Summary
Small molecule inhibitors of amyloid aggregation have potential as treatment for a variety of conditions. In this issue of Chemistry & Biology, Romero et al. (2013) use amyloid-dependent B. subtilis biofilm formation to screen for amyloid inhibitors, identifying compounds that not only inhibit B. subtilis biofilm formation but also ones that disrupt preformed biofilms.
Amyloid fibers are historically associated with diseases such as Alzheimer’s, Huntington’s and the prion diseases. Many proteins can adopt the amyloid conformation—essentially a β-rich repeating structure where the β strands orient perpendicular to the fiber axis. Ordered amyloid protein polymers upset cellular proteostasis because they can inappropriately interact with membranes or sequester chaperone machinery. Therefore, the search is on for ways to ameliorate amyloid-related diseases by targeting amyloid formation. A major hurdle in this endeavor is that disease-associated amyloid formation is sporadic and difficult to faithfully reproduce in model organisms.
However, a growing number of ‘functional’ amyloids have been identified that are assembled by dedicated biogenesis systems. Functional amyloids and their assembly systems have been found in nearly all walks of cellular life, including mammals, fungi and bacteria (Hammer et al. 2008, Fowler et al. 2007). Functional amyloids contribute to cellular biology in various ways, including regulation of melanin synthesis, information transfer, or as a structural component. Furthermore, some of these functional amyloid systems provide a unique platform for understanding how amyloid formation can be directed and controlled so that cellular toxicity is minimized.
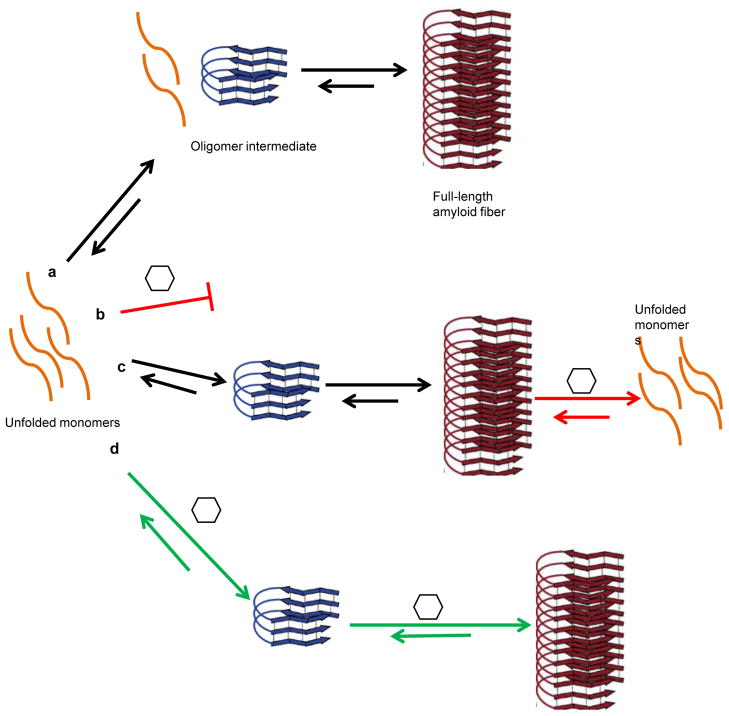
Amyloids are commonly found as the major protein component of the extracellular matrix in bacterial biofilms. Bacteria within the biofilm are protected from environmental insults, including disinfectants and antibiotics, making biofilms a major concern in hospital and industrial settings. Therefore, factors that can disrupt bacterial amyloid formation would be potential lead compounds for targeting bacterial biofilms or amyloid formation in general. The study presented in this issue of Chemistry & Biology by Romero et al. (2013) describes Bacillus subtilis pellicle biofilm as a model system to screen for amyloid inhibitors. The extracellular matrix of B. subtilis biofilms consist of two components, the exopolysaccharide (EPS) and the amyloidogenic protein TasA (Branda et al. 2006). Two small chemical molecules that inhibit biofilm formation by targeting the extracellular amyloid component of B. subtilis biofilms were identified. Importantly, one of the compounds not only inhibited biofilm formation but also disassembled already formed B. subtilis biofilms. Anti-biofilm compounds with distinct modes of action can be used synergistically to increase the potency of inhibition. The two inhibitory compounds described by Romero et al. created an even stronger inhibitory effect when allowed to exert their effect simultaneously. Collaboration between molecules may allow one type of molecule to dissolve pre-formed fibers, while another molecule could discourage new fiber formation (Fig. 1).
Fig. 1. Various modes of action of amyloid modulatory molecules.
a. A typical amyloid pathway. During the rate-limiting, lag phase, unfolded monomeric proteins assemble into conformationally dynamic oligomers. These oligomers can serve as seeds or templates that guide mature amyloid formation.
b. Inhibition at the monomer stage, the protein does not proceed to the oligomer intermediate.
c. A molecule capable of disassembling preformed amyloid fibers.
d. Stimulation of amyloid formation.
Utilizing functional amyloids as a tool for identifying general amyloid inhibitors has produced several promising leads in recent years. Strategic design of small compounds as amyloid modulators and thorough investigation of their effect on numerous amyloidogenic proteins is a promising approach to battle the threat of amyloid influence on health. One example of strategic design is the use of a small library of fluorescent compounds designed to interact with amyloid pathways based on structure-activity information (Chorell et al. 2012). This approach presents an opportunity to gain specific information on the systems studied by observing the interactions of the molecules and the amyloid protein or the cellular compartment. In the case of E. coli and biofilm formation, molecules have been discovered that inhibit both curli formation and type1-pili assembly, two protein structures that contribute to biofilm formation (Cegelski et al. 2009). Finally, another promising approach to screen for amyloid modulators makes use of the E. coli curli export system to assemble extracellular amyloid fibers of human disease associated Huntington or yeast prion proteins (Sivanathan et al. 2012). This provides an opportunity to screen for molecules affecting formation of the amyloid fibers.
The two molecules identified by Romero et al. seem thus far to be general amyloid inhibitors, as they were able to inhibit biofilm formation by Bacillus subtilis, Escherichia coli and Bacillus cereus. However, it is interesting to note that a molecule that inhibits amyloid formation by one protein may have a different effect on other proteins, including the ability to stimulate amyloid formation (Horvath et al. 2012). The two inhibitory molecules had no effect on Staphylococcus aureus biofilm even though S. aureus has been shown to produce functional amyloids that stabilize biofilm architecture (Schwartz et al. 2012). This might suggest that the compounds identified by Romero et al. are specific for certain amyloids or that the conditions in which the S. aureus biofilms were grown for this study do not require an amyloid matrix. Fully understanding of the specificity of these compounds and their biological activities will be a fascinating future direction for this work.
Clearly, Romero et al. have paved the way for capitalizing on a well-understood biofilm system to easily screen and identify new compounds that ameliorate amyloid formation.
Acknowledgments
The authors acknowledge helpful discussions from members of the Chapman lab, as well as support from NIH RO1-AI073847.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Hammer ND, Wang X, Mcguffie B, Chapman M. J Alzheimers Dis. 2008;13:407–419. doi: 10.3233/jad-2008-13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler D, Koulov A, Balch W, Kelly J. Trends Biochem Sci. 2007;32:217–224. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- Chorell E, Pinkner JS, Bengtsson C, Edvinsson S, Cusumano CK, Rosenbaum E, Johansson LB, Hultgren SJ, Almqvist F. Chemistry. 2012;18:4522–4532. doi: 10.1002/chem.201103936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegelski L, Pinkner JS, Hammer ND, Cusumano CK, Hung CS, Chorell E, Aberg V, Walker JN, Seed PC, Almqvist F, Chapman M, Hultgren SJ. Nat Chem Biol. 2009;5:913–919. doi: 10.1038/nchembio.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath I, Weise C, Andersson EK, Chorell E, Sellstedt M, Bengtsson C, Olofsson A, Hultgren S, Chapman MR, Wolf-Watz M, Almqvist F, Wittung-Stafshede P. J Am Chem Soc. 2012;134:3439–3444. doi: 10.1021/ja209829m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, et al. Chem Biol. 2013;20:xxxx–xxxx. [Google Scholar]
- Schwartz K, Syed AK, Stephenson RE, Rickard AH, Boles BR. PLoS Pathog. 2012;8:e1002744. doi: 10.1371/journal.ppat.1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanathan V, Hochschild A. Genes Dev. 2012;26:2659–2667. doi: 10.1101/gad.205310.112. [DOI] [PMC free article] [PubMed] [Google Scholar]