Abstract
Purpose
To evaluate patient-specific immunotherapy with mitumprotimut-T (idiotype keyhole limpet hemocyanin [Id-KLH]) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in CD20+ follicular lymphoma.
Patients and Methods
Patients with treatment-naive or relapsed/refractory disease achieving a complete response (CR), partial response (PR), or stable disease (SD) with four weekly rituximab infusions were randomly assigned to mitumprotimut-T/GM-CSF or placebo/GM-CSF, with doses given monthly for six doses, every 2 months for six doses, and then every 3 months until disease progression (PD). Randomization was stratified by prior therapy (treatment-naive or relapsed/refractory) and response to rituximab (CR/PR or SD). The primary end point was time to progression (TTP) from randomization.
Results
A total of 349 patients were randomly assigned; median age was 54 years, 79% were treatment naive, and 86% had stage III/IV disease. Median TTP was 9.0 months for mitumprotimut-T/GM-CSF and 12.6 months for placebo/GM-CSF (hazard ratio [HR] = 1.384; P = .019). TTP was comparable between the two arms in treatment-naive patients (HR = 1.196; P = .258) and shorter with mitumprotimut-T/GM-CSF in relapsed/refractory disease (HR = 2.265; P = .004). After adjusting for Follicular Lymphoma International Prognostic Index (FLIPI) scores, the difference in TTP between the two arms was no longer significant. Overall objective response rate, rate of response improvement, and duration of response were comparable between the two arms. Toxicity was similar in the two arms; 76% of adverse events were mild or moderate, and 94% of patients had injection site reactions.
Conclusion
TTP was shorter with mitumprotimut-T/GM-CSF compared with placebo/GM-CSF. This difference was possibly due to the imbalance in FLIPI scores.
INTRODUCTION
Despite progress in the treatment of advanced follicular B-cell lymphoma, most patients experience recurrences. The induction of an active immune response to patient-specific tumor antigens could result in more durable remissions and improve treatment outcome.
B cells express a surface immunoglobulin with a specific idiotype (Id) that is unique to each B-cell clone. Because B-cell lymphoma arises from the clonal expansion of a single B cell, the Id protein expressed by the predominant malignant clone could serve as a patient-specific target for active immunotherapy. Early studies have demonstrated that patients with indolent B-cell lymphoma can mount anti-Id immune responses after immunization with patient-specific Id proteins, and durable clinical responses could be achieved in patients first placed into remission with chemotherapy.1,2 To augment the immunogenicity of the Id protein, it has been mixed with chemical adjuvants or conjugated to keyhole limpet hemocyanin (KLH), a strong immunogenic protein, to form an Id-KLH complex.2 Furthermore, the immunomodulatory cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF) has been coadministered with Id-KLH to increase the proportion of immune responders.2,3
Mitumprotimut-T (Specifid; Favrille, San Diego, CA) is a patient-specific Id-KLH therapeutic vaccine in which the Id protein is produced by a proprietary recombinant technology. A phase II trial conducted in 32 patients with relapsed follicular B-cell lymphoma has shown that mitumprotimut-T plus GM-CSF without preceding debulking therapy led to a 15% response rate and durable remissions.4 A subsequent phase II trial investigated mitumprotimut-T plus GM-CSF after rituximab in follicular lymphoma. An objective response was achieved in 27 (77%) of 35 treatment-naive patients and 28 (52%) of 54 patients with relapsed/refractory disease. The event-free survival curves seemed to plateau at 4 years at 40% in treatment-naive patients and 17% in relapsed/refractory disease.5,6 This phase III trial was conducted to confirm these favorable preliminary findings.
PATIENTS AND METHODS
Eligibility
Patients with histologically confirmed CD20+ follicular lymphoma WHO grade 1 to 3 were eligible if they were at least 18 years of age, had an Eastern Cooperative Oncology Group performance status of 0 to 1, granulocytes ≥ 1,500/μL, platelets ≥ 75,000/μL, and hemoglobin ≥ 10 g/dL. Patients had to be candidates for rituximab therapy (ie, be treatment-naive, have experienced relapse after chemotherapy, or have experienced relapse after a response to rituximab more than 6 months). Patients had to have bidimensionally measurable disease and a lymph node accessible for biopsy to produce mitumprotimut-T. Previously treated patients were ineligible if they had received more than two systemic lymphoma therapies (rituximab/chemotherapy given simultaneously were considered a single regimen), more than six courses of fludarabine or any fludarabine within 9 months, rituximab/chemotherapy within 2 years, an anti-CD20–radiolabeled antibody, Id-KLH, or high-dose therapy with stem-cell transplantation. Patients were ineligible if they had a known allergy to GM-CSF, were receiving concurrent immunosuppressive therapy, had a history of CNS lymphoma, were HIV positive, were pregnant or nursing women, or had a serious nonmalignant disease that would compromise protocol objectives.
Procedures and Study Drug Administration
Institutional review boards approved the study at all sites. After signed informed consent was obtained, patients underwent a lymph node biopsy to produce their Id-KLH vaccine.4 Eligible patients received rituximab at 375 mg/m2 weekly for 4 weeks and underwent tumor restaging 2 months later. Patients with stable disease (SD), partial response (PR), or complete response (CR) at restaging were randomly assigned to receive mitumprotimut-T or placebo. Random assignment occurred regardless of successful production of mitumprotimut-T and was performed centrally on a 1:1 schedule using balanced blocks of four, with stratification by prior treatment (treatment-naive v relapsed/refractory disease) and response to rituximab therapy (CR/PR v SD). Mitumprotimut-T (0.5 mg of Id and 0.5 mg of KLH) or placebo was given on day 1 and GM-CSF (Leukine, sargramostim; Bayer HealthCare, Montville, NJ) was given at 250 μg daily on days 1 to 4 of each course. To ensure the integrity of treatment blinding, study drug and placebo prepared at Favrille were sent to an independent distributor that shipped the appropriate vials to clinical sites. Blinded study drug (1 mL) and GM-CSF (0.5 to 1.0 mL) were given subcutaneously, with GM-CSF administered close to the blinded drug injection site. Courses were repeated monthly for six doses, every 2 months for six doses, and then every 3 months until evidence of progressive disease (PD) or unacceptable toxicity was observed.
Computed tomography (CT) scans of the neck, chest, abdomen, and pelvis were obtained at entry and repeated every 3 months for the first 2 years and then every 6 months. On discontinuation of treatment or at the date of data cutoff, copies of all patient CT scans were rendered anonymous and submitted for review by a central radiology group (Synarc, San Francisco, CA). Two radiologists reviewed the CT scans independently to determine disease response and a third radiologist adjudicated discordant cases; all three radiologists were blinded to treatment assignment and clinical outcomes. Characterization of sites of nodal involvement for determination of the Follicular Lymphoma International Prognostic Index (FLIPI) was also performed by central radiology.7 Disease response was assessed by the investigators to manage patients and determine response to rituximab before random assignment and for patient stratification.
Disease response was defined using modified International Workshop Group response criteria.8 Objective responses had to be confirmed at least 4 weeks later. An unconfirmed (u) response was downgraded to the next lower stage (ie, CR/CRu to PR and PR to SD). To qualify for CR, patients had to have a documented negative bone marrow biopsy. For this analysis, patients with CRu are reported as CR. Criteria for PD were met when a new lesion was noted, the sum of the product of the perpendicular diameters (PPD) of all abnormal lymph nodes (SPD) increased by ≥ 50% from previous nadir, the PPD of any single lymph node increased by ≥ 50% from previous nadir, or any previously abnormal lymph node that had returned to normal size increased to more than 1.5 cm in its longest transverse diameter, or to more than 1.0 cm in its longest transverse diameter if the cross-perpendicular diameter was more than 1.0 cm and less than 1.5 cm. Blood samples for immune assays were obtained at baseline and before each blinded study drug course and assays were to be performed as previously described.4
Sample Size Calculation and Statistical Methods
It was calculated that 342 patients had to be randomly assigned to detect a TTP hazard ratio (HR) of 1.545 for control versus mitumprotimut-T, with α = .01, 1-β = .78, and an estimated median TTP of 12.5 and 19.3 months in the control and mitumprotimut-T arms, respectively. The study was to be unblinded when at least 248 PDs were observed or after a total trial duration of 36 months (18 months for patient enrollment and 18 months of follow-up). An unblinded interim efficacy analysis was to be performed by an independent data monitoring board using the secondary efficacy end point of rate of response improvement (RRI) defined below, with the option of terminating the trial if the interim analysis showed a robust result in favor of the treatment group.9 The level of significance for the RRI interim analysis was set using the O'Brien-Fleming stopping rule boundary at 0.005 for the interim analysis and 0.048 for the final analysis.
The intent-to-treat population consisted of all randomly assigned patients. The efficacy-assessable population consisted of randomly assigned patients who received at least one dose of blinded study drug and had both a baseline and at least one follow-up CT scan assessment. The primary efficacy end point was TTP for all patients and for patient subsets according to stratification factors and was measured from the date of random assignment to the date of first documentation of PD, initiation of another therapy for lymphoma, or death as a result of lymphoma. Patients who had not experienced PD at the time of study analysis or who were lost to follow-up were censored at their last CT scan evaluation. Secondary end points included objective response rate (ORR), RRI (defined as the percentage of patients with SD or a PR after rituximab whose response subsequently improved to a PR or CR), duration of response, and safety. Comparisons of time-to-event variables between the two groups in the intent-to-treat population, patient subsets, and posthoc analyses were performed by Cox regression model adjusting for the two stratification factors. Statistical comparisons were two-sided. The study database was locked before unblinding, and all analyses were performed by the sponsor (Favrille).
RESULTS
Patient Disposition and Characteristics
Between July 2004 and January 2006, 495 patients were assessed for eligibility and 364 patients were enrolled and received rituximab. Fifteen patients withdrew during or after rituximab therapy and were not eligible for random assignment, 14 patients because of PD and one patient because of an adverse event. Thus the intent-to-treat population consists of 349 patients: 174 patients randomly assigned to mitumprotimut-T and 175 patients randomly assigned to placebo. Thirty-four randomly assigned patients did not receive blinded study drug, 28 patients because mitumprotimut-T could not be produced, five patients because of PD before start of blinded study drug, and one patient because of withdrawal for personal reasons (Fig 1).
Fig 1.
Patient disposition. GM-CSF, granulocyte-macrophage colony-stimulating factor; Id-KLH, idiotype keyhole limpet hemocyanin; PD, progressive disease.
Demographics and disease status at entry were comparable between the two groups (Table 1). The median age was 54 years (range, 21 to 86 years), 57% were male, 85% had an Eastern Cooperative Oncology Group performance status of 0, 93% had follicular lymphoma WHO grade 1 or 2, 86% had stage III to IV disease, and 79% were treatment naive. There was an imbalance in the distribution of FLIPI risk scores between the two treatment arms, with more high-risk FLIPI patients randomly assigned to mitumprotimut-T and more low-risk FLIPI patients randomly assigned to placebo, and this imbalance was most significant in patients with relapsed/refractory disease.
Table 1.
Patient and Disease Characteristics at Baseline
| Characteristic | Mitumprotimut-T (n = 174) |
Placebo (n = 175) |
||
|---|---|---|---|---|
| No. | %* | No. | %* | |
| Age, years | ||||
| Median | 55.8 | 53.0 | ||
| Range | 22-86 | 21-81 | ||
| < 65 | 137 | 79 | 148 | 85 |
| ≥ 65 | 37 | 21 | 27 | 15 |
| Sex | ||||
| Female | 77 | 44 | 74 | 42 |
| Male | 97 | 56 | 101 | 58 |
| Race | ||||
| White | 160 | 92 | 163 | 93 |
| African-American | 3 | 2 | 4 | 2 |
| Hispanic or Latino | 8 | 5 | 4 | 2 |
| Asian | 3 | 2 | 4 | 2 |
| ECOG PS | ||||
| 0 | 146 | 84 | 152 | 87 |
| 1 | 28 | 16 | 22 | 13 |
| 2 or not reported | 0 | 0 | 1 | 0 |
| WHO grade | ||||
| 1 | 89 | 51 | 91 | 52 |
| 2 | 70 | 40 | 73 | 42 |
| 3-unknown | 15 | 9 | 11 | 6 |
| “B” symptoms | ||||
| Present | 12 | 7 | 23 | 13 |
| Absent | 162 | 93 | 150 | 86 |
| Unknown | 0 | 0 | 2 | 1 |
| Prior therapy | ||||
| T-N | 137 | 79 | 138 | 79 |
| R/R | 37 | 21 | 37 | 21 |
| FLIPI risk group | ||||
| Low | 51/174 | 29 | 78/175 | 45 |
| T-N | 37/137 | 27 | 55/138 | 40 |
| R/R | 14/37 | 38 | 23/37 | 62 |
| Intermediate | 71/174 | 41 | 66/175 | 38 |
| T-N | 55/137 | 40 | 57/138 | 41 |
| R/R | 16/37 | 43 | 9/37 | 24 |
| High | 49/174 | 28 | 29/175 | 17 |
| T-N | 42/137 | 31 | 25/138 | 18 |
| R/R | 7/37 | 19 | 4/37 | 11 |
| Unknown | 3 | 2 | 2 | 1 |
| Ann Arbor stage | ||||
| I | 0 | 0 | 7 | 4 |
| II | 17 | 10 | 21 | 12 |
| III | 75 | 43 | 67 | 38 |
| IV | 80 | 46 | 79 | 45 |
| Unknown | 2 | 1 | 1 | 0 |
| No. of nodal sites | ||||
| Median | 4 | 4 | ||
| Range | 1-6 | 1-6 | ||
| LDH, U | ||||
| Median | 186 | 177 | ||
| Range | 93-691 | 59-496 | ||
| Hemoglobin, g/dL | ||||
| Median | 14.1 | 14.1 | ||
| Range | 8.9-16.7 | 10.5-18.1 | ||
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; T-N, treatment-naive patients; R/R, relapsed refractory disease; FLIPI, Follicular Lymphoma International Prognostic Index; LDH, lactate dehydrogenase.
Percentage may not add to 100% because of rounding.
Efficacy Results
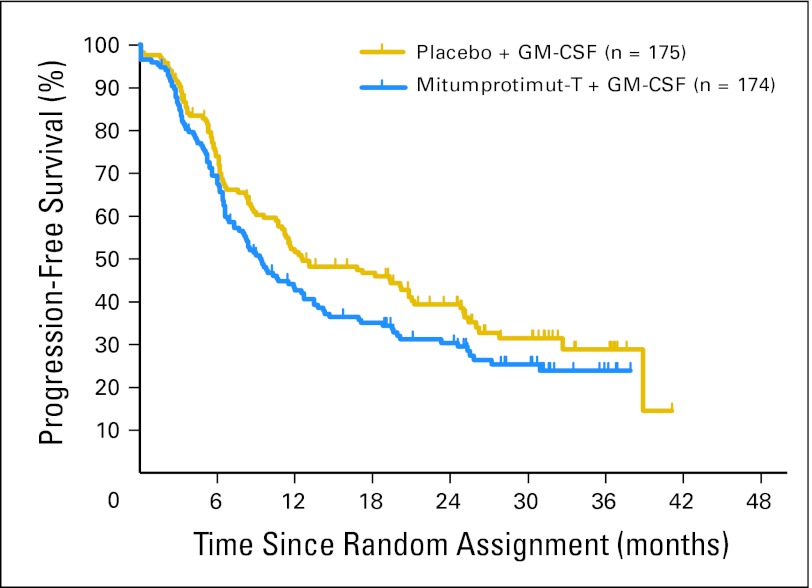
As of March 2008, 215 randomly assigned patients (62%) had experienced PD (113 patients assigned to mitumprotimut-T/GM-CSF and 102 patients assigned to placebo/GM-CSF). The median TTP was 9.0 months (95% CI, 6.2 to 12.5 months) for 174 patients randomly assigned to mitumprotimut-T/GM-CSF and 12.6 months (95% CI, 10.7 to 20.8 months) for 175 patients randomly assigned to placebo/GM-CSF (Fig 2), with a mitumprotimut-T:placebo HR of 1.384 (95% CI, 1.053 to 1.819; P = .019).
Fig 2.
Time to progression: intent-to-treat population. Mitumprotimut-T:placebo hazard ratio of 1.384 (95% CI, 1.053 to 1.819; P = .019). GM-CSF, granulocyte-macrophage colony-stimulating factor.
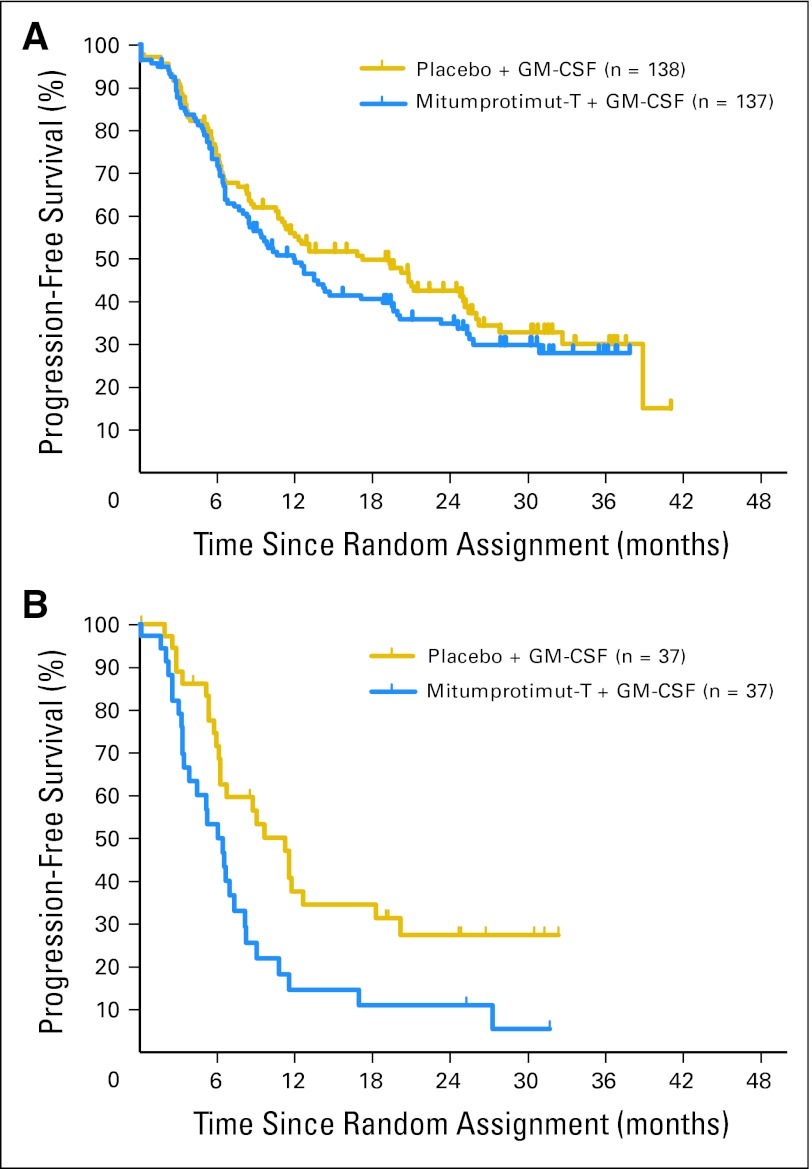
In 275 patients with treatment-naive disease, there was no significant difference in TTP between the two arms (HR = 1.196; P = .258), with median TTP of 11.9 months (95% CI, 8.4 to 17.1 months) for mitumprotimut-T/GM-CSF and 17.2 months (95% CI, 11.0 to 25.0 months) for placebo/GM-CSF (Fig 3A). In 74 patients with relapsed/refractory disease, there was a significant difference in TTP between the two arms (HR = 2.265; P = .004), with median TTP of 6.0 months (95% CI, 3.8 to 7.3 months) for mitumprotimut-T/GM-CSF and 11.2 months (95% CI, 6.2 to 18.2 months) for placebo/GM-CSF (Fig 3B).
Fig 3.
Time to progression by prior therapy: (A) treatment-naive patients and (B) patients with relapsed/refractory disease. Mitumprotimut-T:placebo hazard ratio (HR) = 1.196;P = .258 for treatment-naive patients. Mitumprotimut-T:placebo HR = 2.265, P = .004 for patients with relapsed/refractory disease. GM-CSF, granulocyte-macrophage colony-stimulating factor.
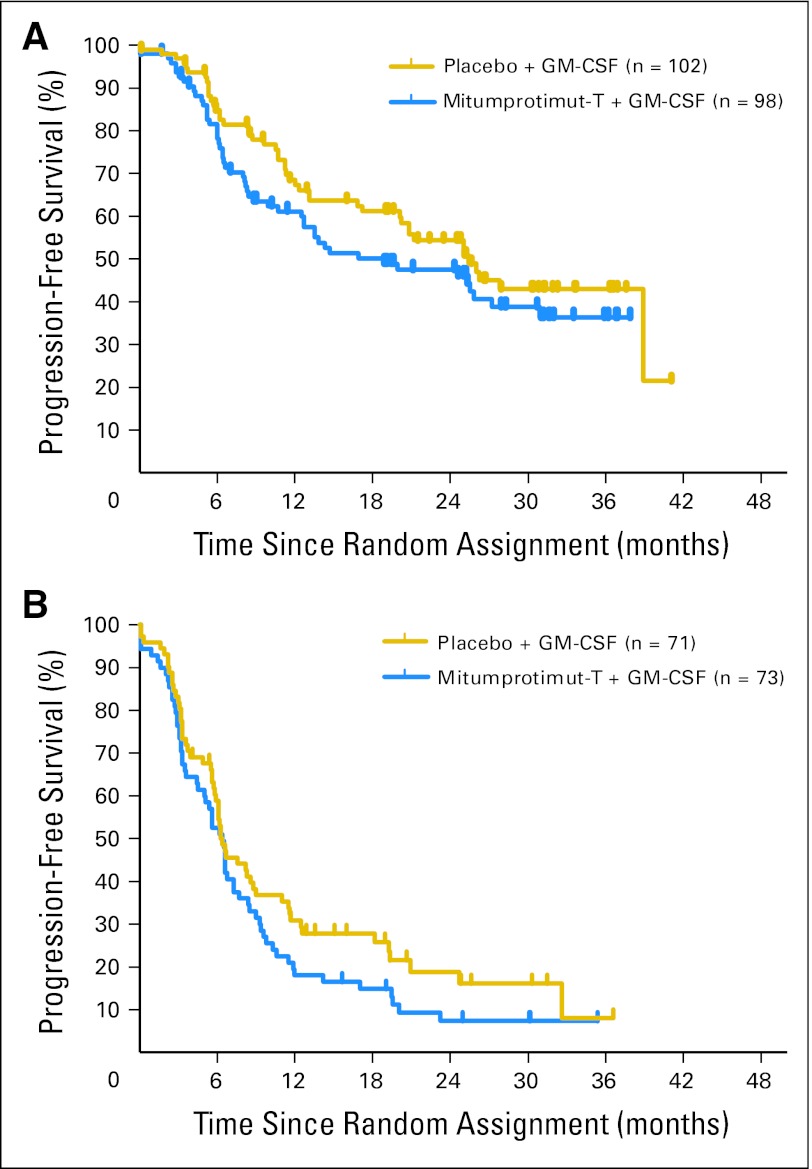
In 205 patients achieving CR or PR to rituximab, there was no significant difference in TTP between the two arms (HR = 1.352; P = .142), with median TTP of 18.8 months (95% CI, 12.5 to 27.2 months) for mitumprotimut-T/GM-CSF and 25.4 months (95% CI, 20.1 month to undetermined) for placebo/GM-CSF (Fig 4A). In 144 patients with SD after rituximab, there was no significant difference in TTP between the two arms (HR = 1.412; P = .068), with median TTP of 6.4 months (95% CI, 4.5 to 73. months) for mitumprotimut-T/GM-CSF and 6.3 months (95% CI, 5.8 to 8.8 months) for placebo/GM-CSF (Fig 4B).
Fig 4.
Time to progression by disease response to rituximab based on investigator's assessment: (A) objective response and (B) stable disease. Mitumprotimut-T:placebo hazard ratio (HR) = 1.352; P = .142 for patients having complete response/partial response. Mitumprotimut-T:placebo HR = 1.412; P = .068 for patients with stable disease. GM-CSF, granulocyte-macrophage colony-stimulating factor.
In 162 treatment-naïve patients achieving CR or PR to rituximab, there was no significant difference in TTP between the two arms (HR = 1.141; P = .570), with median TTP of 25.3 months for mitumprotimut-T/GM-CSF and 25.4 months for placebo/GM-CSF. In 113 treatment-naive patients with SD after rituximab, there was no significant difference in TTP between the two arms (HR = 1.243; P = .310), with median TTP of 6.6 months for mitumprotimut-T/GM-CSF and 6.4 months for placebo/GM-CSF.
When FLIPI was added as a covariate in the Cox regression model, the difference in TTP between the two treatment arms was no longer significant (HR = 1.242; P = .128). There were no significant differences in TTP between the two treatment arms in 78 patients with high-risk FLIPI scores (P = .891) or 266 patients with intermediate/low-risk FLIPI scores (P = .143).
When using the investigator's assessment of disease response, there were no significant differences in TTP in the intent-to-treat population (HR = 1.213; P = .169), with median TTP of 12.7 months (95% CI, 10.3 to 18.4 months) for mitumprotimut-T/GM-CSF and 17.0 months (95% CI, 12.0 to 24.5 months) for placebo/GM-CSF, or in any of the patient subsets. Comparisons of TTP between the two treatment arms for 312 patients comprising the efficacy-assessable population were consistent with those observed in the intent-to-treat population.
There were no significant differences between the two treatment arms in the ORR to rituximab, the ORR postrandomization, and RRI (Table 2). As a result of resource constraints, the immune assays were not performed, and immune responses are not available.
Table 2.
Objective Responses Rate and Rate of Response Improvement
| Response | Tumor Restaging Before Randomization |
Best Response After Randomization |
||||||
|---|---|---|---|---|---|---|---|---|
| Mitumprotimut-T |
Placebo |
Mitumprotimut-T |
Placebo |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Randomly assigned patients | ||||||||
| No. of patients | 174 | 175 | 174 | 175 | ||||
| CR | 29 | 17 | 34 | 19 | 69 | 40 | 81 | 46 |
| PR | 69 | 40 | 68 | 39 | 42 | 24 | 34 | 19 |
| ORR | 98 | 57 | 102 | 58 | 111 | 64 | 115 | 65 |
| Treatment-naive patients | ||||||||
| No. of patients | 137 | 138 | 137 | 138 | ||||
| CR | 23 | 17 | 25 | 18 | 60 | 44 | 66 | 48 |
| PR | 57 | 42 | 55 | 40 | 32 | 23 | 24 | 17 |
| ORR | 80 | 59 | 80 | 58 | 92 | 67 | 90 | 65 |
| Relapsed/refractory disease | ||||||||
| No. of patients | 37 | 37 | 37 | 37 | ||||
| CR | 6 | 16 | 9 | 24 | 9 | 24 | 15 | 41 |
| PR | 12 | 32 | 13 | 35 | 10 | 27 | 10 | 27 |
| ORR | 18 | 48 | 22 | 59 | 19 | 51 | 25 | 68 |
| RRI postrandomization | ||||||||
| No. of patients | ||||||||
| SD to PR | 9/70 | 13 | 7/70 | 10 | ||||
| SD to CR | 4/70 | 6 | 6/70 | 9 | ||||
| PR to CR | 36/69 | 52 | 41/68 | 60 | ||||
| Any RRI | 49/139 | 35 | 54/138 | 39 | ||||
Abbreviations: CR, complete response; PR, partial response; ORR, objective response rate; RRI, rate of response improvement; SD, stable disease.
Safety Results
Study drug exposure was comparable in the two treatment arms, with a mean of 10.4 courses (range, two to 21 courses) in 155 patients given mitumprotimut-T and 10.8 courses (range, one to 21 courses) in 160 patients given placebo. The interval of time between any two blinded study drug doses for the first six doses was comparable between the two treatment arms, with a mean of 33.8 days for mitumprotimut-T and 32.2 days for placebo.
Safety was assessed in all patients who received rituximab and in patients who received blinded study drug/GM-CSF. The type, frequency, and severity of treatment-emergent adverse events were comparable between the two treatment arms (Table 3). The most common adverse event was injection site reaction, reported in 93.6% of patients. Injection site reactions (defined as erythema, pruritus, edema, inflammation, induration, and/or pain) were transient and graded as mild or moderate in 58.7% and 31.7% of patients, respectively. No cumulative toxicities were observed, and there were no drug-related deaths.
Table 3.
Adverse Events Reported in ≥ 10% of Patients*
| Adverse Event | Mitumprotimut-T and GM-CSF (n = 155) |
Placebo and GM-CSF (n = 160) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Injection site reaction | 147 | 95 | 148 | 97 |
| Fatigue | 70 | 45 | 69 | 43 |
| Headache | 45 | 29 | 43 | 27 |
| Fever of any cause | 34 | 22 | 26 | 16 |
| Arthralgia | 30 | 19 | 30 | 19 |
| Chills | 28 | 18 | 24 | 15 |
| Nausea | 27 | 17 | 33 | 21 |
| Myalgia | 27 | 17 | 23 | 14 |
| Influenza-like symptoms | 26 | 17 | 30 | 19 |
| Back pain | 26 | 17 | 38 | 24 |
| Upper respiratory tract infection | 25 | 16 | 27 | 17 |
| Cough | 24 | 16 | 14 | 9 |
| Diarrhea | 23 | 15 | 26 | 16 |
| Pain | 23 | 15 | 24 | 15 |
| Dizziness | 21 | 14 | 13 | 8 |
| Pain in extremity | 17 | 11 | 16 | 10 |
| Dyspnea | 15 | 10 | 16 | 10 |
| Bone pain | 14 | 9 | 14 | 9 |
| Rash | 13 | 8 | 17 | 11 |
| Abdominal pain | 11 | 7 | 18 | 11 |
| Chest pain | 9 | 6 | 19 | 12 |
Abbreviation: GM-CSF, granulocyte-macrophage colony-stimulating factor.
Regardless of relationship to blinded study drug and GM-CSF.
DISCUSSION
This phase III trial evaluated an all-biologic immunotherapeutic approach in follicular lymphoma consisting of passive immunotherapy with rituximab followed by active immunization with a patient-specific vaccine. It was hypothesized that active immunization would extend the time to disease progression after cytoreduction with rituximab. Rituximab was chosen for tumor debulking because it is better tolerated than chemotherapy and, when used alone or with chemotherapy, is the preferred treatment for most patients with follicular lymphoma. Furthermore, studies have shown that rituximab does not suppress T-cell numbers and T-cell immunity, a key contributor to anticancer immune response.10,11 This trial differed from other Id-KLH phase III trials in that it used rituximab debulking instead of chemotherapy, provided for “boosting” doses of vaccines beyond the first six doses in an attempt to maintain or augment immune responses, was open to enrollment of previously untreated patients and those who had experienced relapse, and allowed enrollment of patients with SD after debulking therapy.12,13
Patient characteristics were comparable in the two treatment arms except for the imbalance in FLIPI. When the study was designed, the importance of FLIPI as prognostic factor for time to progression and for patients treated with rituximab alone had not been realized, and patients were not stratified by FLIPI score.7 This inadvertently resulted in markedly more patients with high-risk FLIPI randomly assigned to mitumprotimut-T, particularly in the subset of patients with relapsed/refractory disease and fewer patients with low-risk FLIPI randomly assigned to placebo.
Treatment was usually well tolerated, and most adverse events were consistent with those expected with subcutaneous administration of immunomodulatory agents and with those reported in other Id-KLH trials.2–5,12 The type, incidence, and severity of adverse events were comparable between the two arms, providing additional assurance that blinding was maintained during the trial.
The study did not confirm the hypothesized improvement in TTP with mitumprotimut-T in randomly assigned patients nor in patients who received study drug. There was a significantly inferior TTP in the mitumprotimut-T/GM-CSF arm compared with placebo/GM-CSF. Although it is not possible to exclude a detrimental vaccine effect, this difference can be attributed to the marked imbalance in FLIPI risk group between the two arms because the difference in TTP was no longer significant after adjusting for FLIPI risk group in all, treatment-naive, and relapsed/refractory disease patients.
In the control arm, the observed median TTP of 12.6 months was consistent with the protocol assumptions and the published single-agent rituximab trials. The median TTP of 17.2 months from randomization, or approximately 20 months from the start of rituximab, in treatment-naive patients is within the published range of 18 to 26 months.14–16 The median TTP of 11.2 months from randomization, or approximately 14 months from the start of rituximab, in relapsed/refractory disease is within the published range of 6 to 13 months.17–19
In the mitumprotimut-T arm, the overall ORR was similar to that reported in the phase II trial.5 The median and 3-year TTP, however, were lower than expected and that reported in the phase II trial.6 Whether this is due to enrollment of proportionally more patients with high-risk FLIPI scores, stricter definition of PD, stringent central radiology review, or other unknown factors in unclear.
A randomized phase III trial comparing Id-KLH/GM-CSF with KLH/GM-CSF in 287 previously untreated patients with follicular lymphoma who had achieved an objective response with eight courses of cyclophosphamide, vincristine, and prednisone showed an apparent plateau of progression-free survival (PFS) at 30% at 5 years, but there was no difference in PFS and time to next lymphoma treatment between the two arms, although there was a significant prolongation of PFS in patients mounting an anti-Id humoral immune response.13 The final results of another randomized phase III trial evaluating Id vaccination in patients achieving an objective response to chemotherapy have not been reported.12
Taken together, the results of the two reported phase III trials indicate that immunotherapy with patient-specific Id-KLH and GM-CSF does not improve PFS despite evidence of cellular and humoral anti-Id responses, in contrast to the encouraging results reporter in the smaller earlier single-arm studies.1–6,10,20,21 Whether the absence of improvement is the result of targeting an irrelevant antigen, the weak immunogenicity of Id and inadequacy of GM-CSF as adjuvant, impaired humoral responses after rituximab, presence of immune inhibitors such as regulatory T-cells or transforming growth factor β, vaccination in patients with residual disease, or other reasons is unclear and requires further evaluation.
Acknowledgment
We thank the patients who participated in the trial, the investigators who contributed patients to the study (Appendix, online only), and the study coordinators.
Appendix
The following investigators participated in this study: Steven Allen, North Shore University Hospital, NY; Nancy Bartlett, Washington University in St Louis Medical Center, MO; Patrick Beatty, Montana Cancer Center, MT; Archie Brown, Ochsner Clinical Foundation, LA; Bruce Cheson, Georgetown University Lombardi Comprehensive Cancer Center, DC; Gary Cohen, Greater Baltimore Medical Center, MD; Brenda Cooper, University Hospital of Cleveland, OH; Delva Deauna-Limayo, University of Kansas Medical Center, KS; John Densmore, University of Virginia Health Center, VA; Lou Fehrenbacher, Kaiser Permanente, San Francisco, CA; Roger Fleischman, Markey Cancer Center at University of Kentucky, KY; Andres Forero-Torres, University of Alabama in Birmingham, AL; Arnold Freedman, Dana-Farber Cancer Institute, MA; Jonathan Friedberg, University of Rochester Medical Center, NY; Stephanie Gregory, Rush Presbyterian St Luke's Medical Center, IL; Gerald Gross, Roger Marris Cancer Center, ND; Michael Guarino, Christiana Care Health Services, DE; John Hainsworth, Minnie Pearl, Sarah Cannon Cancer Center, TN; Paul Hamlin, Memorial Sloan-Kettering Cancer Center, NY; Peter Holman, University of California in San Diego Medical Center, CA; David Hurd, Wake Forest University, NC; Nalini Janakiraman, Henry Ford Hospital, MI; Patrick Johnston, Mayo Clinic, MN; Vicky Jones, NorthstarLodge of Yakima, WA; Brad Kahl, University of Wisconsin Health Center, WI; Lawrence Kaplan, University of California in San Francisco Medical Center, CA; Ronald Levy, Stanford University, CA; Stacy Lewis, Providence Cancer Center, OR; James Liebmann, New Mexico Oncology Hematology Consultants, NM; Thomas Lin, The Ohio State University Medical Center, OH; John Lister, Western Pennsylvania University Medical Center, PA; Roger Lyons, Cancer Care Centers of South Texas, TX; Jeffrey Matous, Rocky Mountain Cancer Center, CO; Michael Milder, Swedish Hospital Cancer Institute, WA; Ann Mohrbacher, University of Southern California Keck School of Medicine, CA; Sattva Neelapu, M. D. Anderson Cancer Center, TX; Craig Nichols, Oregon Health Sciences University, OR; John Pancoast, Barret Cancer Center at the University of Cincinnati, OH; Dat Pham, University of Florida in Jacksonville, FL; Luis Pineiro, Baylor University School of Medicine, TX; Javier Pinilla, H. Lee Moffitt Cancer Center, FL; Brad Pohlman, Cleveland Clinic Foundation Taussig Cancer Center, OH; Jonathan Polikoff, Kaiser Permanente in San Diego, CA; Albert Quiery, Geisinger Clinic in Danville, PA; Charles Redfern, Sharp Health Care Clinical Oncology Research, CA; Craig Reeder, Mayo Clinic, AZ; Harold Richter, Boca Raton Community Hospital, FL; Michael Robertson, Indiana University Medical Center, IN; Fred Rosenfelt, Tower Hematology Oncology Medical Group, CA; Gregory Sarna, Cedar Sinai Medical Center, CA; Charles Schiffer, Karmanos Cancer Center, MI; Ilan Shapira, Beth Israel New York Medical Center, NY; Mitchell Smith, Fox Chase Cancer Center, PA; Haluk Tezcan, North Idaho Cancer Center, ID; Mathew Thomas, Mid Dakota Clinic, ND; Peter Wiernik, New York Medical College, NY; Jane Winter, Northwestern University, IL.
Footnotes
Supported and sponsored by Favrille, Inc.
Presented in part at the 48th Annual Meeting of the American Society of Hematology, December 9-12, 2006, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: John F. Bender, Favrille Inc (C); Daniel P. Gold, Favrille Inc (C); Richard G. Ghalie, Favrille Inc (C); Morgan E. Stewart, Favrille Inc (C); Vanessa Esquibel, Favrille Inc (C) Consultant or Advisory Role: Arnold Freedman, Favrille, Inc (C); Sattva S. Neelapu, Favrille Inc. (C); Craig Nichols, Favrille Inc (C); Jane N. Winter, Favrille Inc (C); Paul Hamlin, Favrille Inc (C) Stock Ownership: John F. Bender, Favrille Inc; Daniel P. Gold, Favrille Inc; Vanessa Esquibel, Favrille Honoraria: Paul Hamlin, Favrille Inc Research Funding: Arnold Freedman, Favrille Inc; Craig Nichols, Favrille Inc; Jane N. Winter, Favrille Inc; Michael J. Robertson, Favrille Inc; Benjamin Djulbegovic, Favrille Inc; Sattva S. Neelapu, Favrille Inc; Paul Hamlin, Favrille Inc Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: John F. Bender, Daniel P. Gold, Richard G. Ghalie, Morgan E. Stewart
Administrative support: John F. Bender, Richard G. Ghalie, Vanessa Esquibel
Provision of study materials or patients: Arnold Freedman, Sattva S. Neelapu, Craig Nichols, Michael J. Robertson, Benjamin Djulbegovic, Jane N. Winter, John F. Bender, Daniel P. Gold, Paul Hamlin
Collection and assembly of data: Arnold Freedman, Sattva S. Neelapu, Craig Nichols, Michael J. Robertson, Benjamin Djulbegovic, Jane N. Winter, Paul Hamlin, John F. Bender, Daniel P. Gold, Richard G. Ghalie, Morgan E. Stewart, Vanessa Esquibel
Data analysis and interpretation: Arnold Freedman, Sattva S. Neelapu, Craig Nichols, Michael J. Robertson, Benjamin Djulbegovic, Jane N. Winter, John F. Bender, Daniel P. Gold, Richard G. Ghalie, Morgan E. Stewart, Vanessa Esquibel, Paul Hamlin
Manuscript writing: Arnold Freedman, Sattva S. Neelapu, Craig Nichols, Michael J. Robertson, Benjamin Djulbegovic, Jane N. Winter, John F. Bender, Daniel P. Gold, Richard G. Ghalie, Morgan E. Stewart, Paul Hamlin
Final approval of manuscript: Arnold Freedman, Sattva S. Neelapu, Craig Nichols, Michael J. Robertson, Benjamin Djulbegovic, Jane N. Winter, John F. Bender, Daniel P. Gold, Richard G. Ghalie, Morgan E. Stewart, Vanessa Esquibel, Paul Hamlin
REFERENCES
- 1.Kwak LW, Campbell MJ, Czerwinski DK, et al. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. N Engl J Med. 1992;327:1209–1215. doi: 10.1056/NEJM199210223271705. [DOI] [PubMed] [Google Scholar]
- 2.Hsu FJ, Caspar CB, Czerwinski D, et al. Tumor-specific idiotype vaccines in the treatment of patients with B-cell lymphoma: Long-term results of a clinical trial. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 3.Bendandi M, Gocke CD, Kobrin CB, et al. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma. Nat Med. 1999;5:1171–1177. doi: 10.1038/13928. [DOI] [PubMed] [Google Scholar]
- 4.Redfern CH, Guthrie TH, Bessudo A, et al. Phase II trial of idiotype vaccination in previously treated patients with indolent non-Hodgkin's lymphoma resulting in durable clinical responses. J Clin Oncol. 2006;24:3107–3112. doi: 10.1200/JCO.2005.04.4289. [DOI] [PubMed] [Google Scholar]
- 5.Koc ON, Redfern C, Wiernik PH, et al. Continued late conversion to complete remission (CR/CRu) and durability of remission (DUR) in pts with B-cell follicular lymphoma (FL) treated with rituximab followed by mitumprotimut-T (Id-KLH, FavId) immunotherapy. Blood. 2007;110 abstr 3427. [Google Scholar]
- 6.Koc ON, Redfern C, Wiernik PH, et al. Active immunotherapy with mitumprotimut-T (Specifid, Id-KLH, FavId) following rituximab cytoreduction in patients with follicular B-cell lymphoma: Progression-free survival at 4-year follow-up—Study FavId-04. Blood. 2007;110 abstr 2567. [Google Scholar]
- 7.Solal-Céligny P, Roy P, Colombat P, et al. Follicular Lymphoma International Prognostic Index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas: NCI Sponsored International Working Group. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 9.Freedman A, Hamlin PA, Neelapu S, et al. Phase III trial of active immunotherapy (FavId, Id/KLH) following rituximab induction therapy: Clinical responses in patients with follicular non-Hodgkin's lymphoma. Blood. 2006;108 abstr 2756. [Google Scholar]
- 10.Koc O, Redfern C, Wiernik P, et al. Extended follow-up and analysis with central radiological review of patients receiving FavId (Id/KLH) vaccine following rituximab. Blood. 2005;106 abstr 772. [Google Scholar]
- 11.Neelapu SS, Kwak LW, Kobrin CB, et al. Vaccine-induced tumor-specific immunity despite severe B-cell depletion in mantle cell lymphoma. Nat Med. 2005;11:986–991. doi: 10.1038/nm1290. [DOI] [PubMed] [Google Scholar]
- 12.Stergiou AM, Neelapu SS, Casciano R, et al. BiovaxID vaccine therapy of follicular lymphoma in first remission: Phase III blinded safety update. Blood. 2007;110 abstr 4500. [Google Scholar]
- 13.Levy R, Robertson MJ, Ganjoo K, et al. Results of a phase 3 trial evaluating safety and efficacy of specific immunotherapy, recombinant idiotype (Id) conjugated to KLH (Id-KLH) with GM-CSF, compared to non-specific immunotherapy, KLH with GM-CSF, in patients with follicular non-Hodgkin's lymphoma (fNHL) Proc Am Assoc Cancer Res. 2008 abstr LB-204. [Google Scholar]
- 14.Colombat P, Brousse N, Morschhauser F, et al. Single treatment with rituximab monotherapy for low-tumor burden follicular lymphoma (FL): Survival analyses with extended follow-up of 7 years. Blood. 2006;108 abstr 486. [Google Scholar]
- 15.Witzig TE, Vukov A, Habermann TM, et al. Rituximab therapy for patients with newly diagnosed, advanced-stage follicular grade I non-Hodgkin's lymphoma: A Phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:1103–1108. doi: 10.1200/JCO.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 16.Ghielmini M, Hsu Schmitz SF, Cogliatti SB, et al. Prolonged treatment with rituximab significantly increases event-free survival and response duration compared with the standard weekly x4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 17.Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 18.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: Half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 19.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of Yttrium-90-labeled ibritumomab tioxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]
- 20.Inogès S, Rodriguez-Calvillo M, Zabalegui N, et al. Clinical benefit associated with idiotypic vaccination in patients with follicular lymphoma. J Natl Cancer Inst. 2006;98:1292–1301. doi: 10.1093/jnci/djj358. [DOI] [PubMed] [Google Scholar]
- 21.Timmerman JM, Vose J, Levy R, et al. Long-term follow-up of patients treated in a Phase 2 trial with MyVax personalized immunotherapy (recombinant Id-KLH with GM-CSF) after chemotherapy as initial treatment for follicular non-Hodgkin's lymphoma (NHL) Blood. 2005;106 abstr 2438. [Google Scholar]