Abstract
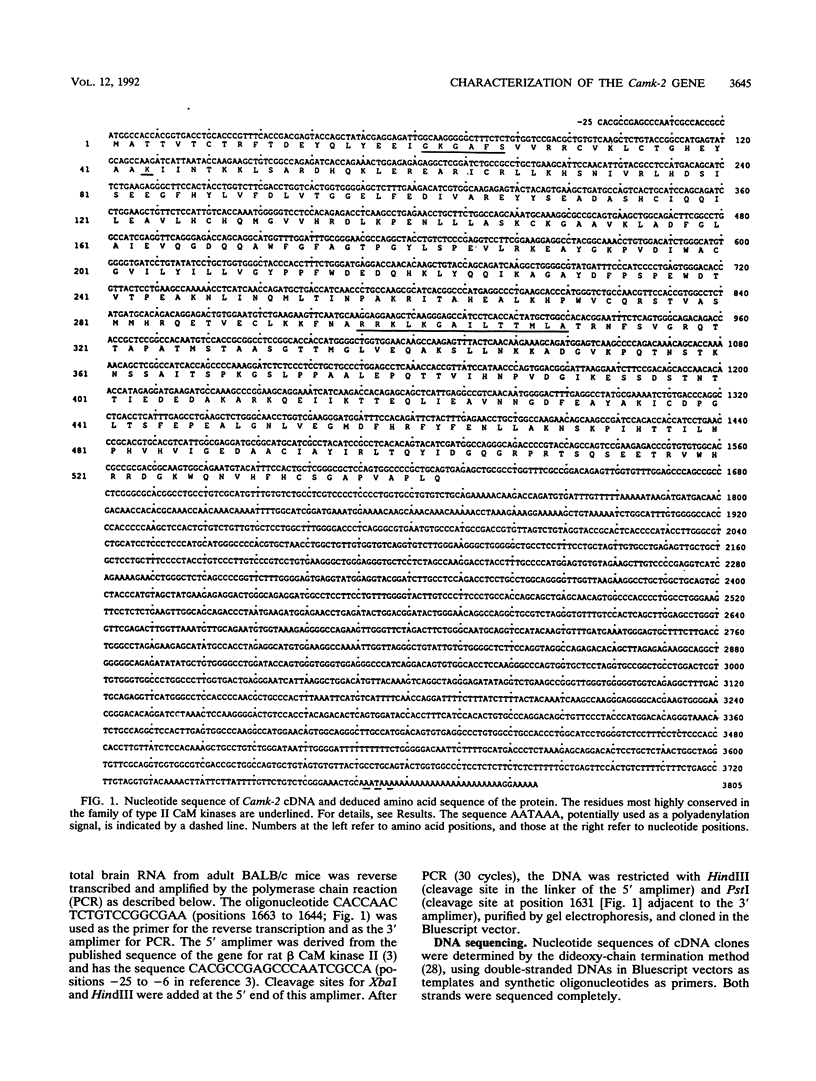
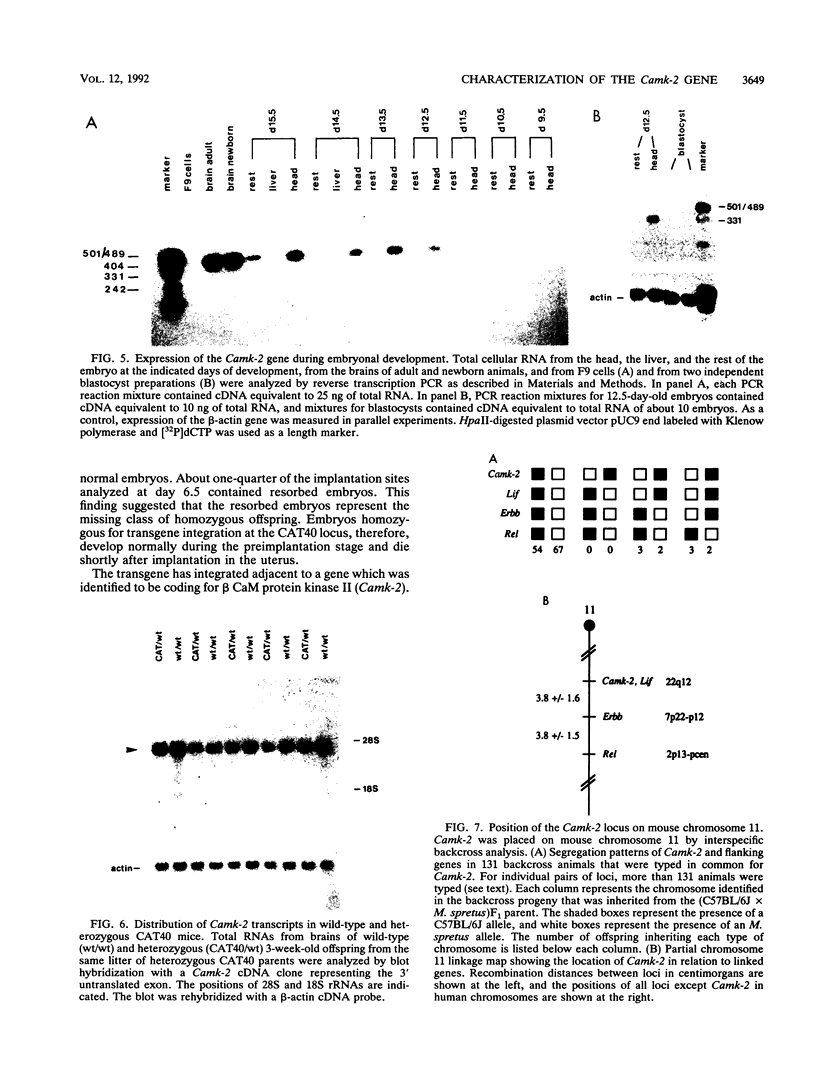
The transgenic mouse strain CAT40 carries in its germ line one copy of a DNA construct consisting of the chloramphenicol acetyltransferase gene and the immunoglobulin heavy-chain enhancer. We show that transgene integration has resulted in a recessive lethal mutation that leads to death of homozygous CAT40 embryos shortly after implantation. The transgene has integrated adjacent to the 3' end of the gene coding for the beta subunit of the brain-specific Ca2+/calmodulin-dependent protein kinase II (Camk-2). The complete cDNA sequence of the Camk-2 gene and most of its exon/intron structure was determined. The deduced amino acid sequence is highly homologous to the previously described rat protein. The chromosomal location of the Camk-2 locus was mapped by interspecific backcross analysis to the proximal region of mouse chromosome 11. This region lacks previously identified recessive embryonic lethal mutations. During embryonic development, Camk-2-specific transcripts are first seen in the head section of 12.5-day-old embryos, and in adult mice the gene is expressed almost exclusively in the brain. Although transcription of the Camk-2 gene in heterozygous CAT40 mice is affected by transgene integration, it is unlikely that this gene is responsible for the mutant phenotype, since it is not expressed in blastocysts and the first transcripts during normal development are detected after the death of homozygous CAT40 embryos. Transgene integration is accompanied by a large deletion of cellular DNA; death is therefore most likely caused by the loss of a gene or genes that are important for early postimplantation development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bennett M. K., Erondu N. E., Kennedy M. B. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J Biol Chem. 1983 Oct 25;258(20):12735–12744. [PubMed] [Google Scholar]
- Bennett M. K., Kennedy M. B. Deduced primary structure of the beta subunit of brain type II Ca2+/calmodulin-dependent protein kinase determined by molecular cloning. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1794–1798. doi: 10.1073/pnas.84.7.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchberg A. M., Brownell E., Nagata S., Jenkins N. A., Copeland N. G. A comprehensive genetic map of murine chromosome 11 reveals extensive linkage conservation between mouse and human. Genetics. 1989 May;122(1):153–161. doi: 10.1093/genetics/122.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulleit R. F., Bennett M. K., Molloy S. S., Hurley J. B., Kennedy M. B. Conserved and variable regions in the subunits of brain type II Ca2+/calmodulin-dependent protein kinase. Neuron. 1988 Mar;1(1):63–72. doi: 10.1016/0896-6273(88)90210-3. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991 Apr;7(4):113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Nishida Y., Mintz B. Early postimplantation embryo lethality due to DNA rearrangements in a transgenic mouse strain. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6020–6024. doi: 10.1073/pnas.83.16.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Goldenring J. R., Gonzalez B., McGuire J. S., Jr, DeLorenzo R. J. Purification and characterization of a calmodulin-dependent kinase from rat brain cytosol able to phosphorylate tubulin and microtubule-associated proteins. J Biol Chem. 1983 Oct 25;258(20):12632–12640. [PubMed] [Google Scholar]
- Hanley R. M., Means A. R., Kemp B. E., Shenolikar S. Mapping of calmodulin-binding domain of Ca2+/calmodulin-dependent protein kinase II from rat brain. Biochem Biophys Res Commun. 1988 Apr 15;152(1):122–128. doi: 10.1016/s0006-291x(88)80688-0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., McGuinness T. L., Greengard P. Evidence that the major postsynaptic density protein is a component of a Ca2+/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1984 Feb;81(3):945–949. doi: 10.1073/pnas.81.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of synaptic transmission in the central nervous system: long-term potentiation. Cell. 1989 Dec 1;59(5):777–787. doi: 10.1016/0092-8674(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Kapiloff M. S., Durgerian S., Tatemoto K., Russo A. F., Hanson P., Schulman H., Rosenfeld M. G. Molecular cloning of a brain-specific calcium/calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5962–5966. doi: 10.1073/pnas.84.16.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. How calmodulin binds its targets: sequence independent recognition of amphiphilic alpha-helices. Trends Biochem Sci. 1990 Feb;15(2):59–64. doi: 10.1016/0968-0004(90)90177-d. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Lai S. P., Van Quill K. R., Westphal H. Tissue-specific expression in transgenic mice of a fused gene containing RSV terminal sequences. Science. 1986 Mar 28;231(4745):1574–1577. doi: 10.1126/science.3006249. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Reik W., Williams G., Barton S., Norris M., Neuberger M., Surani M. A. Provision of the immunoglobulin heavy chain enhancer downstream of a test gene is sufficient to confer lymphoid-specific expression in transgenic mice. Eur J Immunol. 1987 Apr;17(4):465–469. doi: 10.1002/eji.1830170405. [DOI] [PubMed] [Google Scholar]
- Reith A. D., Bernstein A. Molecular basis of mouse developmental mutants. Genes Dev. 1991 Jul;5(7):1115–1123. doi: 10.1101/gad.5.7.1115. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieke A., Harbers K., Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. 1983 Jul 28-Aug 3Nature. 304(5924):315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- Schulman H., Lou L. L. Multifunctional Ca2+/calmodulin-dependent protein kinase: domain structure and regulation. Trends Biochem Sci. 1989 Feb;14(2):62–66. doi: 10.1016/0968-0004(89)90045-5. [DOI] [PubMed] [Google Scholar]
- Schulman H. The multifunctional Ca2+/calmodulin-dependent protein kinase. Adv Second Messenger Phosphoprotein Res. 1988;22:39–112. [PubMed] [Google Scholar]
- Tobimatsu T., Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989 Oct 25;264(30):17907–17912. [PubMed] [Google Scholar]
- Tobimatsu T., Kameshita I., Fujisawa H. Molecular cloning of the cDNA encoding the third polypeptide (gamma) of brain calmodulin-dependent protein kinase II. J Biol Chem. 1988 Nov 5;263(31):16082–16086. [PubMed] [Google Scholar]
- Woychik R. P., Maas R. L., Zeller R., Vogt T. F., Leder P. 'Formins': proteins deduced from the alternative transcripts of the limb deformity gene. Nature. 1990 Aug 30;346(6287):850–853. doi: 10.1038/346850a0. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Stewart T. A., Davis L. G., D'Eustachio P., Leder P. An inherited limb deformity created by insertional mutagenesis in a transgenic mouse. Nature. 1985 Nov 7;318(6041):36–40. doi: 10.1038/318036a0. [DOI] [PubMed] [Google Scholar]