blue), microtubulin (red) and NF-kB (red) in PAECs under HP flow with the absence or presence of circulating taxol. Scale bar =10μm. (B) Quantitative analysis of the cell polarity index showing that circulating taxol significantly decreases the polarity index of cells. (C) Quantitative analysis of the NF-kB translocation showing circulating taxol significantly decreases the NF-kB activation. “*”: Significantly different from the untreated HP condition. “#”: Significantly different from the untreated static condition. (P<0.05)
To confirm the results from gene assays of pro-inflammatory molecules, we carried out the monocyte adhesion assay on the PAECs (Figure 7). Compared to the static condition, the high pulsatility flow significantly increased monocyte adhesion (P<0.05). However, circulating taxol with the high pulsatility flow decreased monocyte adhesion to PAECs, when compared to the untreated flow condition (P<0.05). Therefore, the adhesion of endothelial cells is functionally upregulated by high pulsatility flow with the absence of taxol but downregulated by 10nM of circulating taxol.
Figure 7.

Circulating taxol (10nM) inhibits monocyte adhesion on PAEC after cells under the HP flow condition. Data represent mean ± SEM, n=6 for each group. “*”: Significantly different from the untreated HP condition; “#”Significantly different from the untreated static condition. (P<0.05)
Discussion
We have demonstrated that extended perfusion of endothelial cells with low pulsatility flow, a normal physiological flow condition in small arteries or microvasculature, inhibits NF-κB activation in cells, whereas high pulsatility flow with the same mean flow rate continuously stimulates NF-κB activation over the time. The NF-κB responses to flow pulsatility, as shown here, correlated well with PAEC inflammatory responses in our previous study14. Our results on cells pre-treated with drugs that inhibit high synthesis-breakdown dynamics of cytoskeletal elements revealed that high pulsatility flow-induced NF-κB activation depended on cytoskeletal dynamics which reorganized cell structure in the presence of flow and initiated signal transduction. Taxol, for example, reduced PAEC polarity, which led to decreased NF-κB activation, proinflammatory molecule expression and monocyte adhesion on the endothelium under high pulsatility flow.
The present study is the first to show that high pulsatility flow, a unidirectional, rapidly-changing flow with a physiological mean flow shear, induces cell overpolarization leading to continuous NF-κB activation which contributes to the pro-inflammatory responses in downstream PAECs. It is well known that flow patterns critically regulate endothelial inflammation and NF-κB activation in vitro. The majority of previous studies have focused on the effects of flows with low magnitude, spatially-disturbed or reversal flow, and have established how these atherosclerosis-associated flow conditions lead to inflammatory events through the disoriented cytoskeleton 46–48. This study examined cellular mechanisms underlying a unique flow condition that is associated with vessel stiffening. Our results suggested that when exposed to high pulsatility flow induced by upstream stiffening, undeformable or less deformable small vessels were susceptible to inflammation due to cytoskeletal remodeling, cell overpolarization and sustained activation of NF-κB. Different from other flow-induced inflammatory responses, endothelial cells were highly oriented to the flow direction. Several other studies also supported the idea that if the artery has high distensibility, high pulsatility flow may actually be cytoprotective, but the same stimulus has an adverse effect in a non-compliant vessel. 49, 50 Only recently have the researchers started to couple arterial stiffening, flow pulsatility and vascular cell dysfunction in vascular-related diseases 2, 39, 51, but the studies are mostly in vivo and lack mechanistic understandings. Our study underpins the importance of stiffening-induced flow on the endothelium, and supports the idea that the stiffening-induced flow which contains excessive hemodynamic pulsatility energy induces sequential intracellular changes that activate endothelial dysfunction.
Our results on time-dependent activation of NF-κB in endothelial cells in response to low pulsatility flow are consistent with previous studies showing that flows with 10–30dyne/cm2 shear stress triggered transient activation of NF-κB in ECs and downregulated the activation at later times as cells adapted to flow33. The time-dependent changes of NF-κB activation are related to the changes in inflammatory gene expression. Being mainly atheroprotective and anti-inflammatory, these flows can also downregulate NF-κB activation and endothelial inflammation after initial cell adaptation52. Similarly, we observed 24hr exposure to low pulsatility flow led cells to 45% suppression of NF-κB activation, following a short-term activation. However, the new finding here is that high pulsatility flow resulted in higher and continuous NF-κB activation in PAECs as evidenced by greater NF-κB translocation at early time points (>5 fold increase at 30min when compared to the low pulsatility flow), and was continuously enhanced for 24hr.
It is known that the cytoskeleton supports cell shape and changes protein polymerization and depolymerization in response to extracellular mechanical forces until a new equilibrium state is reached.53,54, 55 The present study suggested that dynamics of both actin microfilaments and microtubules played important roles in mediating high pulse flow-induced inflammatory signaling pathways, through their effects on cell structural reorganization under flow. Though the role of cytoskeletal elements in high pulsatility flow-induced endothelial biology is a new finding here, our results are consistent with previous studies which found cytoskeletal elements involved in shear stress-induced cell shape, polarization, and intracellular signaling56, 57,32, 58. In particular, it was shown that pulsatile flow induced faster cell alignment and more cell elongation,31 Our results further suggest that pathologic high flow pulsatility overpolarized or oversheared endothelial cells and led to NF-kB activation and inflammatory events, whereas normal pulsatile flow that moderately polarized cells could actually protect cells. One intriguing finding here is that inhibiting the synthesis-breakdown dynamics of cytoskeletal proteins, F-actin and microtubulin, reduced NF-κB activation and pro-inflammatory response under high pulsatility flow, but inhibiting F-actin but not microtubule dynamics increased NF-κB activation (Figure 4) under low pulsatility flow. The possible reasons for such distinct responses might be due to (a) the cell polarization level; (b) distinct roles of actin and microtubule in maintaining stability of the cytoskeletal structure according to the tense grity theory53. An optimal cell polarization might exist to maintain endothelial anti-inflammation, In addition to their importance to cell structure reorganization in response to flow stimuli, the high synthesis-breakdown rates of cytoskeletal proteins are also known to be crucial to cell adhesion. Though the mean flow rates kept the same in different pulsatile flow conditions, the hemodynamic energy of high pulsatility flow was much higher than that of low pulsatility flow, thus imposing greater pulls to detach cells as energy is defined as the ability to exert pulls or pushes against forces (i.e. cell adhesion force) along a path of a certain length. According to the calculation method provided previously to quantify energy of pulsatile pressure flow waveforms59, the surplus (extra) energy contributed by the pulsatile or dynamic component of the high pulsatility flow exceed 10000 ergs/cm3. Therefore, applying cytoskeletal drugs needs to consider their effects on flow sensing as well as effects on cell adhesion.
Our results showed that taxol inhibited inflammatory response and NF-κB activation under high pulsatility flow through protecting the cells from over-shearing. In fact, the drugs used here, cytochalasin, phalloidin, nocodazole and taxol, all have known effects on anti-inflammatory responses from endothelial cells under different circumstances 45, 60, 61, and some have been used in vivo as anti-inflammatory drugs. It is tempting to speculate that taxol could exert anti-inflammatory effects in conditions where high pulsatility flow might induce and perpetuate inflammation such as pulmonary hypertension. Though several publications showed that taxol induced inflammatory signaling, our results and other studies demonstrated that the microtubule-stabilizing agent might also attenuate inflammation. Mirzapoiazova et al showed significant anti-inflammatory effects of taxol.45 It requires a much higher concentration of taxol (~30 μM) to induce inflammatory signal than that (10 nM) to stabilize microtubule. It seems that anti-inflammatory function of taxol is achieved at a much lower concentration in certain pathological circumstances; we used 1nM statically and 10nM in the circulation. Microtubule stabilization with taxol may affect the contractile cytoskeleton in a way that preserves the cell–cell junction in the endothelium.45 Similarly, taxol likely contributed to cell-cell contact integrity of the cells under high pulsatility flow challenge. In addition, recent studies have highlighted that high flow shear stress through reducing a-tubulin might be a leading cause of endothelial-to-mesenchymal transition (EndoMT), a new mechanism of vascular pathological conditions such as fibrosis.62, 63 Taxol was also shown to maintain endothelial cell structure under high shear and decreaseflow-inducedEndoMT.64–66 As the present study showed cells under high pulsatility flow elongated and exhibited a morphology closer to mesenchymal phenotype (i.e. fibroblasts) rather than normal cobble-stone endothelial morphology. It would be interesting to further explore the potential role of high pulsatility flow and taxol treatment in vascular fibrosis.
Several limitations of the study are acknowledged. First, by studying the flow pulsatility effects on isolated PAECs, the experiments did not account for the important cell-substrate interactions which play an important role in vascular mechanotransduction67. Admittedly, matrix proteins could significantly influence flow pulsatility effects on endothelial cell in vitro and in vivo. Second, we acknowledge the limitation of mimicking taxol effects in the vasculature with circulating taxol-containing media. With systemic drug administration in vivo, the taxol concentration fluctuates in the blood stream, and taxol also affects leukocyte activity.68 Thus, a closer comparison to potential medical use of taxol for treatment of pulmonary hypertension may need to involve locally-released taxol technology. Finally, as this study only employed cells from neonatal calves, the implication of the results might be limited to pediatric pulmonary hypertension conditions. Further studies need to confirm whether high pulsatility flow also has similar effects on adult calf cells or human cells.
In conclusion, our study is the first to demonstrate that high pulsatility flow induces early and continuous activation of NF-κB activation through endothelial cell overpolarization. Taxol, a microtubule-stabilizing agent, used at a low concentration effectively reduced PAEC structural reorganization, NF-κB activation, proinflammatory gene expression and monocyte adhesion.
Acknowledgments
This study was funded in part by grants from American Heart Association (SDG2110049 to W.T.), the Children’s Hospital at Denver and Colorado Clinical and Translational Science Institute (TCH and CCTSI, KL2 Award to W.T.) and the NIH (HL K25 097246 to W.T., HL-14985-36 to K.R.S.)
References
- 1.Zoungas S, Asmar RP. Arterial stiffness and cardiovascular outcome. Clin Exp Pharmacol Physiol. 2007;34:647–651. doi: 10.1111/j.1440-1681.2007.04654.x. [DOI] [PubMed] [Google Scholar]
- 2.Zachariah JP, et al. Circulating vascular growth factors and central hemodynamic load in the community. Hypertension. 2012;59:773–779. doi: 10.1161/HYPERTENSIONAHA.111.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 4.Kingwell BA, Ahimastos AA. Arterial stiffness and coronary ischemic disease. Adv Cardiol. 2007;44:125–138. doi: 10.1159/000096725. [DOI] [PubMed] [Google Scholar]
- 5.Adji A, O’Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. 2011;24:5–17. doi: 10.1038/ajh.2010.192. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto J, Ito S. Central pulse pressure and aortic stiffness determine renal hemodynamics: pathophysiological implication for microalbuminuria in hypertension. Hypertension. 2011;58:839–846. doi: 10.1161/HYPERTENSIONAHA.111.177469. [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke MF. Brain microbleeds, amyloid plaques, intellectual deterioration, and arterial stiffness. Hypertension. 2008;51:e20. doi: 10.1161/HYPERTENSIONAHA.107.109199. author reply e21. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell GF, et al. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 9.Safar ME. Peripheral pulse pressure, large arteries, and microvessels. Hypertension. 2004;44:121–122. doi: 10.1161/01.HYP.0000135448.73199.75. [DOI] [PubMed] [Google Scholar]
- 10.London G, et al. Arterial aging and arterial disease: interplay between central hemodynamics, cardiac work, and organ flow- implications for CKD and cardiovascular disease. Kidney Int Sup. 2011;1:10–12. doi: 10.1038/kisup.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobs RW, Muvarak NE, Eickhoff JC, Chesler NC. Linked mechanical and biological aspects of remodeling in mouse pulmonary arteries with hypoxia-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288:H1209–1217. doi: 10.1152/ajpheart.01129.2003. [DOI] [PubMed] [Google Scholar]
- 12.Hunter K, et al. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–174. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorgulu S, et al. A new echocardiographic approach in assessing pulmonary vascular bed in patients with congenital heart disease: pulmonary artery stiffness. Anadolu Kardiyol Derg. 2003;3:92–97. [PubMed] [Google Scholar]
- 14.Li M, Scott DE, Shandas R, et al. High pulsatility flow induces adhesion molecule and cytokine mRNA expression in distal pulmonary artery endothelial cells. Ann Biomed Eng. 2009;37:1082–1092. doi: 10.1007/s10439-009-9684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Maki-Petaja KM, Wilkinson IB. Anti-inflammatory drugs and statins for arterial stiffness reduction. Curr Pharm Des. 2009;15:290–303. doi: 10.2174/138161209787354221. [DOI] [PubMed] [Google Scholar]
- 17.Orr AW, Hahn C, Blackman BR, Schwartz MA. p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaik SS, Soltau TD, Chaturvedi G, et al. Low intensity shear stress increases endothelial ELR+ CXC chemokine production via a focal adhesion kinase-p38{beta} MAPK-NF-{kappa}B pathway. J Biol Chem. 2009;284:5945–5955. doi: 10.1074/jbc.M807205200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis ME, Grumbach IM, Fukai T, et al. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J Biol Chem. 2004;279:163–168. doi: 10.1074/jbc.M307528200. [DOI] [PubMed] [Google Scholar]
- 20.Petzold T, Orr AW, Hahn C, et al. Focal adhesion kinase modulates activation of NF-kappaB by flow in endothelial cells. Am J Physiol Cell Physiol. 2009;297:C814–822. doi: 10.1152/ajpcell.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–1224. doi: 10.1152/ajpheart.01047.2006. [DOI] [PubMed] [Google Scholar]
- 22.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714–1718. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 23.Dai G, Kaazempur-Mofrad MR, Natarajan S, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monaco C, Paleolog E. Nuclear factor kappaB: a potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc Res. 2004;61:671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 25.Chiu JJ, Chen LJ, Chang SF, et al. Shear stress inhibits smooth muscle cell-induced inflammatory gene expression in endothelial cells: role of NF-kappaB. Arterioscler Thromb Vasc Biol. 2005;25:963–969. doi: 10.1161/01.ATV.0000159703.43374.19. [DOI] [PubMed] [Google Scholar]
- 26.Eng E, Ballermann BJ. Diminished NF-kappaB activation and PDGF-B expression in glomerular endothelial cells subjected to chronic shear stress. Microvasc Res. 2003;65:137–144. doi: 10.1016/s0026-2862(03)00004-9. [DOI] [PubMed] [Google Scholar]
- 27.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol. 2001;281:L529–533. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 28.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. Faseb J. 2006;20:811–827. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 29.Barbee KA, Mundel T, Lal R, Davies PF. Subcellular distribution of shear stress at the surface of flow-aligned and nonaligned endothelial monolayers. Am J Physiol. 1995;268:H1765–1772. doi: 10.1152/ajpheart.1995.268.4.H1765. [DOI] [PubMed] [Google Scholar]
- 30.Helmke BP, Rosen AB, Davies PF. Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys J. 2003;84:2691–2699. doi: 10.1016/S0006-3495(03)75074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsiai TK, et al. Endothelial cell dynamics under pulsating flows: significance of high versus low shear stress slew rates (d (tau)/dt) Ann Biomed Eng. 2002;30:646–656. doi: 10.1114/1.1484222. [DOI] [PubMed] [Google Scholar]
- 32.Cheng M, Wu J, Liu X, et al. Low shear stress-induced interleukin-8 mRNA expression in endothelial cells is mechanotransduced by integrins and the cytoskeleton. Endothelium. 2007;14:265–273. doi: 10.1080/10623320701678169. [DOI] [PubMed] [Google Scholar]
- 33.Imberti B, Morigi M, Zoja C, et al. Shear stress-induced cytoskeleton rearrangement mediates NF-kappaB-dependent endothelial expression of ICAM-1. Microvasc Res. 2000;60:182–188. doi: 10.1006/mvre.2000.2260. [DOI] [PubMed] [Google Scholar]
- 34.Miao H, et al. Effects of flow patterns on the localization and expression of VE-cadherin at vascular endothelial cell junctions: in vivo and in vitro investigations. J Vasc Res. 2005;42:77–89. doi: 10.1159/000083094. [DOI] [PubMed] [Google Scholar]
- 35.Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis. 2008;11:169–182. doi: 10.1007/s10456-007-9087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeom M, et al. Lactoferrin inhibits the inflammatory and angiogenic activation of bovine aortic endothelial cells. Inflamm Res. 2011;60:475–482. doi: 10.1007/s00011-010-0294-1. [DOI] [PubMed] [Google Scholar]
- 37.Tsao PS, Lewis NP, Alpert S, Cooke JP. Exposure to shear stress alters endothelial adhesiveness. Role of nitric oxide. Circulation. 1995;92:3513–3519. doi: 10.1161/01.cir.92.12.3513. [DOI] [PubMed] [Google Scholar]
- 38.Panaritis V, et al. Pulsatility index of temporal and renal arteries as an early finding of arteriopathy in diabetic patients. Ann Vasc Surg. 2005;19:80–83. doi: 10.1007/s10016-004-0134-2. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GF, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang BT, et al. Three-dimensional hemodynamics in the human pulmonary arteries under resting and exercise conditions. Ann Biomed Eng. 2011;39:347–358. doi: 10.1007/s10439-010-0124-1. [DOI] [PubMed] [Google Scholar]
- 41.Paz R, Mohiaddin RH, Longmore DB. Magnetic resonance assessment of the pulmonary arterial trunk anatomy, flow, pulsatility and distensibility. Eur Heart J. 1993;14:1524–1530. doi: 10.1093/eurheartj/14.11.1524. [DOI] [PubMed] [Google Scholar]
- 42.Reuben SR. Compliance of the human pulmonary arterial system in disease. Circ Res. 1971;29:40–50. doi: 10.1161/01.res.29.1.40. [DOI] [PubMed] [Google Scholar]
- 43.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 44.Katoh K, Noda Y. Distribution of cytoskeletal components in endothelial cells in the Guinea pig renal artery. Int J Cell Biol. 2012;2012:439349. doi: 10.1155/2012/439349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirzapoiazova T, Kolosova IA, Moreno L, et al. Suppression of endotoxin-induced inflammation by taxol. Eur Respir J. 2007;30:429–435. doi: 10.1183/09031936.00154206. [DOI] [PubMed] [Google Scholar]
- 46.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39–40. 41–33. [PubMed] [Google Scholar]
- 47.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 48.Passerini AG, Polacek DC, Shi C, et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng X, Haldar S, Deshpande S, Irani K, Kass DA. Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension. 2003;41:378–381. doi: 10.1161/01.hyp.0000049624.99844.3d. [DOI] [PubMed] [Google Scholar]
- 50.Qiu Y, Tarbell JM. Interaction between wall shear stress and circumferential strain affects endothelial cell biochemical production. J Vasc Res. 2000;37:147–157. doi: 10.1159/000025726. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Partridge J, et al. Laminar shear stress acts as a switch to regulate divergent functions of NF-kappaB in endothelial cells. FASEB J. 2007;21:3553–3561. doi: 10.1096/fj.06-8059com. [DOI] [PubMed] [Google Scholar]
- 53.Ingber DE. Tensegrity and mechanotransduction. J Bodyw Mov Ther. 2008;12:198–200. doi: 10.1016/j.jbmt.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buxbaum RE, Heidemann SR. A thermodynamic model for force integration and microtubule assembly during axonal elongation. J Theor Biol. 1988;134:379–390. doi: 10.1016/s0022-5193(88)80068-7. [DOI] [PubMed] [Google Scholar]
- 55.Putnam AJ, Schultz K, Mooney DJ. Control of microtubule assembly by extracellular matrix and externally applied strain. Am J Physiol Cell Physiol. 2001;280:C556–564. doi: 10.1152/ajpcell.2001.280.3.C556. [DOI] [PubMed] [Google Scholar]
- 56.McCue S, Dajnowiec D, Xu F, et al. Shear stress regulates forward and reverse planar cell polarity of vascular endothelium in vivo and in vitro. Circ Res. 2006;98:939–946. doi: 10.1161/01.RES.0000216595.15868.55. [DOI] [PubMed] [Google Scholar]
- 57.Loufrani L, Henrion D. Role of the cytoskeleton in flow (shear stress)-induced dilation and remodeling in resistance arteries. Med Biol Eng Comput. 2008;46:451–460. doi: 10.1007/s11517-008-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vartanian KB, Berny MA, McCarty OJ, et al. Cytoskeletal structure regulates endothelial cell immunogenicity independent of fluid shear stress. Am J Physiol Cell Physiol. 2010;298:C333–341. doi: 10.1152/ajpcell.00340.2009. [DOI] [PubMed] [Google Scholar]
- 59.Undar A, et al. Precise quantification of pressure flow waveforms of a pulsatile ventricular assist device. ASAIO J. 2005;51:56–59. doi: 10.1097/01.mat.0000150326.51377.a0. [DOI] [PubMed] [Google Scholar]
- 60.Salu KJ, Bosmans JM, Huang Y, et al. Effects of cytochalasin D-eluting stents on intimal hyperplasia in a porcine coronary artery model. Cardiovasc Res. 2006;69:536–544. doi: 10.1016/j.cardiores.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 61.Redd MJ, Kelly G, Dunn G, et al. Imaging macrophage chemotaxis in vivo: studies of microtubule function in zebrafish wound inflammation. Cell Motil Cytoskeleton. 2006;63:415–422. doi: 10.1002/cm.20133. [DOI] [PubMed] [Google Scholar]
- 62.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am J Pathol. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 64.Egorova AD, van der Heiden K, Poelmann RE, Hierck BP. Primary cilia as biomechanical sensors in regulating endothelial function. Differentiation. 2012;83:S56–61. doi: 10.1016/j.diff.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Hierck BP, et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 66.Egorova AD, et al. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res. 2011;108:1093–1101. doi: 10.1161/CIRCRESAHA.110.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res. 2009;104:995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li T, Hu J, Thomas JA, Li L. Differential induction of apoptosis by LPS and taxol in monocytic cells. Mol Immunol. 2005;42:1049–1055. doi: 10.1016/j.molimm.2004.09.032. [DOI] [PubMed] [Google Scholar]