Abstract
Purpose
Neoadjuvant pemetrexed plus cisplatin was administered, followed by extrapleural pneumonectomy (EPP) and hemithoracic radiation (RT), to assess the feasibility and efficacy of trimodality therapy in stage I to III malignant pleural mesothelioma.
Patients and Methods
Requirements included stage T1-3 N0-2 disease, no prior surgical resection, adequate organ function (including predicted postoperative forced expiratory volume in 1 second ≥ 35%), and performance status 0 to 1. Patients received pemetrexed 500 mg/m2 plus cisplatin 75 mg/m2 for four cycles. Patients without disease progression underwent EPP followed by RT (54 Gy). The primary end point was pathologic complete response (pCR) rate.
Results
Seventy-seven patients received chemotherapy. All four cycles were administered to 83% of patients. The radiologic response rate was 32.5% (95% CI, 22.2 to 44.1). Fifty-seven patients proceeded to EPP, which was completed in 54 patients. Three pCRs were observed (5% of EPP). Forty of 44 patients completed irradiation. Median survival in the overall population was 16.8 months (95% CI, 13.6 to 23.2 months; censorship, 33.8%). Patients completing all therapy had a median survival of 29.1 months and a 2-year survival rate of 61.2%. Radiologic response of complete or partial response was associated with a median survival of 26.0 months compared with 13.9 months for patients with stable disease or progressive disease (P = .05).
Conclusion
This multicenter trial showed that trimodality therapy with neoadjuvant pemetrexed plus cisplatin is feasible with a reasonable long-term survival rate, particularly for patients who completed all therapy. Radiologic response to chemotherapy, but not sex, histology, disease stage, or nodal status, was associated with improved survival.
INTRODUCTION
Malignant pleural mesothelioma (MPM) is a rare, locally aggressive disease of poor prognosis and increasing incidence within industrialized nations.1–3 Among patients with stage I to III disease, there is currently no universally accepted treatment approach. The diffuse thoracic involvement characteristic of MPM renders surgical4 and radiotherapeutic5 approaches ineffective when used independently. Although extrapleural pneumonectomy (EPP) is a preferred surgical option at institutions specializing in MPM, median survival among patients receiving EPP alone is less than 10 months.6,7
Adjuvant hemithoracic radiation has been studied as a way of improving local control after EPP, especially because a higher dose of radiation can be achieved without risk of pneumonitis, as the lung has been removed. Memorial Sloan-Kettering Cancer Center investigators conducted a prospective trial in which patients underwent EPP (n = 54) followed by high-dose (54 Gy) external-beam radiation therapy (RT) to the ipsilateral hemithorax.8 This therapy resulted in a dramatic reduction in local relapse and prolonged survival in patients with early-stage disease. Local relapse occurred in two patients, local and distant relapse occurred in five patients, and distant only relapse occurred in 30 patients. Median survival was 33.8 months for stages I and II and 10 months for stages III and IV (P = .04). EPP followed by adjuvant chemoradiotherapy was studied at Brigham and Women's Hospital (Boston, MA) in 183 patients with MPM.9 The perioperative mortality rate was 3.8%. Survival in the remaining 176 patients was 38% at 2 years and 15% at 5 years, and the median survival was 19 months.
The concept of neoadjuvant chemotherapy in MPM was extrapolated from results of patients with stage IIIA non–small-cell lung cancer and based on the difficulty of administering adjuvant therapy to patients undergoing pneumonectomy.10 Initially, the combination of gemcitabine and cisplatin was identified as an active regimen in MPM,11 and this led to two trials using this regimen as neoadjuvant therapy before EPP and RT.12,13 Ultimately, pemetrexed (Alimta; Eli Lilly, Indianapolis, IN) and cisplatin became the standard first-line regimen for MPM based on a phase III trial showing that it improved survival over treatment with cisplatin alone.14 Thus we chose pemetrexed and cisplatin as the induction chemotherapy regimen before EPP and RT in this multicenter trial testing the feasibility and efficacy of trimodality therapy for stage I to III MPM.
PATIENTS AND METHODS
Eligibility Criteria
Patients with histologically confirmed MPM and clinical stage I to III disease (T1-3, N0-2, M0) were included in this study.15 Positron emission tomography scan and mediastinoscopy were not mandated, but could be performed at the discretion of the treating physician. Patients were required to be age ≥ 18 years of age and have an Eastern Cooperative Oncology Group performance status of 0 to 1. Before enrollment, consultation with a thoracic surgeon was required to determine EPP suitability. Respiratory reserve was determined by pulmonary function tests and quantitative ventilation/perfusion scans, and patients needed a predicted postoperative forced expiratory volume in 1 second ≥ 35%. Cardiac function and absence of coronary artery disease was documented by radionucleotide or echocardiographic stress testing. Patients were also required to have adequate organ and bone marrow function, including an estimated creatinine clearance ≥ 45 mL/min (estimated by Cockcroft and Gault).16
Exclusion criteria included prior systemic chemotherapy, prior surgical resection of mesothelioma (with the exception of chemical pleurodesis), prior RT, serious concomitant disorders, and second primary malignancy. The institutional review board of each study site approved the protocol before enrolling patients. This study was performed in compliance with the principles of good clinical practice, the Helsinki Declaration, and federal and institutional guidelines.
Treatment Plan
Neoadjuvant chemotherapy consisted of pemetrexed 500 mg/m2 plus cisplatin 75 mg/m2 once every 21 days for four cycles. Folic acid supplementation, 400 to 1,000 μg or equivalent, was administered beginning 1 to 2 weeks before the first dose of pemetrexed and continued daily until therapy discontinuation. Vitamin B12 1,000 μg was administered 1 to 2 weeks before the first dose of study therapy and repeated every 9 weeks until therapy discontinuation. Dexamethasone was administered for 3 days each cycle, beginning 1 day before pemetrexed dosing. Standard dose adjustments were required for hematologic, renal, and neural toxicity.
All patients underwent attempted EPP between 3 and 8 weeks after the completion of chemotherapy, unless there was objective evidence of disease progression or significant deterioration of functional status. EPP consisted of en-bloc removal of the pleura, lung, diaphragm, and pericardium in all patients without gross invasion of the chest wall or other vital structures.17 Every effort was made to carry out a complete resection. The diaphragmatic and pericardial defects were reconstructed with absorbable (Dexon; U.S. Surgical, Norwalk, CT) or nonabsorbable (preferably Gore-Tex; W.L. Gore & Associates, Flagstaff, AZ) patches as required. Mediastinal lymph node dissection was performed for staging purposes. This included levels 4 and 10 on the right side and levels 5 and 6 on the left side. In both cases, levels 7 (subcarinal lymph nodes) were removed during mobilization of the mainstem bronchus. Additional lymph nodes at levels 8, 9, and in the internal mammary region were identified and biopsied if possible. When exploration at thoracotomy revealed unresectable disease, the operation was aborted.
Patients with resectable disease received adjuvant hemithoracic RT starting 4 to 12 weeks (preferably within 8 weeks) after EPP. A total of 54 Gy in 30 fractions of 1.8 Gy per day were administered. Intensity-modulated RT was allowed by the protocol. After 22 fractions totaling 39.6 Gy, the spinal cord (and therefore the mediastinum) was removed from the field. From the beginning of treatment, Lipowitz's alloy blocks were used to shield the stomach, kidney, heart, and liver from photon irradiation. Blocked areas were treated with electron radiation with customized lead cut-outs conforming exactly to the size of the blocked areas projected onto the skin surface. Interruptions or delays were permitted only for febrile neutropenia or grade 3 and 4 esophagitis, mucositis, or pneumonitis. On completion of all therapy, patients had computed tomography scans 1 month after completion of irradiation and then every 3 months for up to 2 years to monitor for recurrence.
Study End Points and Statistical Considerations
The primary end point of this study was the pathologic complete response (pCR) rate, which was evaluated at the time of EPP. The planned enrollment of this study was 77 patients. This assumed that up to 25% of patients would be lost to follow-up or attrition before surgery. Using a one-sided exact binomial test (with α = 0.025), a sample size of 60 patients would provide 80% power to test whether the true pathologic complete response (pCR) rate was ≤ 1% (H0), versus a true pCR rate of 7% (HA).
Radiologic response rate was assessed by modified Response Evaluation Criteria in Solid Tumors criteria, which have been validated in mesothelioma.18 Secondary end points of this study included overall survival (OS) and progression-free survival (PFS); these were analyzed using Kaplan-Meier methods19 and were censored at the date of the last follow-up visit for patients who were still alive and had not experienced disease progression. Chemotherapy toxicity was evaluated using National Cancer Institute Common Toxicity Criteria version 2.0.20 Surgical morbidity was any Common Toxicity Criteria grade 3 or 4 event within 30 days of surgery. RT safety was evaluated using Acute Radiation Morbidity criteria.21
RESULTS
Patients
Patient disposition is summarized in Figure 1. Between September 17, 2003, and March 15, 2006, 82 patients were screened for eligibility at nine centers in the United States. Five patients were screen failures and did not participate. The remaining 77 patients who received at least one dose of chemotherapy were included in the intent-to-treat (ITT) population. Of these, 57 patients proceeded to EPP (completed in 54 patients), 44 patients started RT, and 40 patients completed all therapy.
Fig 1.
Patient disposition.
Baseline demographics are listed in Table 1. Patients had a median age of 63 years. Most patients were men (72.7%) and white (92.2%) and had epithelial histology (80.5%). One patient with stage IV disease was treated and included in the ITT population in violation of the protocol.
Table 1.
Baseline Demographics and Clinical Characteristics of Intent-to-Treat Population
| Characteristic | No. of Patients (N = 77) | % |
|---|---|---|
| Age, years | ||
| Median | 63.0 | |
| Range | 34-78 | |
| Sex | ||
| Male | 56 | 72.7 |
| Female | 21 | 27.3 |
| Race | ||
| White | 71 | 92.2 |
| Other | 6 | 7.8 |
| ECOG PS | ||
| 0 | 28 | 36.4 |
| 1 | 47 | 61.0 |
| 2 | 2 | 2.6 |
| Histology | ||
| Epithelial | 62 | 80.5 |
| Mixed | 2 | 2.6 |
| Sarcomatoid | 1 | 1.3 |
| Indeterminate | 12 | 15.6 |
| Clinical stage | ||
| IA | 3 | 3.9 |
| IB | 3 | 3.9 |
| II | 33 | 42.9 |
| III | 35 | 45.5 |
| IV | 1 | 1.3 |
| Unavailable | 2 | 2.6 |
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Chemotherapy
Patients in the study received a total of 278 cycles of chemotherapy (mean, 3.6 cycles; median, 4.0 cycles). Sixty-four patients (83.1%) received the protocol-specified four cycles of treatment. Five patients (6.5%) received a dose reduction. Mean dose-intensity was 97.9% for pemetrexed (range, 73.1% to 116.0%) and 97.5% for cisplatin (range, 72.2% to 116.7%).
Radiologic response to chemotherapy included one patient with complete response (1.3%), 24 patients with partial response (31.2%), 36 patients with stable disease (46.8%), five patients with progressive disease (6.5%), and 11 patients with unknown or unavailable response (14.3%). The overall response rate was 32.5% (95% CI, 22.2 to 44.1). Response did not vary by clinical stage. Chemotherapy toxicity is summarized in Table 2. Grade 3 or 4 neutropenia occurred in 3.9% of patients and febrile neutropenia occurred in 2.6% of patients. Notable grade 3 or 4 nonhematologic toxicities included pneumonia (5.2%), chest pain (3.9%), and pulmonary embolism (2.6%).
Table 2.
Chemotherapy Toxicity (N = 77)
| Toxicity | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Hematologic | ||||||||
| Anemia | 8 | 10.4 | 8 | 10.4 | 1 | 1.3 | 0 | 0 |
| Febrile neutropenia | — | — | 1 | 1.3 | 1 | 1.3 | ||
| Neutropenia | 2 | 2.6 | 3 | 3.9 | 2 | 2.6 | 1 | 1.3 |
| Thrombocytopenia | 2 | 2.6 | 1 | 1.3 | 0 | 0 | 0 | 0 |
| Nonhematologic | ||||||||
| Alopecia | 7 | 9.1 | 1 | 1.3 | — | — | ||
| Constipation | 30 | 39.0 | 15 | 19.5 | 0 | 0.0 | 0 | 0.0 |
| Chest pain | 3 | 3.9 | 4 | 5.2 | 2 | 2.6 | 1 | 1.3 |
| Dehydration | 4 | 5.2 | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 |
| Dyspnea | 3 | 3.9 | 7 | 9.1 | 2 | 2.6 | 0 | 0.0 |
| Fatigue | 29 | 37.7 | 21 | 27.3 | 1 | 1.3 | 0 | 0.0 |
| Nausea | 42 | 54.5 | 14 | 18.2 | 1 | 1.3 | 0 | 0.0 |
| Pneumonia | 0 | 0.0 | 0 | 0.0 | 4 | 5.2 | 0 | 0.0 |
| Pulmonary embolism | — | — | — | 2 | 2.6 | |||
| Rash | 18 | 23.4 | 0 | 0.0 | — | — | ||
| Vomiting | 20 | 26.0 | 12 | 15.6 | 2 | 2.6 | 0 | 0.0 |
Surgery and RT
Of the 57 patients considered for surgery, 54 patients underwent EPP (27 right-sided and 27 left-sided), for a surgical completion rate of 94.7% (70.1% of ITT population). Three patients (5.3% of EPP population, 95% CI, 1% to 15%) had a pCR. Compared with initial clinical stage, pathologic stage at time of surgery was improved for 15.8%, unchanged for 42.1%, worsened for 28.1%, and unknown for 14.0%. Two patients with a pCR had a survival time less than the median (one death as a result of respiratory failure and one death as a result of progressive disease); however, one patient with a pCR (a 61-year-old male with stage IA disease at study entry) was alive at 25 months at the time of study conclusion. Two patient deaths occurred within 30 days of EPP (one bronchopleural fistula and one sepsis). The most common grade 3 or 4 adverse events after surgery included atrial fibrillation (10.5% of EPP), pain (7.0%), dyspnea (5.3%), anemia (5.5%), and sepsis (3.5%).
Among the 44 patients receiving RT, median dose was 45.9 Gy (range, .28 to 60 Gy). Radiation pneumonitis occurred in two patients, causing one fatality in a patient who received intensity-modulated RT. Grade 3 acute Radiation Therapy Oncology Group toxicities included two patients with upper gastrointestinal toxicity, one patient with larynx toxicity, one patient with skin toxicity, and one patient with lung toxicity.
Survival, PFS, and Recurrence
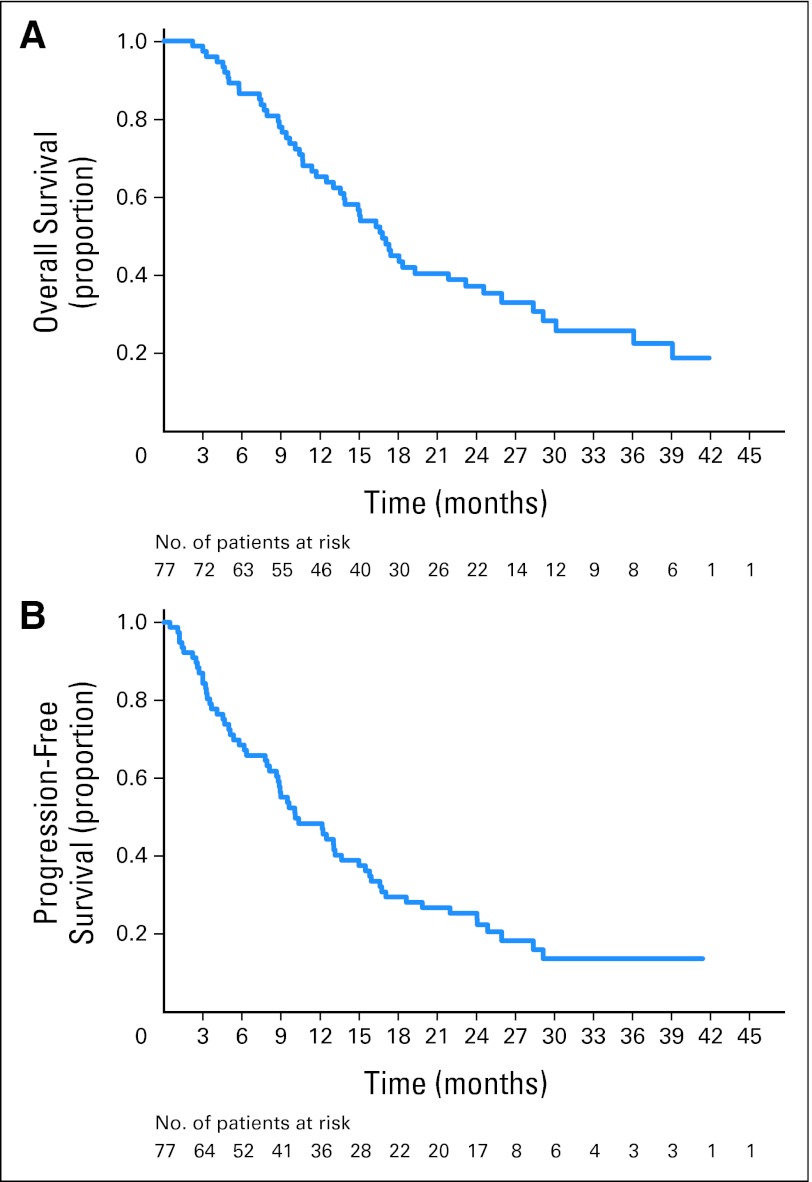
OS and PFS from this study are summarized in Figure 2. Median survival in the ITT population was 16.8 months (95% CI, 13.6 to 23.2 months; censorship, 33.8%); 1-year survival was 65.2%, and 2-year survival was 37.2%. Median PFS was 10.1 months (95% CI, 8.6 to 15.0 months; censorship, 19.5%); PFS rates were 48.4% at 1 year (95% CI, 36.8% to 59.1%) and 25.5% at 2 years (95% CI, 16.2% to 35.7%). Median survival for individual patient subgroups is summarized in Table 3. Among those who underwent EPP, median survival was 21.9 months (95% CI, 16.8 to 29.1 months). Among patients who completed RT, median survival was 29.1 months, 1-year survival was 90.0%, and 2-year survival was 61.2%. On the basis of a univariate subgroup analysis, radiologic response of complete or partial response was associated with a median survival of 26.0 months compared with 13.9 months for patients with stable disease or progressive disease (P = .05) in the ITT population, though no difference was seen in the EPP or RT populations. Survival was not significantly different by histology, nodal status, sex, or clinical stage. Twenty-three patients in the EPP population (40.4%) had documented recurrent disease (eight local only, 12 metastatic only, and three local and metastatic). The most common sites of relapse were pleural effusion (n = 7), pleural rind (n = 6), lung (n = 5), and lymph nodes (n = 2). Median time to relapse was 18.3 months (95% CI, 11.99 months to not estimable). Relapse-free rates among EPP patients were 63.8% at 1 year and 38.9% at 2 years.
Fig 2.
(A) Kaplan-Meier overall survival, intent-to-treat (ITT) population (median, 16.8 months; 95% CI, 3.6 to 23.2 months). (B) Kaplan-Meier progression-free survival, ITT population (median, 10.1 months; 95% CI, 8.6 to 15.0 months).
Table 3.
Median Survival by Patient Subgroup
| Factor | ITT Population (N = 77) |
EPP Population (N = 57) |
RT Completed (N = 40) |
|||
|---|---|---|---|---|---|---|
| Months | No. of Patients in Subgroup | Months | No. of Patients in Subgroup | Months | No. of Patients in Subgroup | |
| Overall survival, months | ||||||
| Median | 16.8 | 21.9 | 29.1 | |||
| 95% CI | 13.6 to 23.2 | 16.8 to 29.1 | 19.3 to — | |||
| By histology | ||||||
| Epithelial | 17.4 | 62 | 24.6 | 47 | 36.1 | 34 |
| Other histology | 13.8 | 15 | 17.5 | 10 | 20.8 | 6 |
| By nodal status | ||||||
| N0 | 17.1 | 45 | 24.6 | 32 | 28.4 | 24 |
| N1 or N2 | 16.6 | 20 | 18.1 | 15 | 29.1 | 9 |
| By radiologic response | ||||||
| CR or PR | 26.0* | 25 | 26.0 | 24 | 30.1 | 17 |
| SD or PD | 13.9* | 41 | 16.8 | 26 | 28.4 | 18 |
| By sex | ||||||
| Male | 16.8 | 56 | 19.3 | 40 | 28.4 | 27 |
| Female | 17.3 | 21 | 26.0 | 17 | 30.1 | 13 |
| By initial clinical stage | ||||||
| I or II | 17.3 | 39 | 24.6 | 29 | 28.4 | 23 |
| III or IV | 16.8 | 36 | 19.3 | 26 | NA | 16 |
Abbreviations: ITT, intent to treat; EPP, extrapleural pneumonectomy; RT, radiation therapy; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NA, not assessable.
Denotes survival differences between strata with log-rank P ≤ .05.
DISCUSSION
The optimal treatment for fit patients with stage I to III MPM remains a matter of debate. Because most patients with MPM present at the time of diagnosis with disease confined to the hemithorax, local therapies, such as surgical resection, seem appropriate. However, many surgeons advocate against surgery for this disease. Even with a marked reduction in surgical morbidity from EPP performed by experienced surgeons, relapse rates remain unacceptably high. To lower the risk of relapse in the thoracic cavity, hemithoracic radiation has been used. Adequate radiation doses are achievable because the lung is surgically absent, and this technique improves local control rates.8 However, survival remains poor because patients develop metastatic disease, primarily to the contralateral pleura or lung, or the peritoneum. Once a chemotherapy regimen was identified that demonstrated a reasonable level of activity in MPM (gemcitabine and cisplatin), the logical step was to add chemotherapy to the surgery and radiation to treat the disease systemically. Several studies using gemcitabine and cisplatin along with surgery and radiation in a combined-modality approach for resectable MPM have been reported, and those results are summarized in Table 4.13,22–25 Ultimately, pemetrexed and cisplatin emerged as the standard first-line regimen for MPM, and that chemotherapy regimen was selected for the induction therapy in this trial. To our knowledge, this is the largest prospective trial of multimodality therapy for early-stage MPM reported, and it was conducted by specialized centers in the United States.
Table 4.
Trimodality With Extrapleural Pneumonectomy and Radiation Therapy for Malignant Pleural Mesothelioma
| Author | No. of Patients | Stage of Disease | Chemotherapy Regimens Used | No. With EPP | % of ITT | Key Results |
|---|---|---|---|---|---|---|
| Weder et al13 | 19 | I-III | Neoadjuvant gemcitabine 1,000 mg/m2 days 1, 8, 15 plus cisplatin 80 mg/m2 day 1, 28-day cycle × 3 | 16 | 84 | RR = 32%, OS = 23 months for ITT |
| Flores et al22 | 19 | III-IV | Neoadjuvant gemcitabine 1,250 mg/m2 days 1, 8 plus cisplatin 75 mg/m2 day 8, 21-day cycle × 4 | 8 | 42 | PR = 26%, SD = 32%, OS = 19 months for ITT |
| Weder et al23 | 61 | I-III | Neoadjuvant gemcitabine 1000 mg/m2 days 1, 8, 15 plus cisplatin 80 mg/m2 day 1, 28-day cycle X 3 | 45 | 74 | OS = 19.8 months for ITT and 23 months for EPP |
| Rea et al24 | 21 | I-III | Neoadjuvant gemcitabine 1,000 mg/m2 days 1, 8, 15 plus carboplatin AUC 5 day 1, 28-day cycle × 3 | 17 | 81 | PR = 33%, SD = 67%, OS = 25.5 months for ITT |
| Batirel et al25 | 20 | I-III | Adjuvant gemcitabine 1,250 mg/m2 days 1, 8 plus cisplatin 75 mg/m2 day 1, 21-day cycle × 3. After 2005, adjuvant pemetrexed 500 mg/m2 day 1 plus cisplatin 75 mg/m2 day 1 × 3 | 16 | 80 | OS = 17.2 months for ITT and 23.9 months for EPP |
| Current study | 77 | I-III | Neoadjuvant pemetrexed 500 mg/m2 day 1 plus cisplatin 75 mg/m2 day 1 × 4 | 54 | 70 | pCR = 5.3%, RR = 32.5%, OS = 16.8 months for ITT and 21.9 months for EPP |
Abbreviations: EPP, extrapleural pneumonectomy; ITT, intent to treat; RR, response rate; OS, overall survival; PR, partial response; SD, stable disease; AUC, area under the curve; pCR, pathologic complete response.
This study demonstrates the feasibility of this trimodality approach using pemetrexed and cisplatin as a neoadjuvant chemotherapy regimen. We chose to administer the chemotherapy before surgery to improve tolerance (because many patients tolerate chemotherapy poorly after pneumonectomy) and to allow assessment for response. We observed an excellent rate of chemotherapy delivery; 83% of patients completed all four planned cycles of induction therapy. Similarly, a high rate of chemotherapy delivery before surgery was noted in the study by Weder et al,23 in which 95% of patients completed three cycles of gemcitabine/cisplatin. The toxicities of chemotherapy, which were rarely severe, were comparable to those reported in the prior phase III trial among patients receiving vitamin supplementation.14 Neoadjuvant chemotherapy did not impact surgical risk. The surgical mortality rate of 3.7% and the rate of other surgical complications such as atrial fibrillation were in line with previous reports. The toxicity from hemithoracic radiation also compared favorably with prior reports. Pneumonitis was a major concern, particularly after reports emerged of fatal pneumonitis when intensity-modulated RT techniques were used in the adjuvant setting after EPP,26 and one patient died from this in the current study.
Pemetrexed and cisplatin was efficacious as an induction regimen. The primary end point of the study, pCR, was met, with three patients (5%) obtaining a pCR. This did not confer long-term survival to two patients, although one patient with a pCR was alive at 25 months when this study concluded. The observed radiologic response rate was 33%. Although this seems slightly less than the 41% response rate reported in the phase III trial, response is particularly difficult to assess in this population with early-stage mesothelioma. Many patients on this study presented with just a thin rim of pleural thickening or loculated fluid collections, complicating our ability to measure response. Other means of assessment may add to our ability to monitor chemotherapy activity, such as positron emission tomography scans (which were not mandated in this study, but were performed in a proportion of patients) or serum markers, such as soluble mesothelin-related protein27 or osteopontin.28
The median survival for all patients enrolled onto this trial was 16.8 months, which was lower than the survival of 19.8 months reported in the only other multicenter trial as reported by Weder et al.23 Comparison of results across trials should be made cautiously, because outcomes in this setting may be particularly influenced by the effects of patient selection.23,29 When only patients evaluated for EPP are considered, median survival was 21.9 months. Reports of trimodality therapy that have focused exclusively on the subset of patients undergoing EPP have generally described survival in the range of 20 to 26 months.9,22,23,25,30,31 One limitation of the current study may be that a mediastinoscopy was not required for staging, and data regarding how many patients underwent one was not captured.
Despite this disappointing median survival, the 2-year survival was 37%, and approximately 20% of patients were estimated to survive more than 3 years. This suggests that a subgroup of patients is more likely to benefit from this aggressive approach. To categorize those patients, we performed an exploratory subgroup analysis. Previous reports in surgically managed MPM have identified factors such as disease histology, sex, and nodal status to be associated with survival outcomes. Within the ITT population of the current study, univariate analysis of patient subgroups indicated that radiologic response, but not other factors, was associated with improved survival.25,32 Complete or partial response was associated with nearly twice the median survival when compared with stable or progressive disease (26.0 months to 13.9 months; P = .05).
So is this multimodality treatment program an acceptable approach for patients with early-stage MPM? We believe it is, but put forward several caveats. These patients were highly selected on the basis of their stage of disease, their performance status, and their cardiopulmonary reserve. Furthermore, they were managed at centers that treat high volumes of patients with mesothelioma. As just described, the treatment algorithm with induction chemotherapy, EPP, and then hemithoracic radiation is feasible and effective, but only a subgroup of patients experience long survival. Perhaps response to chemotherapy is one surrogate for selecting optimal patients, which additionally argues for its use before surgery, but this would need to be validated in other studies. Gene profiling of tumors has also been proposed as a method for determining prognosis and selecting appropriate patients for surgery.33
Future and ongoing studies will additionally evaluate the role of surgery in this disease. A retrospective analysis of surgical databases from three institutions suggests that survival for patients undergoing pleurectomy/decortication were similar to those for patients undergoing EPP.34 Perhaps this less-aggressive surgery is adequate, though the challenges of radiating the pleura with the lung intact after surgery would likely result in higher rates of pneumonitis. Taking the question about surgery to the next level, the Medical Research Council is conducting the Mesothelioma and Radical Surgery trial, which randomly assigns patients after chemotherapy to EPP or no surgery. Until these issues are sorted out, however, the approach outlined in this study is reasonable for this select group of fit patients with early-stage MPM.
Acknowledgment
We thank the following investigators for their participation in this study: David Jablons, University of California at San Francisco; Mark Krasna, St Joseph Medical Center, Towson, MD; and Julie Brahmer, Sidney Kimmel Cancer Center at Johns Hopkins University, Baltimore, MD.
Footnotes
Supported by Eli Lilly.
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, May 13-17, 2005, Orlando, FL; and 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trials repository link available on JCO.org.
Clinical trial information can be found for the following: NCT00087698.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Matthew Monberg, Eli Lilly (C); Coleman K. Obasaju, Eli Lilly (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: Harvey I. Pass, Eli Lilly; Hedy L. Kindler, Eli Lilly Research Funding: Lee M. Krug, Eli Lilly; Hedy L. Kindler, Eli Lilly; Nicholas J. Vogelzang, Eli Lilly Expert Testimony: None Other Remuneration: Hedy L. Kindler, Eli Lilly
AUTHOR CONTRIBUTIONS
Conception and design: Lee M. Krug, Harvey I. Pass, Valerie W. Rusch, Hedy L. Kindler, David J. Sugarbaker, Kenneth E. Rosenzweig, Raja Flores, Joseph S. Friedberg, Katherine Pisters, Coleman K. Obasaju, Nicholas J. Vogelzang
Financial support: Coleman K. Obasaju
Administrative support: Matthew Monberg
Provision of study materials or patients: Lee M. Krug, Harvey I. Pass, Valerie W. Rusch, Hedy L. Kindler, David J. Sugarbaker, Kenneth E. Rosenzweig, Raja Flores, Joseph S. Friedberg, Katherine Pisters, Nicholas J. Vogelzang
Collection and assembly of data: Lee M. Krug, Hedy L. Kindler, David J. Sugarbaker, Matthew Monberg, Coleman K. Obasaju
Data analysis and interpretation: Lee M. Krug, Harvey I. Pass, Valerie W. Rusch, Hedy L. Kindler, David J. Sugarbaker, Kenneth E. Rosenzweig, Matthew Monberg, Coleman K. Obasaju, Nicholas J. Vogelzang
Manuscript writing: Lee M. Krug, Harvey I. Pass, Matthew Monberg, Coleman K. Obasaju
Final approval of manuscript: Lee M. Krug, Harvey I. Pass, Valerie W. Rusch, Hedy L. Kindler, David J. Sugarbaker, Kenneth E. Rosenzweig, Raja Flores, Joseph S. Friedberg, Katherine Pisters, Matthew Monberg, Coleman K. Obasaju, Nicholas J. Vogelzang
REFERENCES
- 1.Peto J, Decarli A, La Vecchia C, et al. The European mesothelioma epidemic. Br J Cancer. 1999;79:666–672. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol. 1997;145:211–218. doi: 10.1093/oxfordjournals.aje.a009093. [DOI] [PubMed] [Google Scholar]
- 3.Zellos L, Christiani DC. Epidemiology, biologic behavior, and natural history of mesothelioma. Thorac Surg Clin. 2004;14:469–477. doi: 10.1016/j.thorsurg.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 4.van Ruth S, Baas P, Zoetmulder FA. Surgical treatment of malignant pleural mesothelioma: A review. Chest. 2003;123:551–561. doi: 10.1378/chest.123.2.551. [DOI] [PubMed] [Google Scholar]
- 5.Baldini EH. External beam radiation therapy for the treatment of pleural mesothelioma. Thorac Surg Clin. 2004;14:543–548. doi: 10.1016/S1547-4127(04)00108-2. [DOI] [PubMed] [Google Scholar]
- 6.Pass HI, Kranda K, Temeck BK, et al. Surgically debulked malignant pleural mesothelioma: Results and prognostic factors. Ann Surg Oncol. 1997;4:215–222. doi: 10.1007/BF02306613. [DOI] [PubMed] [Google Scholar]
- 7.Rusch VW, Venkatraman E. The importance of surgical staging in the treatment of malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 1996;111:815–825. doi: 10.1016/s0022-5223(96)70342-2. discussion 825-826. [DOI] [PubMed] [Google Scholar]
- 8.Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;122:788–795. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 9.Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J Thorac Cardiovasc Surg. 1999;117:54–63. doi: 10.1016/s0022-5223(99)70469-1. discussion 63-65. [DOI] [PubMed] [Google Scholar]
- 10.Pisters KM, Ginsberg RJ, Giroux DJ, et al. Induction chemotherapy before surgery for early-stage lung cancer: A novel approach—Bimodality Lung Oncology Team. J Thorac Cardiovasc Surg. 2000;119:429–439. doi: 10.1016/s0022-5223(00)70120-6. [DOI] [PubMed] [Google Scholar]
- 11.Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: A phase II study. J Clin Oncol. 1999;17:25–30. doi: 10.1200/JCO.1999.17.1.25. [DOI] [PubMed] [Google Scholar]
- 12.Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: A phase II trial. J Thorac Oncol. 2006;1:289–295. [PubMed] [Google Scholar]
- 13.Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol. 2004;22:3451–3457. doi: 10.1200/JCO.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 14.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer. AJCC Cancer Staging Handbook. New York, NY: Springer Verlag; 2002. [Google Scholar]
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Rusch V. Mesothelioma and less common pleural tumors. In: Pearson F, Cooper J, Deslauriers J, editors. Thoracic Surgery. ed 2. Philadelphia, PA: Chuchill Livingstone; 2002. pp. 1241–1263. [Google Scholar]
- 18.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15:257–260. doi: 10.1093/annonc/mdh059. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.National Cancer Institute. NCI Common Toxicity Criteria Version 2.0. Bethesda, MD: National Cancer Institute Cancer Therapy Evaluation Program; 1999. [Google Scholar]
- 21.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 22.Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol. 2007;2:957–965. doi: 10.1097/JTO.0b013e31815608d9. [DOI] [PubMed] [Google Scholar]
- 23.Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol. 2007;18:1196–1202. doi: 10.1093/annonc/mdm093. [DOI] [PubMed] [Google Scholar]
- 24.Rea F, Marulli G, Bortolotti L, et al. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): Feasibility and results. Lung Cancer. 2007;57:89–95. doi: 10.1016/j.lungcan.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol. 2008;3:499–504. doi: 10.1097/JTO.0b013e31816fca1b. [DOI] [PubMed] [Google Scholar]
- 26.Allen AM, Czerminska M, Janne PA, et al. Fatal pneumonitis associated with intensity-modulated radiation therapy for mesothelioma. Int J Radiat Oncol Biol Phys. 2006;65:640–645. doi: 10.1016/j.ijrobp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362:1612–1616. doi: 10.1016/S0140-6736(03)14794-0. [DOI] [PubMed] [Google Scholar]
- 28.Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 29.Jänne PA, Baldini EH. Patterns of failure following surgical resection for malignant pleural mesothelioma. Thorac Surg Clin. 2004;14:567–573. doi: 10.1016/j.thorsurg.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 30.de Perrot M, Uy K, Anraku M, et al. Impact of lymph node metastasis on outcome after extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2007;133:111–116. doi: 10.1016/j.jtcvs.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Stewart DJ, Martin-Ucar A, Pilling JE, et al. The effect of extent of local resection on patterns of disease progression in malignant pleural mesothelioma. Ann Thorac Surg. 2004;78:245–252. doi: 10.1016/j.athoracsur.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 32.Rusch VW, Venkatraman ES. Important prognostic factors in patients with malignant pleural mesothelioma, managed surgically. Ann Thorac Surg. 1999;68:1799–1804. doi: 10.1016/s0003-4975(99)01038-3. [DOI] [PubMed] [Google Scholar]
- 33.Sugarbaker DJ, Richards WG, Gordon GJ, et al. Transcriptome sequencing of malignant pleural mesothelioma tumors. Proc Natl Acad Sci U S A. 2008;105:3521–3526. doi: 10.1073/pnas.0712399105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J Thorac Cardiovasc Surg. 2008;135:620–626. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]