Abstract
Purpose
Brentuximab vedotin is an antibody-drug conjugate (ADC) that selectively delivers monomethyl auristatin E, an antimicrotubule agent, into CD30-expressing cells. In phase I studies, brentuximab vedotin demonstrated significant activity with a favorable safety profile in patients with relapsed or refractory CD30-positive lymphomas.
Patients and Methods
In this multinational, open-label, phase II study, the efficacy and safety of brentuximab vedotin were evaluated in patients with relapsed or refractory Hodgkin's lymphoma (HL) after autologous stem-cell transplantation (auto-SCT). Patients had histologically documented CD30-positive HL by central pathology review. A total of 102 patients were treated with brentuximab vedotin 1.8 mg/kg by intravenous infusion every 3 weeks. In the absence of disease progression or prohibitive toxicity, patients received a maximum of 16 cycles. The primary end point was the overall objective response rate (ORR) determined by an independent radiology review facility.
Results
The ORR was 75% with complete remission (CR) in 34% of patients. The median progression-free survival time for all patients was 5.6 months, and the median duration of response for those in CR was 20.5 months. After a median observation time of more than 1.5 years, 31 patients were alive and free of documented progressive disease. The most common treatment-related adverse events were peripheral sensory neuropathy, nausea, fatigue, neutropenia, and diarrhea.
Conclusion
The ADC brentuximab vedotin was associated with manageable toxicity and induced objective responses in 75% of patients with relapsed or refractory HL after auto-SCT. Durable CRs approaching 2 years were observed, supporting study in earlier lines of therapy.
INTRODUCTION
Improvements in the use of combined chemotherapy and radiotherapy in advanced-stage, newly diagnosed Hodgkin's lymphoma (HL) have resulted in durable remission rates of approximately 60% to 80%.1,2 However, a large fraction of patients with HL are not cured. The standard of care for patients with relapsed or refractory HL is salvage chemotherapy followed by autologous stem-cell transplantation (auto-SCT), which can induce long-term remissions in approximately 50% of patients.3,4 For patients who experience relapse or progressive HL within 1 year after auto-SCT, the prognosis is exceedingly poor, with a median survival time of approximately 1.2 years.5 This relatively young patient population has no currently available standard of care and represents an urgent unmet medical need.
The malignant Hodgkin's Reed-Sternberg cells of classical HL are characterized by the expression of CD30, a member of the tumor necrosis factor superfamily.6,7 Because normal CD30 expression is restricted to a relatively small proportion of activated B cells, T cells, and eosinophils, it represents an ideal target for monoclonal antibody therapy.6–8
Brentuximab vedotin (SGN-35) is an antibody-drug conjugate (ADC) comprising an anti-CD30 antibody conjugated by a protease cleavable linker to the potent antimicrotubule agent, monomethyl auristatin E (MMAE). Binding of the ADC to CD30 on the cell surface initiates internalization of the ADC-CD30 complex, which then traffics to the lysosomal compartment, releasing MMAE via proteolytic cleavage.9 Binding of MMAE to tubulin disrupts the microtubule network, induces cell cycle arrest, and results in apoptotic death of the CD30-expressing tumor cell.10
In a phase I study that enrolled 45 patients with relapsed or refractory CD30-positive lymphomas, the maximally tolerated dose of brentuximab vedotin was determined to be 1.8 mg/kg delivered by intravenous infusion every 3 weeks.11 Treatments were reasonably well tolerated, with the most common adverse events being fatigue, pyrexia, diarrhea, nausea, neutropenia, and peripheral neuropathy. Because a large proportion of patients achieved objective responses in this study, brentuximab vedotin was evaluated in a larger homogeneous population of patients with HL who had relapsed or refractory disease after auto-SCT. The primary end point of this pivotal study was the overall objective response rate (ORR) as determined by an independent review facility (IRF).
PATIENTS AND METHODS
Patient Eligibility
Inclusion criteria for this study were a diagnosis of relapsed or refractory HL after high-dose chemotherapy and auto-SCT, histologically documented CD30-positive Hodgkin's Reed-Sternberg cells by central pathology review, and age 12 years or older. Patients had measurable disease ≥ 1.5 cm by computed tomography (CT), fluorodeoxyglucose-avid disease by positron emission tomography (PET), and an Eastern Cooperative Oncology Group performance status score of 0 or 1. Other inclusion criteria were absolute neutrophil count ≥ 1,000/μL, platelet count ≥ 50,000/μL, serum creatinine ≤ 1.5× the upper limit of normal, and ALT and AST ≤ 2.5× the upper limit of normal. Patients could not be pregnant and could not previously have received allogeneic stem-cell transplantation (SCT).
Study Design and Treatment
This open-label, phase II study was conducted at 25 centers within the United States, Canada, and Europe (ClinicalTrials.gov identifier: NCT00848926). Patients were recruited from February 2009 through August 2009. Primary data analysis was performed after the last patient's end of treatment visit, which occurred in August 2010. A subsequent data cutoff was performed in March 2011 to assess the durability of clinical responses and resolution of peripheral neuropathy. These data are presented in this article.
The study was approved by the institutional review board at each study site, and written informed consent was obtained from all patients before any study-specific procedures, per the Declaration of Helsinki. The dose of brentuximab vedotin was 1.8 mg/kg, administered intravenously once every 3 weeks over 30 minutes on an outpatient basis for up to 16 infusions.
Study Assessments
Baseline evaluations included documentation of disease-related signs and symptoms, a physical examination, bone marrow biopsy, and radiographic studies, including CT of the neck, chest, abdomen, and pelvis and PET scan. The best clinical response was determined by both investigators and an IRF (CoreLab Partners, formerly known as RadPharm; Princeton, NJ) according to the Revised Response Criteria for Malignant Lymphoma.12 Response was assessed by CT scans at cycles 2, 4, 7, 10, 13, and 16 and by PET scans at cycles 4 and 7. After discontinuing treatment, long-term follow-up assessments (including survival and disease status information) were performed every 12 weeks until either patient death or study closure. Patients who discontinued study treatment with stable disease or better had CT scans performed every 12 weeks until disease progression.
An independent data monitoring committee assessed the safety of study participants during the trial and monitored the overall study conduct. Safety monitoring included the recording of adverse events and physical examination findings, vital signs, and routine hematology and serum chemistries. Adverse events were summarized using the Medical Dictionary for Regulatory Activities, version 13.0, and graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events, version 3.
Statistical Analysis
The primary end point of this pivotal study was the ORR per IRF. Secondary end points included duration of response by IRF, complete remission (CR) rate by IRF, and progression-free survival (PFS) by IRF, as well as overall survival (OS) and the incidence and severity of adverse events. PFS was defined as the time from start of study treatment to the first documentation of objective tumor progression or to death as a result of any cause, whichever came first. For this analysis, patients were censored at the time of their last radiologic assessment if they were given another treatment before documentation of progression, with the exception of subsequent SCT as the first therapy after discontinuing brentuximab vedotin. OS was defined as the time from start of study treatment to date of death as a result of any cause.
One hundred patients were planned to be enrolled onto this study. With a sample size of 100 patients, a 29% ORR enables exclusion of a rate of ≤ 20% with 95% confidence.
The ORR per IRF and its two-sided 95% exact CI were calculated. The median and two-sided 95% CIs for duration of response, PFS, and OS were estimated using the Kaplan-Meier method.
In addition to independent review, assessment of efficacy by the study investigators was collected as a protocol-defined exploratory analysis. A κ coefficient was calculated to characterize the agreement in objective response and best response assessments between IRF and investigator.
A prespecified comparison of intrapatient PFS (PFS achieved with the most recent prior systemic therapy after auto-SCT v PFS per investigator with brentuximab vedotin) was performed using a correlated survival analysis.13 PFS achieved with the most recent prior systemic therapy after auto-SCT was determined using progression as documented by the treating physician in the patient's medical record. Post hoc subgroup analyses of PFS by best response and PFS in the subgroup of patients who achieved a CR by IRF and then either did or did not undergo an allogeneic SCT were performed using the Kaplan-Meier method.
RESULTS
Patients
Patient characteristics are listed in Table 1. One hundred two patients were enrolled, 48 males (47%) and 54 females (53%). The median age was 31 years (range, 15 to 77 years), and 87% of the patients were white. Seventy-one percent of the patients had primary refractory disease, and 42% had disease that was refractory to the most recent prior therapy. The median number of prior chemotherapy regimens excluding auto-SCT was 3.5 (range, one to 13 regimens). Sixty-six percent of patients had received prior radiation therapy, and all 102 patients had undergone auto-SCT. The median time to relapse after auto-SCT was 6.7 months (range, 0 to 131 months). The majority of patients (71%) had experienced relapse within a year of receiving their auto-SCT.
Table 1.
Demographics and Baseline Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 31 | |
| Range | 15-77 | |
| Sex | ||
| Male | 48 | 47 |
| Female | 54 | 53 |
| Race | ||
| Asian | 7 | 7 |
| Black or African American | 5 | 5 |
| White | 89 | 87 |
| Other | 1 | 1 |
| ECOG performance status* | ||
| 0 | 42 | 41 |
| 1 | 60 | 59 |
| Baseline “B” symptoms | 35 | 34 |
| Bone marrow involvement | 8 | 8 |
| Prior radiation | 67 | 66 |
| No. of prior chemotherapy regimens | ||
| Median | 3.5 | |
| Range | 1-13 | |
| Primary refractory disease† | 72 | 71 |
| Disease status relative to most recent prior therapy‡ | ||
| Relapsed | 59 | 58 |
| Refractory | 43 | 42 |
| Best response achieved with most recent systemic regimen | ||
| Complete response | 12 | 12 |
| Partial response | 35 | 34 |
| Stable disease | 23 | 23 |
| Progressive disease | 26 | 25 |
| Unknown/other | 6 | 6 |
| No. of prior auto-SCTs | ||
| 1 | 91 | 89 |
| 2 | 11 | 11 |
| Time from auto-SCT to first post-transplantation relapse, months | ||
| Median | 6.7 | |
| Range | 0-131 | |
| Time from initial diagnosis to first dose of study drug, months | ||
| Median | 39.90 | |
| Range | 11.8-219.7 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; auto-SCT, autologous stem-cell transplantation.
ECOG performance scores range from 0 (normal activity) to 5 (death), with higher scores indicating more severe disability.
Primary refractory disease is defined as failure to obtain a complete remission with front-line therapy or relapse within 3 months of front-line therapy.
Relapsed indicates best response of complete or partial remission to most recent prior therapy, and refractory indicates best response of stable or progressive disease to most recent prior therapy.
Efficacy
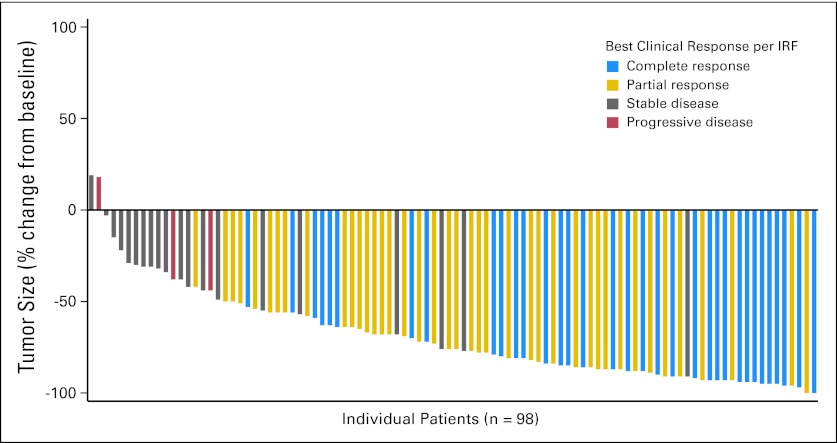
Tumor reductions were observed in 94% of patients (Fig 1). The ORR was 75% (95% CI, 64.9% to 82.6%); 34% of all patients achieved a CR (95% CI, 25.2% to 44.4%), and the overall disease control rate (CR + partial remission + stable disease) was 96% (95% CI, 90.3% to 98.9%; Table 2). The median time to objective response was 5.7 weeks (range, 5.1 to 56 weeks), and the median time to CR was 12 weeks (range, 5.1 to 56 weeks); these medians approximate the timing of the first postbaseline CT and the first postbaseline PET assessments, respectively.
Fig 1.
Maximum percent reduction in the sum of the product of diameters in individual patients (n = 98) per Cheson et al.12 Tumor size reductions were observed in 96 (94%) of 102 patients. Four patients were not included in the analysis; three patients had no measurable lesions per independent review facility (IRF), and one patient had no postbaseline scans.
Table 2.
Key Response Results
| Parameter | No. of Patients (N = 102) | % |
|---|---|---|
| Objective response | 76 | 75 |
| Complete remission | 35 | 34 |
| Partial remission | 41 | 40 |
| Stable disease | 22 | 22 |
| Progressive disease | 3 | 3 |
| Not evaluable | 1 | 1 |
| Duration of objective response, months | ||
| Median | 6.7 | |
| 95% CI | 3.6 to 14.8 | |
| Duration of response for patients with complete remission, months (n = 35) | ||
| Median | 20.5 | |
| 95% CI | 10.8 to NE | |
| Progression-free survival, months | ||
| Median | 5.6 | |
| 95% CI | 5.0 to 9.0 | |
| Overall survival, months | ||
| Median | 22.4 | |
| 95% CI | 21.7 to NE | |
Abbreviation: NE, not estimable.
For patients who had an objective response, the median duration of response was 6.7 months (95% CI, 3.6 to 14.8 months). The median duration of response for patients who achieved a CR was 20.5 months (95% CI, 10.8 months to not estimable; Table 2). Analyses of efficacy by subgroups (including age, sex, race, baseline tumor size [sum of the product of diameters], relapsed v refractory disease status, and primary refractory disease) did not reveal any group of patients who did not achieve clinically meaningful antitumor activity.
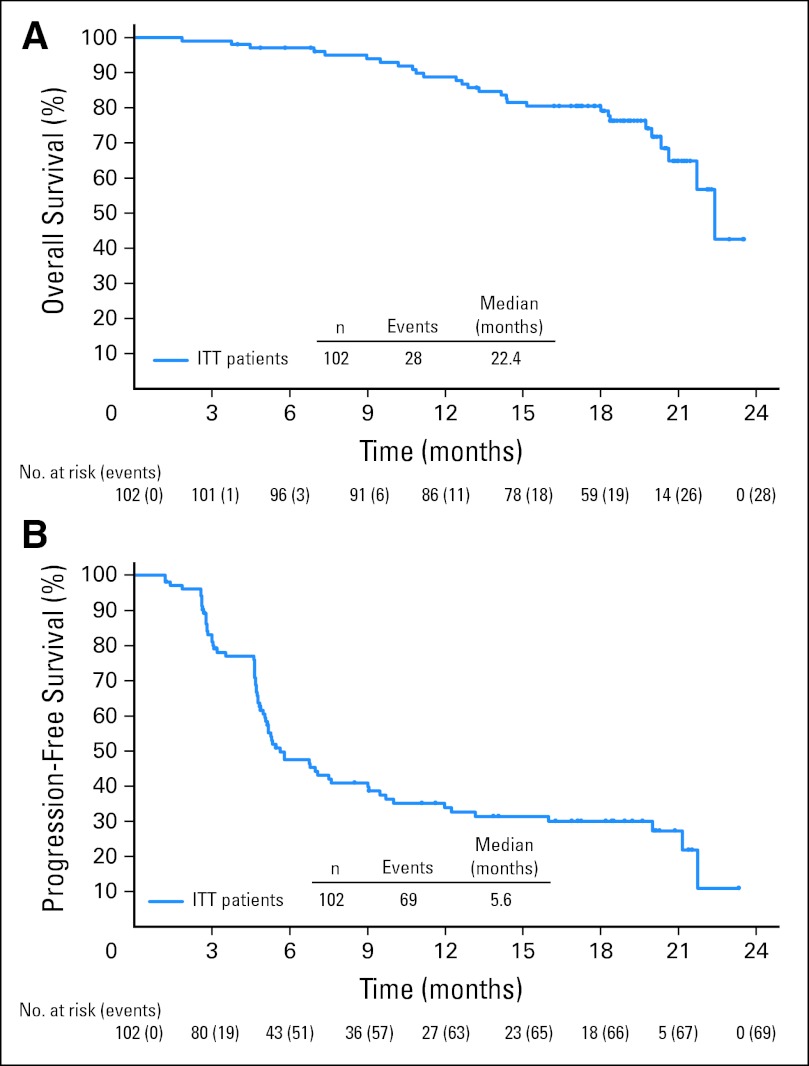
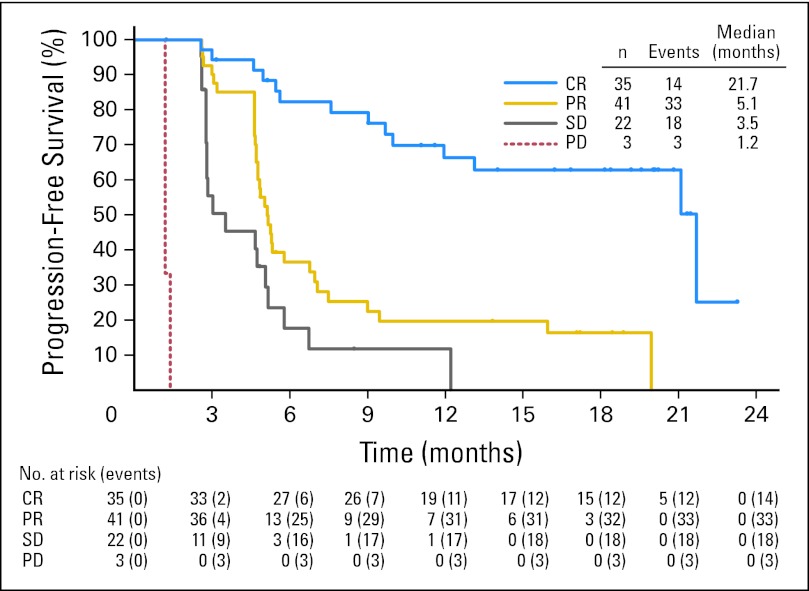
Thirty-one of 102 patients were alive and free of documented progressive disease after a median observation time of 18.5 months (range, 1.8 to 23.5 months). Twenty-eight of 102 patients were known to have died, and the estimated 12-month survival was 89% (95% CI, 83% to 95%). A Kaplan-Meier plot of OS is presented in Figure 2A. The estimated median PFS for all patients on this study was 5.6 months (95% CI, 5.0 to 9.0 months; Fig 2B). The median PFS for patients who achieved a CR with brentuximab vedotin was 21.7 months, which was notably longer than the median PFS for patients who did not obtain a CR (Appendix Fig A1, online only).
Fig 2.
Secondary end points of overall survival (A) and progression-free survival (B). ITT, intent to treat.
At the time of this analysis, eight patients who obtained responses with brentuximab vedotin (five patients with CR and three patients with partial remission per IRF) had received an allogeneic SCT immediately after brentuximab vedotin and before any evidence of tumor progression. The median PFS in the five patients who had a CR and underwent subsequent allogeneic SCT was 21.1 months. The median PFS in the 30 patients who achieved a CR but did not receive an allogeneic SCT was 21.7 months. For patients who received another therapy after discontinuing brentuximab vedotin, this was at the discretion of the investigator who made such decisions independently of central radiologic review. Subsequent therapies included both single-agent and multiagent regimens; however, PFS was censored before receipt of any subsequent therapy.
Assessment of response by the study investigators supported the efficacy analysis by independent review. By investigator assessment, the ORR was 72% (95% CI, 61.8% to 80.1%); 33% of patients achieved a CR (95% CI, 24.3% to 43.4%), and the overall disease control rate was 99% (95% CI, 94.7% to 100%). Assessment of objective response was concordant between IRF and investigator for 89 (87%) of 102 patients. The κ coefficient, a statistical measure of agreement between two observations, was 0.68, which suggested good concordance between the assessors.
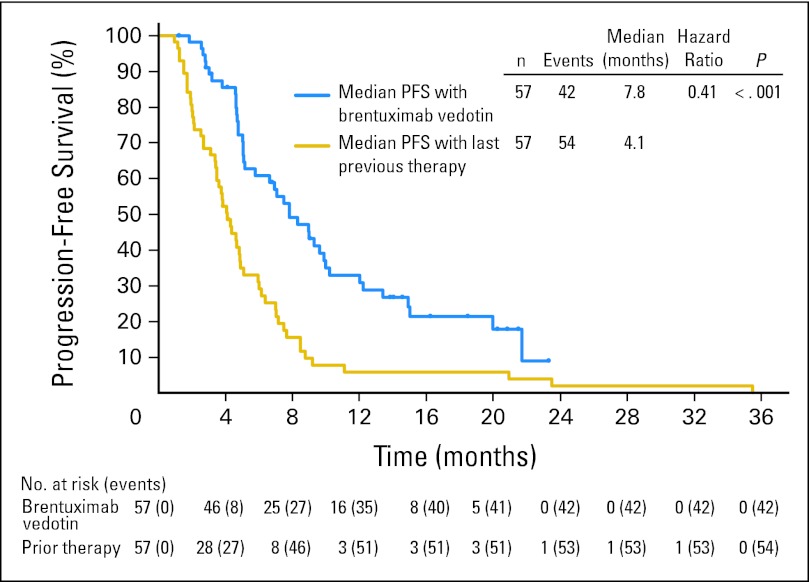
A subset of patients (57 of 102 patients) had received a systemic therapy at the time of relapse after auto-SCT. A preplanned analysis was performed in this subset of patients to compare the PFS achieved with the most recent prior systemic therapy with that achieved with brentuximab vedotin. The median PFS achieved with the most recent prior systemic therapy was 4.1 months (95% CI, 3.4 to 4.9 months) by investigator assessment. When these same patients subsequently received brentuximab vedotin, the median PFS was 7.8 months (95% CI, 5.2 to 9.9 months; Fig 3). Using a correlated survival analysis, the hazard ratio was 0.41, indicating that PFS was significantly prolonged with brentuximab vedotin compared with the prior systemic therapy (P < .001). This was equivalent to a 60% decrease in the hazard of progression or death for patients after initiating brentuximab vedotin treatment.
Fig 3.
Progression-free survival (PFS) achieved with brentuximab vedotin compared with PFS achieved with the last prior therapy. Data shown are median PFS as assessed by investigator in the subset of patients (n = 57) who received systemic therapy after autologous stem-cell transplantation and before receiving brentuximab vedotin.
Safety
All patients enrolled onto this study received at least one infusion of brentuximab vedotin. The median number of cycles was nine (range, one to 16 cycles), the mean number of cycles was 10, and the median relative dose-intensity was 96%.
The most common (≥ 10%) treatment-related adverse events were peripheral sensory neuropathy (42%), nausea (35%), fatigue (34%), neutropenia (19%), diarrhea (18%), pyrexia (14%), vomiting (13%), arthralgia (12%), pruritus (12%), myalgia (11%), peripheral motor neuropathy (11%), and alopecia (10%; Table 3). A total of 56 patients (55%) experienced adverse events of grade 3 or higher. Besides peripheral sensory neuropathy (8%), the majority of grade 3 or higher adverse events were laboratory abnormalities including neutropenia (20%), thrombocytopenia (8%), and anemia (6%). No cases of febrile neutropenia were observed. There were no deaths within 30 days from the last drug administration, and no deaths were attributed to the study drug.
Table 3.
Drug-Related Adverse Events Reported by ≥ 10% of Patients and Grade 3 or 4 Incidence of These Events Regardless of Relationship to Brentuximab Vedotin
| Adverse Event | Events Related to Brentuximab Vedotin (any grade) |
Any Grade 3 Events |
Any Grade 4 Events |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Peripheral sensory neuropathy | 43 | 42 | 8 | 8 | 0 | 0 |
| Nausea | 36 | 35 | 0 | 0 | 0 | 0 |
| Fatigue | 35 | 34 | 2 | 2 | 0 | 0 |
| Neutropenia | 19 | 19 | 14 | 14 | 6 | 6 |
| Diarrhea | 18 | 18 | 1 | 1 | 0 | 0 |
| Pyrexia | 14 | 14 | 2 | 2 | 0 | 0 |
| Vomiting | 13 | 13 | 0 | 0 | 0 | 0 |
| Arthralgia | 12 | 12 | 0 | 0 | 0 | 0 |
| Pruritus | 12 | 12 | 0 | 0 | 0 | 0 |
| Myalgia | 11 | 11 | 0 | 0 | 0 | 0 |
| Peripheral motor neuropathy | 11 | 11 | 1 | 1 | 0 | 0 |
| Alopecia | 10 | 10 | 0 | 0 | 0 | 0 |
Twenty patients had adverse events that led to treatment discontinuation; the most common of these were peripheral sensory neuropathy in six patients and peripheral motor neuropathy in three patients. Doses of brentuximab vedotin were delayed because of adverse events in 47% of patients. Overall, 8% of doses were delayed. The most common events leading to dose delays were neutropenia (16%) and peripheral sensory neuropathy (13%). Doses of brentuximab vedotin were prospectively reduced from 1.8 to 1.2 mg/kg in 11 patients; 10 of the 11 patients received a dose reduction because of peripheral neuropathy, and the other patient had a dose reduction because of grade 4 thrombocytopenia.
Fifty-six patients experienced peripheral neuropathy events (as identified by Standardized Medical Dictionary for Regulatory Activities Query) of any grade; 20% of patients had peripheral neuropathy events with a worst severity of grade 2, 11% had events with a worst severity of grade 3, and there were no grade 4 events. The majority of events were grade 1 or 2 peripheral sensory neuropathy characterized by numbness and tingling of the fingers and toes. The median time to onset of any peripheral neuropathy event was 12.4 weeks; the median times to onset of grade 2 and grade 3 peripheral neuropathy events were 27.3 and 38.0 weeks, respectively. Eighty percent of patients had either resolution or some improvement (of one grade or more) of peripheral neuropathy. Complete resolution of all events of peripheral neuropathy occurred in 50% of patients. The median time to improvement or resolution was 13.2 weeks.
DISCUSSION
In this pivotal, phase II, multicenter trial of brentuximab vedotin monotherapy, 75% of patients achieved an objective response and 34% obtained a CR as determined by independent review. Patients had disease that was particularly refractory to prior treatments as evidenced by the fact that 71% of patients did not achieve a CR or had experienced relapse within 3 months of front-line therapy. Furthermore, these patients had a poor prognosis because the median time to relapse after auto-SCT was only 6.7 months. In this context, the rates of overall response and durable CR are notable for a single-agent therapy after the failure of prior combination chemotherapy and auto-SCT. Although historically, each successive treatment delivered to a patient with multiply relapsed lymphoma tends to result in diminishing progression-free intervals, the PFS achieved with brentuximab vedotin was significantly longer than that achieved with the most recent prior therapy in the subset of patients who had received a systemic therapy after auto-SCT.
These results compare favorably with response rates achieved in the Cancer and Leukemia Group B study of the multiagent regimen of gemcitabine, vinorelbine, and pegylated liposomal doxorubicin in patients with disease relapse after auto-SCT (ORR, 75% [27 of 36 patients]; CR, 17% [six of 36 patients]).14 Of note, more than one third of patients achieved a CR with single-agent brentuximab vedotin, and the antitumor activity was obtained without the characteristic toxicity of combination chemotherapy regimens such as gemcitabine, vinorelbine, and pegylated liposomal doxorubicin, which was associated with grade 3 or 4 neutropenia, thrombocytopenia, and febrile neutropenia in 51%, 43%, and 11% of patients after auto-SCT, respectively.14
Most adverse events associated with brentuximab vedotin were managed through standard supportive care, and the most common events were typically grade 1 or 2. The most clinically meaningful adverse event in the current study was cumulative grade 1 or 2 peripheral neuropathy. Neuropathy typically developed after prolonged exposure to the drug, with a median onset of grade 2 at 27.3 weeks. The neuropathy was also largely reversible; 80% of the patients had either resolution of events or improvement after treatment was completed or discontinued or the dose was reduced. Given that the cytotoxic component of brentuximab vedotin is a potent antimicrotubule agent, the peripheral neuropathy observed was consistent with a class effect of antimicrotubule drugs.15,16 Patients in this study were also predisposed to developing peripheral neuropathy given that they had been exposed to multiple prior chemotherapy regimens and 23% had peripheral neuropathy at the time of study entry.
In this study, brentuximab vedotin was administered for a maximum of 16 cycles; the actual median and mean durations of treatment were nine and 10 cycles, respectively. Although the majority of responses occurred early in the course of treatment, one CR was initially documented after approximately 1 year of therapy. Eighteen patients received all 16 cycles of treatment. Although currently unknown, the optimal duration of therapy should strike a balance between maintaining tumor control and minimizing toxicity, which may be best achieved with judicious application of dose delays and dose reductions if toxicity develops.
Allogeneic SCT seems to be another viable treatment option with promising results for patients who have highly treatment-refractory HL for whom salvage therapy obtains a state of minimal to undetectable disease.17–20 At the time of data cutoff, eight patients from this study had received an allogeneic SCT as their first subsequent therapy after obtaining responses to brentuximab vedotin. All eight patients are alive and remain in follow-up at the time of data cutoff, with a PFS that is indistinguishable from patients who did not receive a transplantation. Further follow-up is ongoing.
Although patients enrolled onto this study had a poor prognosis and had previously received multiple chemotherapy regimens, almost all achieved tumor regression, more than one third obtained a CR, and 31 patients remain alive without documented progression. Outpatient therapy with brentuximab vedotin was associated with manageable toxicities. Observed peripheral neuropathy was predominantly sensory in nature and largely reversible. These results provide support for clinical evaluation in patients with earlier stage disease. A phase I study is evaluating the combination of brentuximab vedotin and multiagent chemotherapy in untreated, newly diagnosed patients with HL. In addition, a placebo-controlled, randomized, phase III trial is evaluating the effect of brentuximab vedotin on PFS and OS in high-risk patients with HL in the post–auto-SCT setting.
Acknowledgment
We acknowledge the patients who participated in the study and the study staff who helped to take care of them. We also acknowledge Eilidh Williamson for medical writing assistance under the sponsorship of Seattle Genetics.
Appendix
Fig A1.
Progression-free survival of patients according to best response. CR, complete remission; PD, progressive disease; PR, partial remission; SD, stable disease.
Footnotes
Processed as a Rapid Communication manuscript. See accompanying editorial on page 2171 and articles on pages 2190 and 2197; listen to the podcast by Dr Soiffer at www.jco.org/podcasts
Supported by Seattle Genetics, Bothell, WA.
Presented in part at the 52nd Annual Meeting of the American Society of Hematology, December 4-7, 2010, Orlando, FL; the 47th Annual Meeting of the American Society of Clinical Oncology, June 3-7, 2011, Chicago, IL; 11th International Conference on Malignant Lymphoma, June 15-18, 2011, Lugano, Switzerland.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00848926.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Emily K. Larsen, Seattle Genetics (C); Dana A. Kennedy, Seattle Genetics (C); Eric L. Sievers, Seattle Genetics (C) Consultant or Advisory Role: Anas Younes, Seattle Genetics (C); Ajay K. Gopal, Seattle Genetics (C); Scott E. Smith, Cephalon (C), Spectrum Pharmaceuticals (C), Celgene (C); Kerry J. Savage, Seattle Genetics (C); Bruce D. Cheson, Seattle Genetics (C); Sven de Vos, Seattle Genetics (C); Craig H. Moskowitz, Seattle Genetics (C); Robert Chen, Seattle Genetics (C) Stock Ownership: Emily K. Larsen, Seattle Genetics; Dana A. Kennedy, Seattle Genetics; Eric L. Sievers, Seattle Genetics Honoraria: Anas Younes, Seattle Genetics, Novartis, sanofi-aventis; Ajay K. Gopal, Seattle Genetics, Millennium Pharmaceuticals; Kerry J. Savage, Seattle Genetics; Andreas Engert, Seattle Genetics, Millennium Pharmaceuticals, Takeda Research Funding: Anas Younes, Seattle Genetics, Novartis, Genentech; Ajay K. Gopal, Seattle Genetics; Scott E. Smith, Seattle Genetics; Stephen M. Ansell, Seattle Genetics; Joseph D. Rosenblatt, Seattle Genetics; Kerry J. Savage, Seattle Genetics; Radhakrishnan Ramchandren, Seattle Genetics; Nancy L. Bartlett, Seattle Genetics; Bruce D. Cheson, Seattle Genetics; Sven de Vos, Seattle Genetics; Andres Forero-Torres, Seattle Genetics; Craig H. Moskowitz, Seattle Genetics; Joseph M. Connors, Seattle Genetics; Andreas Engert, Seattle Genetics, Millennium Pharmaceuticals; Robert Chen, Seattle Genetics Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Anas Younes, Ajay K. Gopal, Radhakrishnan Ramchandren, Craig H. Moskowitz, Joseph M. Connors, Andreas Engert, Dana A. Kennedy, Eric L. Sievers, Robert Chen
Provision of study materials or patients: Anas Younes, Ajay K. Gopal, Scott E. Smith, Stephen M. Ansell, Joseph D. Rosenblatt, Kerry J. Savage, Radhakrishnan Ramchandren, Nancy L. Bartlett, Bruce D. Cheson, Sven de Vos, Andres Forero-Torres, Craig H. Moskowitz, Robert Chen
Collection and assembly of data: Anas Younes, Ajay K. Gopal, Scott E. Smith, Stephen M. Ansell, Joseph D. Rosenblatt, Radhakrishnan Ramchandren, Nancy L. Bartlett, Sven de Vos, Andres Forero-Torres, Joseph M. Connors, Andreas Engert, Dana A. Kennedy, Eric L. Sievers, Robert Chen
Data analysis and interpretation: Anas Younes, Ajay K. Gopal, Scott E. Smith, Stephen M. Ansell, Joseph D. Rosenblatt, Kerry J. Savage, Radhakrishnan Ramchandren, Nancy L. Bartlett, Bruce D. Cheson, Sven de Vos, Joseph M. Connors, Emily K. Larsen, Dana A. Kennedy, Eric L. Sievers, Robert Chen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Connors JM. State-of-the-art therapeutics: Hodgkin's lymphoma. J Clin Oncol. 2005;23:6400–6408. doi: 10.1200/JCO.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's disease. N Engl J Med. 2003;348:2386–2395. doi: 10.1056/NEJMoa022473. [Erratum: N Engl J Med 353:744, 2005] [DOI] [PubMed] [Google Scholar]
- 3.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 4.Majhail NS, Weisdorf DJ, Defor TE, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1065–1072. doi: 10.1016/j.bbmt.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Horning S, Fanale M, deVos S, et al. Defining a population of Hodgkin lymphoma patients for novel therapeutics: An international effort. Ann Oncol. 2008;19:118. abstr. [Google Scholar]
- 6.Dürkop H, Latza U, Hummel M, et al. Molecular cloning and expression of a new member of the nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 7.Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: A new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood. 1995;85:1–14. [PubMed] [Google Scholar]
- 8.Matsumoto K, Terakawa M, Miura K, et al. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004;172:2186–2193. doi: 10.4049/jimmunol.172.4.2186. [DOI] [PubMed] [Google Scholar]
- 9.Sutherland MS, Sanderson RJ, Gordon KA, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281:10540–10547. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- 10.Francisco JA, Cerveny CG, Meyer DL, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102:1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]
- 11.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 14.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 15.Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24:1633–1642. doi: 10.1200/JCO.2005.04.0543. [DOI] [PubMed] [Google Scholar]
- 16.Swain SM, Arezzo JC. Neuropathy associated with microtubule inhibitors: Diagnosis, incidence, and management. Clin Adv Hematol Oncol. 2008;6:455–467. [PubMed] [Google Scholar]
- 17.Anderlini P, Saliba R, Acholonu S, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin's lymphoma: The updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93:257–264. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarina B, Castagna L, Farina L, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: A retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115:3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 19.Claviez A, Canals C, Dierickx D, et al. Allogeneic hematopoietic stem cell transplantation in children and adolescents with recurrent and refractory Hodgkin lymphoma: An analysis of the European Group for Blood and Marrow Transplantation. Blood. 2009;114:2060–2067. doi: 10.1182/blood-2008-11-189399. [DOI] [PubMed] [Google Scholar]
- 20.Chen R, Palmer JM, Popplewell L, et al. Reduced intensity allogeneic hematopoietic cell transplantation can induce durable remission in heavily pretreated relapsed Hodgkin lymphoma. Ann Hematol. 2011;90:803–808. doi: 10.1007/s00277-010-1146-3. [DOI] [PubMed] [Google Scholar]