Abstract
The interaction between the linker for activation of T cells (LAT) with phospholipase C (PLC-γ1) is important for T cell receptor (TCR)-mediated Ca2+ signaling and MAPK activation. Knock-in mice harboring a mutation at the PLC-γ1 binding site (Y136) of LAT develop a severe lymphoproliferative syndrome. These mice have defective thymic development and selection and lack natural regulatory T cells, implicating a breakdown of both central and peripheral tolerance. To bypass this developmental defect, we developed a conditional knock-in line in which only LATY136F is expressed in mature T cells after deletion of the wildtype LAT allele. Analysis of LATY136F T cells indicated that the interaction between LAT and PLC-γ1 plays an important role in TCR-mediated signaling, proliferation, and IL-2 production. Furthermore, the deletion of LAT induced development of the lymphoproliferative syndrome in these mice. Although Foxp3+ natural Treg cells were present in these mice after deletion, they were unable to suppress the proliferation of conventional T cells. Our data indicated that the binding of LAT to PLC-γ1 is essential for the suppressive function of CD4+CD25+ regulatory T cells.
Keywords: T cells, cell activation, cell proliferation, signal transduction
Introduction
The linker for activation of T cells (LAT) is a transmembrane adaptor protein that plays a central role in linking TCR engagement to the activation of downstream signaling events, such as Ras-Erk activation and calcium mobilization. Upon T cell activation, LAT is phosphorylated by ZAP-70 and binds to Grb2, Gads, PLC-γ1, and other signaling proteins (1-5). Among nine conserved tyrosines in the cytoplasmic domain of LAT, four membrane-distal tyrosines are most important in LAT function during thymocyte development and TCR-mediated signaling (4, 6-8). Three of these tyrosines (Y171, Y191 and Y226 in human LAT) bind to Grb2 upon phosphorylation. Grb2 recruits Sos, a guanine nucleotide exchange factor (RasGEF), to the plasma membrane for activation of Ras (4, 9). Interestingly, TCR-mediated Ras activation also requires RasGRP1, another RasGEF that primes the activity of Sos (10). Two of these tyrosines, Y171 and Y191, also bind to Gads, which constitutively interacts with SLP-76. In addition, one critical tyrosine, Y132, associates with PLC-γ1; the interaction of LAT with PLC-γ1 and the Gads-SLP-76 complex is important for the activation of PLC-γ1. Upon activation, PLC-γ1 hydrolyzes phosphatidylinositol 4,5 biphosphate (PIP2) to generate diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). While DAG activates PKC and RasGRP1, IP3 binds to IP3 receptors on the endoplasmic reticulum (ER), inducing the flux of Ca2+ from the ER into the cytoplasm (11, 12). As a result of the transient elevation in the cytoplasmic Ca2+ levels, CRAC (calcium release activated calcium) channels are activated, allowing for the entry of Ca2+ from the extracellular environment. This influx eventually leads to the dephosphorylation and translocation of NFAT proteins to the nucleus (13).
The importance of LAT in T cell activation has been clearly demonstrated in LAT-deficient Jurkat cells. TCR-mediated Erk activation, Ca2+ mobilization, and NFAT translocation are all defective in these cells (5). Studies using LAT-deficient Jurkat cells also show that LAT interaction with PLC-γ1 through Y132 is important in TCR-mediated calcium flux and Ras activation (4). This interaction is also critical for thymocyte development and the control of T cell homeostasis and autoimmunity (14, 15). In LAT knock-in mice with a mutation at the PLC-γ1 binding site (Y136 in murine LAT), thymocyte development is partially blocked at the DN3 (CD25+CD44−) stage, likely due to impaired pre-TCR signaling, a necessity for the transition from the DN3 stage to the DN4 (CD25−CD44−) stage. Consequently, very few DP (CD4+CD8+) cells are present in the thymus. Despite the severe developmental defect, mature CD4+ T cells are present in these mice; however, they undergo uncontrolled proliferation in the periphery which results in the development of splenomegaly and lymphadenopathy (14, 16). The majority of these CD4+ T cells exhibit an effector/memory (CD44+CD62Llo) phenotype and produce high levels of Th2 cytokines, specifically IL-4 and IL-5. As a result of the dominance of Th2 cytokines, B cells are highly activated and secrete large amounts of IgE and IgG1(14). Recent studies indicate that the dominance of Th2 cells in these mice is dependent on STAT6. Interestingly, in the absence of STAT6, LATY136F mice still develop a lymphoproliferative disease; however, the T cell compartment is dominated by Th1 and CD8+ T cells (17).
Several studies have investigated the cause of the autoimmune disease observed in LATY136F mice. One early study suggests that autoimmunity in these mice is likely caused by defective thymic selection. In this study, by using an HY transgenic TCR model, it was shown that both positive selection and negative selection are impaired in LATY136F mice (18). A defect in negative selection may allow autoreactive T cells to survive and exit the thymus, contributing to the development of autoimmune disease. In addition to impaired negative selection, previous studies from our lab show that the LATY136F mice lack naturally arising T regulatory cells (CD4+CD25+ cells)(19). Correspondingly, the level of Foxp3 RNA in thymocytes and peripheral CD4+ T cells is dramatically reduced. In addition, Foxp3 protein is hardly detectable in CD4+ T cells. However, another study using Foxp3EGFP knock-in mice clearly shows that Foxp3+ cells, as indicated by GFP expression, are present in these mice (20). The GFP intensity in CD4+ LATY136F T cells appears to be lower than that in normal Treg cells, suggesting that LAT-mediated signaling is important for maintaining Foxp3 expression during thymocyte development or in the periphery. Additionally, data from our lab show that adoptive transfer of normal Treg cells into these mice can prevent the development of lymphoproliferative disease (19). These data suggest that the unchecked expansion of T cells observed in LATY136F mice may also be due to a lack of peripheral tolerance.
The defect in thymocyte selection processes in LATY136F mice is an obstacle in studying the role of the LAT-PLC-γ1 interaction solely in mature T cells. In this study, we used LAT conditional knock-in mice to examine the role of the LAT-PLC-γ1 interaction in the regulation of TCR-mediated signaling, Treg cell function and T cell homeostasis. We utilized the ERCre transgenic system in which a floxed gene can be deleted upon tamoxifen treatment. ERCre+LATf/f (f=floxed allele) and LATm/+ (m=Y136F form of lat) mice were crossed to produce ERCreLATf/+ and ERCreLATf/m mice. T cells should develop normally in these mice prior to treatment with tamoxifen. After injection, the floxed allele is deleted and T cells express either wild-type (WT) or Y136F LAT. Our results show that the LAT-PLC-γ1 interaction is required for optimal signaling and function in mature T cells. In contrast to previously published studies, mature Y136F mutated T cells were able to flux calcium, albeit at a reduced level. These T cells also failed to proliferate and produce IL-2 upon stimulation via the TCR. Treatment with tamoxifen induced the development of lymphoproliferative disease in ERCreLATf/m mice, similar to the disease observed in LATY136F mice, as indicated by uncontrolled expansion of CD4+ T cells, hyperactivation of both CD4+ T cells and B cells, and overproduction of Th2 cytokines. Furthermore, CD4+CD25+Foxp3+ Treg cells were present in the periphery of tamoxifen-treated ERCreLATf/m mice. However, they were not functional in vivo or in vitro. Our data indicate that the LAT-PLC-γ1 interaction is required for normal Treg cell function.
Materials and Methods
Mice
LATf/f mice were generated as previously described (21). These mice were crossed with ERCre transgenic mice (kindly provided by Dr. Thomas Ludwig, Columbia University, NY). LATf/f mice were first crossed with ERCre+ mice to produce ERCre+LATf/f mice, which were then bred with LATm/+ mice (m=Y136F) to generate ERCreLATf/m and ERCreLATf/+ mice. All mice were used in accordance with the National Institutes of Health guidelines. The procedures described in this study were reviewed and approved by the Duke University Institutional Animal Care and Use Committee. Mice were housed in specific pathogen-free conditions at the Duke University Animal Care facility.
Antibodies and FACS analysis
Texas Red conjugated to anti-IgM was purchased from Southern Biotech. Biotinylated CD69 and TCRβ were from BD Biosciences. 7AAD was purchased from Invitrogen. Thy1.1 Pacific Blue was purchased from BioLegend. All other antibodies used to stain cell markers for FACS analyses were obtained from eBioscience. For staining of cell surface markers, cells were incubated with different combinations of antibodies for approximately 30 minutes on ice and washed in PBS with 2% FBS before FACS analysis. For intracellular staining of cytokines, splenocytes were stimulated with 40 ng/ml of PMA and 500 ng/ml of ionomycin for 1 hour. After addition of Golgi-Stop, cells were stimulated for an additional 4 hours. For other intracellular staining, specifically Foxp3 and CTLA-4, cells were incubated with antibodies and fixed using the Foxp3 staining buffer set (eBioscience) per the manufacturer's guidelines. Samples were analyzed on FACSDiva or FACSCanto II (BD Biosciences). FACS plots shown were analyzed on the Flowjo software. TCR-mediated calcium flux was done as previously described(21).
CD4+ T cell proliferation, IL-2 production, and ELISA
CD4+ T cells were purified by positive selection using a CD4 microbead selection kit (Miltenyi Biotec). For T cell proliferation, 2×105 purified T cells were cultured in a 96-well plate coated with αCD3 (5μg/ml) with or without soluble αCD28 (0.5μg/ml). PMA (40ng/ml) plus ionomycin (500ng/ml) stimulation was used as a control to bypass TCR-mediated signaling. Cells were cultured in triplicate for 36 to 48 hours at 37°C and were then pulsed with 1μCi of [3H]thymidine for an additional 6 hours. Cells were harvested and [3H]thymidine incorporation was measured by a liquid scintillation luminescence counter (Perkin Elmer). For IL-2 production, the supernatants from the above culture conditions were harvested and used for ELISAs using IL-2 capture and detection antibodies according to the manufacturer's protocol (eBioscience). For serum antibody detection, serum was serially diluted. ELISAs for IgG1 and IgM was done using 96 well plates coated with unlabelled anti-mouse (H+L) antibody (Southern Biotech).
Western blots
T cells activated in vitro were used in the analysis of TCR-mediated signaling. Briefly, splenocytes were cultured in αCD3 antibody (2C11)-coated plates in the presence of murine IL-2 (10 ng/ml) for 2 days. Cells were then moved into new flasks to expand for 3 more days in the presence of IL-2. T cells were purified by negative selection using microbeads (Miltenyi Biotec) and were rested in medium without IL-2 for 6 hr before being incubated with biotinylated anti-CD3, anti-CD4 and anti-CD8, washed, and treated with streptavidin for the indicated time points at 37°C. Cells were lysed with RIPA buffer containing a cocktail of protease inhibitors. For Western blotting, samples were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were blotted with different primary antibodies as indicated in each figure and were then probed with either goat anti-mouse or anti-rabbit Ig conjugated with Alexa Fluor680 (Molecular Probes) or IRDye 800 (Rockland). Membranes were visualized and quantified with an infrared fluorescence imaging system (LI-COR Bioscience).
Reconstitution of LAT−/− mice and deletion of LAT
Single-cell suspensions were prepared from the lymph nodes and spleens of ERCreLATf/m or ERCreLATf/+ mice. T cells were enriched by negative selection. Cells were first incubated with biotinylated αB220, αGr1, αMac-1, αCD11c, αNK1.1 (all from eBioscience) on ice for 30 minutes, washed. Non-T cells were removed by using streptavidin-Dynabeads (Invitrogen). 20×106 cells were injected into each 6 week-old LAT−/− recipient via tail vein injections. After 5 weeks, blood was collected from the recipients and FACS analysis was done to ensure successful reconstitution.
To delete the floxed LAT in vivo, 1.5mg of tamoxifen dissolved in corn oil was injected intraperitoneally into mice for two consecutive days. For long-term deletion, the same dose of tamoxifen was administered once a week thereafter. For LAT deletion in vitro, cells were incubated with 4OHT (50nm) for 96 hours following activation with anti-CD3 as described above.
In vitro suppression assay
CD4+CD25+ cells (Treg cells) and Thy1.1+CD4+CD25− T cells (responders) were purified using a regulatory T cell isolation kit (StemCell Technologies). Responders were labeled with 5μM CFDA-SE for 10 minutes and washed three times with 5%FBS/PBS. 2×104 responders were cultured with 1μg/ml αCD3 (2C11), 4×104 APCs (splenocytes from LAT−/− mice), and Treg cells at different ratios as indicated in Figure 5. Cells were cultured for 66 to 72 hours, followed by FACS analysis.
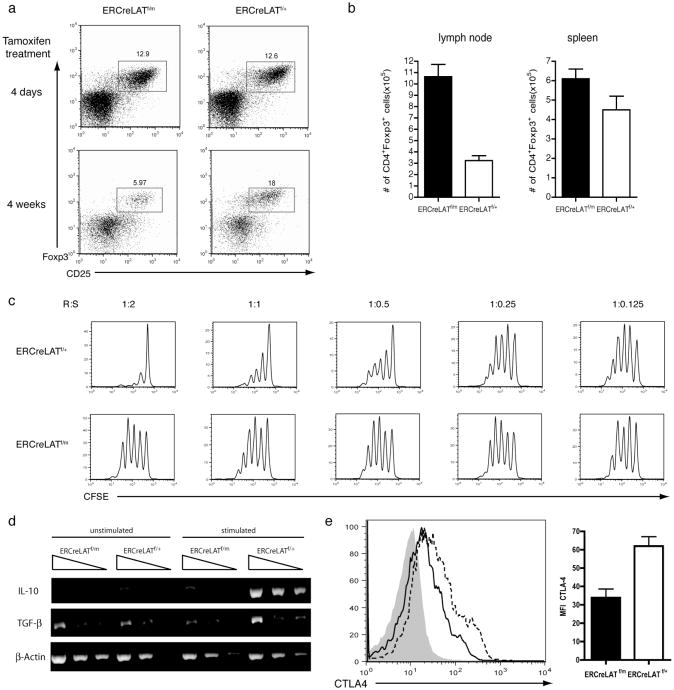
Figure 5. Impaired suppressive function of ERCreLATf/mCD4+Foxp3+ cells.
(a) CD25 and Foxp3 expression in CD4+GFP+ cells in ERCreLATf/m and ERCreLATf/+ mice 4 days after tamoxifen treatment (upper panel) and in LAT-/- mice reconstituted with ERCreLATf/m and ERCreLATf/+ T cells after 4 weeks of maintained tamoxifen treatment (lower panel). 3 mice were analyzed for each genotype. The figure shown is one representative of 4 experiments performed.
(b) Total number of cells from the mesenteric, inguinal, brachial and axillary lymph nodes (left panel) and the spleen (right panel) of ERCreLATf/m and ERCreLATf/+ mice were analyzed 4 days after tamoxifen treatment.
(c) In vitro suppression assay using various ratios of CD4+CD25− responder cells (R) to CD4+CD25+ suppressor cells (S) from ERCreLATf/m and ERCreLATf/+ mice 4-5 days after tamoxifen treatment. Proliferation of wildtype Thy1.1+CD4+CD25- responder cells is shown as indicated by CFSE dilution. The figure shown is one representative of 3 experiments performed.
(d) Expression of IL-10 and TGFβ in ERCreLATf/m and ERCreLATf/+ CD4+CD25+ T cells after one hour of anti-CD3 stimulation.
(e) CTLA-4 expression on GFP+CD4+Foxp3+ cells from the lymph nodes of ERCreLATf/m mice (solid line) and ERCreLATf/+ mice (dashed line) 4-5 days after tamoxifen treatment. GFP+CD4+Foxp3- cells were used as a control (filled area).
Semi-quantitative PCR
CD4+CD25+ and CD4+CD25− T cells were purified as above and were stimulated with plate-bound αCD3 and soluble αCD28 (2μg/ml) for one hour and were then used for RNA extraction. The following primers were used in RT-PCR: 5′-CTATGCTGCCTGCTCTTACTGAC-3′ and 5′-CGGAGAGAGGTACAAACGAGG-3′ for IL-10; 5′-TGCTGCTTTCTCCCTCAACCT-3′ and 5′-CACTGCTTCCCGAATGTCTGA-3′ for TGF-β; 5′-TGTTTGAGACCTTCAACACC-3′ and TAGGAGCCAGAGCAGTAATC-3′ for β-Actin.
Results
The generation of ERCreLATf/m mice
The LATY136F mice exhibit a partial block at the DN3 stage during thymocyte development (14, 16). Positive and negative selection are also impaired in these mice (18). To study the function of the LAT-PLC-γ1 interaction in TCR-mediated signaling and regulation of T cell homeostasis and autoimmunity, we sought to bypass this developmental block by using LAT knock-in mice we previously generated (21). In these mice, exons 7-11 are flanked by two Loxp sites. Deletion of these exons by Cre leads to the production of non-functional LAT. In addition, cells with the lat gene deleted are marked by the expression of GFP. To be able to induce deletion of LAT, we crossed ERCre mice, in which Cre recombinase is fused to a mutated estrogen receptor (ER) ligand-binding domain, with LAT knock-in mice (LATf/f) and LATY136F mice (LATm/+) to generate ERCreLATf/+ and ERCreLATf/m mice. In these mice, T cells should develop normally before injection of tamoxifen due to the presence of the WT allele. After injection, the floxed allele is deleted and T cells should express either Y136F or wild-type (WT) LAT. As shown in Figure 1a (upper panel), prior to tamoxifen treatment, both ERCreLATf/m and ERCreLATf/+ mice showed normal percentages of DN, DP, and SP T cells in the thymus, indicating that the floxed LAT allele is able to support normal thymocyte development. In addition, the percentages of CD4+ and CD8+ T cells in the spleens of these mice were normal (Figure 1a, lower panel). In LATY136F mice, expression of the TCR on peripheral T cells is very low (14) while in ERCreLATf/m and ERCreLATf/+ mice, peripheral CD4+ T cells expressed normal surface levels of TCRβ (data not shown).
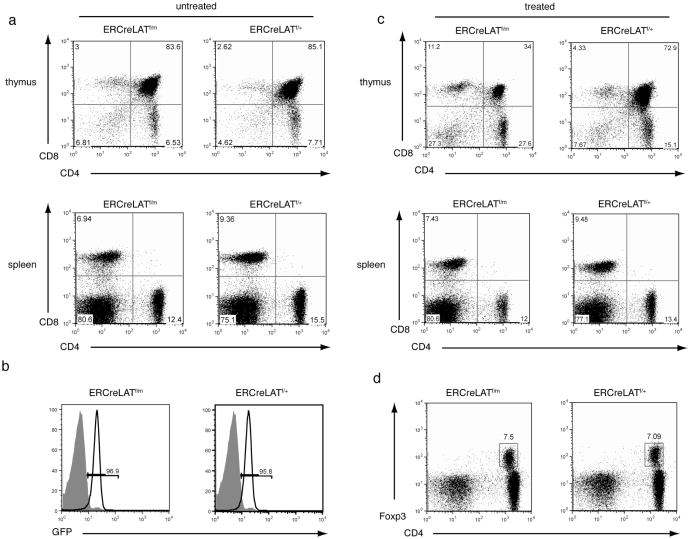
Figure 1. T cell development in ERCreLATf/m mice.
(a-b) CD4 and CD8 expression on thymocytes (upper panel) and splenocytes (lower panel) from untreated (a) and tamoxifen-treated (b) ERCreLATf/m and ERCreLATf/+ littermates.
(c) Efficient deletion of LAT after tamoxifen treatment. GFP expression on CD4+ T cells was analyzed. The shaded area represents CD4+ T cells from a C57BL/6 mouse.
(d) Expression of CD4 and Foxp3 on GFP+ cells from the lymph nodes of ERCreLATf/m and ERCreLATf/+ littermates 4-5 days after tamoxifen treatment. Data shown are representative of three mice of each genotype.
At day 4 after two consecutive injections of tamoxifen, the floxed LAT allele was successfully deleted as indicated by the expression of GFP on over 95% of splenic T cells (Figure 1b). This ERCre transgenic line has been used successfully to delete genes in B cells (22). In our system, tamoxifen-induced deletion affected thymocyte development in ERCreLATf/m mice. There was a reduced percentage of DP thymocytes accompanied by an increase in the frequency of DN thymocytes. The percentages of peripheral CD4+ and CD8+ T cells were similar to those in ERCreLATf/+ mice (Figure 1c, lower panel). Previously, our studies have shown that LATY136F mice lack CD4+CD25+ Treg cells (19). At day 4 after tamoxifen injection, the percentage of CD4+Foxp3+ cells in ERCreLATf/m mice was similar to that in ERCreLATf/+ mice (Figure 1d). Together, these data showed that we have successfully established an experimental system, which allows us to delete LAT in mature T cells and bypass the developmental block in LATY136F mice. These mice will be used to analyze the importance of the LAT-PLC-γ1 interaction in the control of T cell activation and autoimmunity.
The LAT-PLC-γ1 interaction in TCR-mediated signaling and T cell activation
Previously published data indicates that TCR-mediated phosphorylation of LAT and PLC-γ1 is impaired in T cells from LATY136F mice. Consequently, these mutant T cells fail to flux calcium upon TCR engagement (14). These results are similar to previously published data using Jurkat cells (4). However, TCR-mediated Erk activation is surprisingly normal in LATY136F CD4+ T cells (14, 16). Because of the low levels of TCR surface expression (14, 16), these T cells are not suitable to study the role of the LAT-PLC-γ1 interaction in primary T cells. To prepare a large number of cells for biochemical analysis, we deleted the floxed allele in vitro. To this end, splenocytes from ERCreLATf/m and ERCreLATf/+ mice were activated with anti-CD3 for 2 days and then cultured in IL-2 medium in the presence of hydroxyltamoxifen for 3-4 days. These T cells were rested for 6 hours in medium without IL-2 and used for analysis of TCR-mediated signaling. As shown in Figure 2a, surface expression of the TCR on ERCreLATf/m T cells was comparable to that on T cells from ERCreLATf/+ mice. While overall tyrosine phosphorylation of proteins was seemingly unaffected by the Y136F mutation (Figure 2b), TCR-mediated PLC-γ1 phosphorylation and Erk activation were significantly decreased in ERCreLATf/m T cells when compared to the ERCreLATf/+ control (Figure 2c). We next analyzed TCR-mediated calcium mobilization in these cells. In contrast to published data showing that LATY136F T cells fail to mobilize calcium (14), ERCreLATf/m T cells were able to flux some calcium, albeit reduced when compared with ERCreLATf/+ T cells (Figure 2d). Stimulation with ionomycin, a Ca2+ ionophore that bypasses TCR proximal signaling, induced similar levels of calcium flux. We next investigated the ability of ERCreLATf/m CD4+ T cells to produce IL-2. TCR-mediated IL-2 production was impaired in ERCreLATf/m CD4+ T cells as shown in Figure 2e. In addition, TCR-mediated proliferation, assayed by [3H]thymidine incorporation, was impaired in ERCreLATf/m CD4+ T cells while PMA and ionomycin-induced proliferation was similar (Figure 2f). Together, these results suggested that the LAT-PLC-γ1 interaction plays an important role in TCR-mediated PLC-γ1 activation, calcium mobilization, Erk phosphorylation, IL-2 production, and cell proliferation.
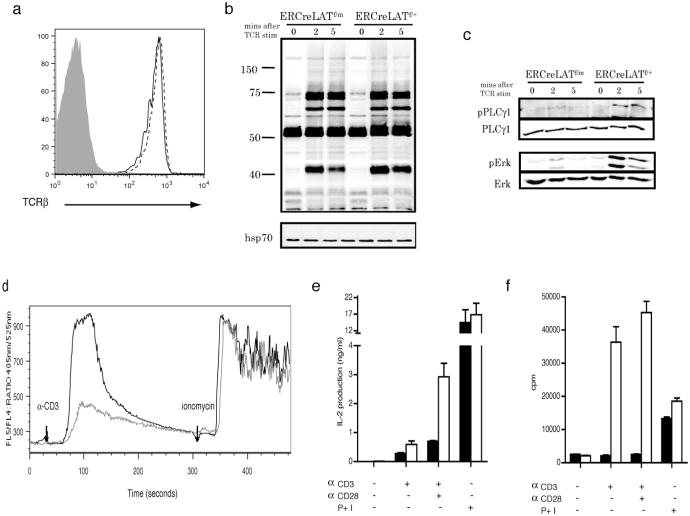
Figure 2. Defective TCR-mediated signaling and function in mature ERCreLATf/m T cells.
Analysis of T cells from ERCreLATf/m and ERCreLATf/+ mice 4-5 days after tamoxifen injections.
(a) TCRβ expression on ERCreLATf/m (solid line) and ERCreLATf/+ (dotted line) in GFP+CD4+ lymph node cells. Shaded area represents TCRβ expression on B cells.
(b)Western blot analysis showing total tyrosine phosphorylation in ERCreLATf/m and ERCreLATf/+ activated T cells after anti-CD3 stimulation. Data shown are a representative of 3 independent experiments.
(c) Western blots showing phosphorylation of PLC-γ1 and Erk1/2.
(d) Calcium flux in ERCreLATf/m (gray line) and ERCreLATf/+ (black line) GFP+CD4+ cells in response to TCR stimulation.
(e) IL-2 production in ERCreLATf/m (filled bars) and ERCreLATf/+ (empty bars) CD4+ cells. The figure is a representative of 4 independent experiments.
(f) Proliferation in ERCreLATf/m (filled bars) and ERCreLATf/+ (empty bars) CD4+ cells.
Development of the lymphoproliferative syndrome in ERCreLATf/m mice after tamoxifen treatment
LATY136F mice develop an autoimmune-like disease characterized by splenomegaly, lymphadenopathy, eosinophilia, and infiltration of lymphocytes into various tissues. CD4+ T cells from these mice undergo uncontrolled expansion and produce a copious amount of Th2 cytokines. As a result, B cells are also activated and undergo isotype-switching, producing high levels of IgG1 and IgE. Since we planned to study the LAT-PLCγ1 interaction in the control of autoimmunity, we first investigated whether ERCreLATf/m mice could be induced to develop a disease similar to that observed in LATY136F mice. ERCreLATf/m and ERCreLATf/+ mice were treated with tamoxifen once a week for four weeks to induce and maintain deletion of the floxed allele. In contrast to ERCreLATf/+ mice, ERCreLATf/m mice exhibited a similar phenotype to what is observed in LATY136F mice, as evidenced by enlarged lymph nodes and spleens (data not shown). Long-term treatment with tamoxifen induced an early block in thymocyte development in ERCreLATf/m mice, as indicated by a drastically reduced percentage of DP thymocytes and an accumulation of DN cells. The percentage of DN3 thymocytes (CD25+CD44−) was also increased in ERCreLATf/m mice (Figure 3a). This result indicated that tamoxifen-induced deletion could happen in early progenitor cells. This block in thymocyte development is similar to that in the LATY136F mice. In the periphery, CD4+ T cells outnumbered CD8+ T cells in ERCreLATf/m mice, although CD4+ T cells did not expand as much as in LATY136F mice (Figure 3b and 3c). The numbers of CD4+ and B220+ cells were increased by approximately two fold and the number of CD8+ cells was significantly decreased (Figure 3b). Most of those CD4+ T cells were CD44+CD62Llo/−, a phenotype of activated/memory T cells (Figure 3c). Additionally, these CD4+ T cells produced a high level of intracellular IL-4 (Figure 3d). B cells were hyperactivated and underwent isotype switching, as indicated by a high expression level of MHC Class II and the excessive production of IgG1 and IgE antibodies into the serum, respectively (data not shown). In LATY136F mice, CD4+CD25+ Treg cells are absent in the periphery (19). Interestingly, in ERCreLATf/m mice treated with tamoxifen, CD4+CD25+Foxp3+ cells were present and the percentage of these cells was similar to that in ERCreLATf/+ mice (Figure 3e). This result suggested that these regulatory T cells are not able to suppress the expansion of CD4+ effector cells. Collectively, these data indicated that the LATY136F phenotype can be recapitulated in tamoxifen-treated ERCreLATf/m mice. Moreover, the LAT-PLC-γ1 interaction might not be important in the survival of Treg cells in the periphery.
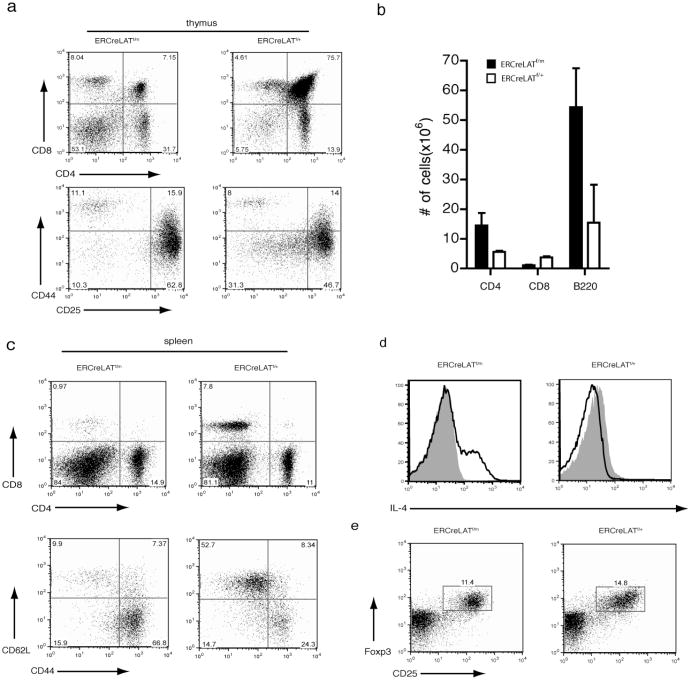
Figure 3. Recapitulation of the LATY136F phenotype in ERCreLATf/m mice 3 weeks after deletion of floxed LAT.
Analysis of ERCreLATf/m and ERCreLATf/+ mice after 3-4 weeks of tamoxifen treatment.
(a) CD4 and CD8 expression on GFP+ thymocytes from ERCreLATf/m and ERCreLATf/+ mice (upper panel). CD25 and CD44 expression profile on DN thymocytes (lower panel). (b) Total numbers of CD4+, CD8+ and B220+ cells in the spleens of ERCreLATf/m and ERCreLATf/+ mice. The figure shown is one representative of 5 mice analyzed.
(c) CD4 vs CD8 (upper panel) and CD62L vs CD44 surface expression in GFP+CD4+ splenocytes.
(d) Intracellular IL-4 expression in GFP+CD4+ splenocytes after P+I stimulation. The shaded area represents unstimulated controls.
(e) Surface CD25 and intracellular Foxp3 expression in GFP+CD4+ lymph node cells.
Deletion of the floxed allele in mature ERCreLATf/m T cells
Our data above showed that deletion of the floxed allele in ERCreLATf/m mice led to the development of an autoimmune syndrome similar to that in LATY136F mice; however, thymic selection may still be affected in ERCreLATf/m mice after 4 weeks of tamoxifen treatment, allowing for the escape of autoreactive T cells into the periphery. To determine whether impaired thymic selection is the cause for autoimmunity, we established a system in which LAT−/− mice were reconstituted with untreated mature ERCreLATf/m or ERCreLATf/+ T cells. Approximately 20×106 T cells were adoptively transferred into LAT−/− mice via tail vein injection. We waited about 5 weeks to allow expansion of these T cells by homeostatic proliferation prior to tamoxifen treatment. To ensure complete deletion of the floxed allele, we injected tamoxifen once a week for four weeks before analysis. As shown in Figure 4a, mice reconstituted with ERCreLATf/m T cells had splenomegaly after tamoxifen treatment. Lymph nodes were also enlarged in these mice. Without tamoxifen treatment, the size of the lymph nodes and spleen in these mice were normal (data not shown). In addition, mice reconstituted with ERCreLATf/+ T cells treated with tamoxifen also had spleens and lymph nodes similar in size to those of B6 mice (data not shown). These enlarged lymphoid organs were partially due to the expansion of CD4+ T cells present in the mice reconstituted with ERCreLATf/m cells (Figure 4a, right panel and Figure 4b), whereas the total number of CD8+ T cells was not significantly affected by the Y136F mutation. Analysis of the ERCreLATf/m CD4+ T cells showed that these cells also downregulated TCRβ on their cell surfaces like the LATY136F T cells (Figure 4c), suggesting that LAT-mediated signaling is required for maintaining cell surface expression of the TCR. Additionally, CD69, an early cell surface marker for activated T cells, was upregulated on the ERCreLATf/m CD4+ T cells compared with ERCreLATf/+ CD4+ T cells (data not shown). These data suggested that mature CD4+ T cells expressing the LATY136F mutant are hyperproliferative compared with T cells expressing wildtype LAT.
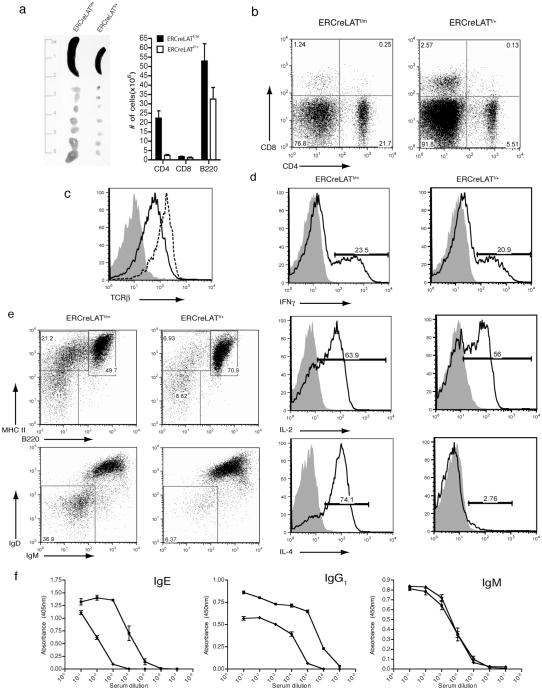
Figure 4. The presence of hyper-activated ERCreLATf/m T cells in the periphery of reconstituted LAT−/− mice.
Analysis of LAT−/− mice reconstituted with ERCreLATf/m or ERCreLATf/+ T cells and treated with tamoxifen for 4-5 weeks.
(a) Total numbers of CD4+, CD8+ and B220+ cells in the spleen of tamoxifen treated LAT−/− recipients reconstituted with ERCreLATf/m or ERCreLATf/+ T cells.
(b) CD4 and CD8 expression on splenocytes. 3 mice of each genotype were analyzed. Data shown is a representative of one of 4 such experiments.
(c) TCRβ expression on GFP+ CD4+ cells in LAT−/− recipients that received cells from either ERCreLATf/m (solid line) or ERCreLATf/+ (dotted line). The shaded area represents a negative control.
(d) IFN-γ, IL-2 and IL-4 production in GFP+CD4+ cells after P+I stimulation. Unstimulated controls (shaded area) and stimulated cells (solid line) are depicted.
(e) B220 and MHC Class II expression on lymph node cells (top panel). IgD and IgM expression on B220+ cells (bottom panel).
(f) Blood serum levels of IgE, IgG1 and IgM from LAT−/− recipients injected with T cells from ERCreLATf/m (squares) and ERCreLATf/+ (diamonds) mice.
We next investigated whether these hyperactivated T cells also produce a large amount of Th2 cytokines. Splenocytes from mice reconstituted with ERCreLATf/m or ERCreLATf/+ T cells were stimulated with PMA and ionomycin for 5 hours and the production of cytokines in these cells was analyzed by intracellular staining. As shown in Figure 4d, ERCreLATf/+ and ERCreLATf/m CD4+ T cells produced similar levels of IFNγ and IL-2 ; however, ERCreLATf/m CD4+ T cells produced much more IL-4. More than 70% of the ERCreLATf/m CD4+ T cells produced IL-4 compared to approximately 3% of the control cells (Figure 4d, lower panel). B cells were also highly activated in the mice reconstituted with ERCreLATf/m T cells as many of them had downregulated B220 and upregulated MHC Class II molecules (Figure 4e, upper panel). In addition, almost one third of the B220+ cells expressed low levels of surface IgD and IgM (Figure 4e, lower panel), indicating that many of these B cells had undergone maturation and isotype switching. In accordance with the above results, the serum concentrations of IgE and IgG1, but not IgM, were elevated in mice reconstituted with ERCreLATf/m T cells (Figure 4f). These results demonstrated that, despite undergoing normal thymic selection, ERCreLATf/m CD4 T cells still exhibited uncontrolled expansion and produced high levels of IL-4.
Defective suppressive function of ERCreLATf/m regulatory T cells
Our previous data show that CD4+CD25+ Treg cells are largely absent in LATY136F mice. Adoptive transfer of WT Treg cells into LATY136F mice suppresses the development of the autoimmune syndrome in these mice, suggesting that the absence of these Treg cells is the cause of this disease(19). However, LAT−/− mice that received ERCreLATf/m T cells and underwent tamoxifen treatment still exhibited signs of autoimmunity, as evidenced by hyperactivation of T and B cells, despite the fact that Treg cells were present. As shown in Figure 5a (upper panel), a similar percentage of Treg cells was present in ERCreLATf/m mice 4-5 days after tamoxifen treatment. Four weeks after deletion of LAT, about 6% of GFP+CD4+ T cells in the LAT−/− mice that received ERCreLATf/m T cells were CD25+Foxp3+ regulatory T cells, as compared to 18% in mice reconstituted with ERCreLATf/+ T cells (Figure 5a, lower panel). However, the total number of regulatory T cells in the lymph nodes of mice reconstituted with ERCreLATf/m T cells was approximately three fold more than those in mice reconstituted with ERCreLATf/+ T cells (Figure 5b). There was also an increase in the number of Treg cells in the spleen. These data suggested that the LAT-PLCγ1 interaction is not required for Treg cell survival; however, it might be important for the function of Treg cells to suppress proliferation of conventional T cells and to control autoimmunity.
To examine the function of ERCreLATf/m Treg cells, CD4+CD25+ T cells were purified from ERCreLATf/m and ERCreLATf/+ mice 5 days after treatment with tamoxifen and used in the assay for Treg cell function. CFSE-labeled wildtype Thy1.1+CD4+CD25− conventional T cells were used as responder cells. As shown in Figure 5c, anti-CD3-induced proliferation of responder cells could be suppressed efficiently by ERCreLATf//+ Treg cells in the presence of APCs, but not by ERCreLATf/m Treg cells. IL-10 and TGF-β are two suppressive cytokines that are produced by Treg cells. Compared with ERCreLATf/+ Treg cells, ERCreLATf/m Treg cells had reduced expression of IL-10 and TGF-β RNA after stimulation via the TCR (Figure 5d). These data suggested that the LAT-PLC-γ1 interaction in regulatory T cells plays an important role in the production of suppressive cytokines and in the suppression of conventional T cell proliferation.
Recent data indicate that the expression of CTLA-4 on Treg cells is essential for their ability to suppress proliferation of conventional T cells (23, 24). Furthermore, mice with CTLA-4 deleted in Foxp3+ cells develop a severe autoimmune disease (23). Hence, we next examined whether CTLA-4 expression on ERCreLATf/m Treg cells is normal. As shown in Figure 5e, 5 days after tamoxifen treatment, the mean fluorescence intensity (MFI) of CTLA-4 in Treg cells from ERCreLATf/m mice was reduced by approximately two-fold when compared with the CTLA-4 in ERCreLATf/+ Treg cells. This result suggested that the LAT-PLC-γ1 interaction is required for maintaining CTLA-4 expression in Treg cells.
Discussion
Our data demonstrate that the LAT-PLC-γ1 interaction is essential for efficient signaling in mature CD4 T cells in response to TCR crosslinking. Specifically, this interaction is important for TCR-mediated Erk activation, Ca2+ flux, IL-2 production, and proliferation. Moreover, when LAT−/− mice were reconstituted with ERCreLATf/m T cells and treated with tamoxifen, they developed a disease similar to that observed in LATY136F mice. Though regulatory T cells were present in ERCreLATf/m mice, our in vitro data show that they were nonfunctional, demonstrating that the LAT-PLC-γ1 interaction is not necessary for the survival of regulatory T cells in the periphery, but is essential for Treg cell function.
LATY136F T cells have a partial block at the DN3 stage of thymocyte development as well as defects in negative and positive selection (14, 16, 18). Our experimental model allowed for the analysis of T cells mutated at tyrosine 136 of LAT after they had undergone normal development and thymic selection. We were therefore able to assess the effects of this mutation specifically on signaling within mature T cells as well as on the function of regulatory T cells without the complications of any pre-existing developmental defects. Our results showed that phosphorylation of PLC-γ1 was defective in ERCreLATf/m T cells when stimulated via the TCR. Consequently, calcium flux was impaired in these mature T cells. These results are similar to those seen in studies using LAT-deficient Jurkat cells expressing human LAT mutated at Tyr132(4, 8). However, in contrast to LATY136F knock-in CD4+ T cells, calcium flux was not completely absent in ERCreLATf/m T cells; this difference can be attributed to the normal TCR expression level on the surfaces of ERCreLATf/m T cells, whereas LATY136F T cells express very low TCR levels (14, 16). The residual calcium flux may be a result of the activation of PLC-γ1 through the association of the Gads-SLP76-PLC-γ1 complex to LAT via the direct binding of Gads to LAT. In addition to causing defects in TCR-mediated PLC-γ1 activation and Ca2+ signaling, the Y136F mutation also affected Erk activation in mature T cells, as phosphorylation of Erk was decreased in ERCreLATf/m T cells when compared to the ERCreLATf/+ control. This is not surprising as it has been shown that the phosphorylation and activation of PLC-γ1 are important for RasGRP1 and MAPK activation (4, 25). However, Sommers et al found that LATY136F peripheral T cells exhibited normal Erk phosphorylation in response to stimulation via the TCR, even though TCR surface expression is very low on these cells (14). This Erk activation occurs despite the lack of LAT and PLC-γ1 phosphorylation and the abrogation of calcium flux in these T cells. The reason for the difference observed between Erk phosphorylation in LATY136F and ERCreLATf/m T cells could be that LATY136F T cells are sensitized by the presence of excess cytokines in the autoimmune environment, allowing for Erk activation upon TCR engagement. It is therefore likely that Erk activation in LATY136F T cells occurred in a LAT-independent manner, possibly through a cytokine-mediated Jak-Stat signaling pathway (26).
Three to four weeks after tamoxifen treatment, ERCreLATf/m mice developed a similar phenotype to that in six-week-old LATY136F mice. Thymocyte development was impaired in ERCreLATf/m mice, including a partial block from the DN3 to the DN4 stage and a subsequently low percentage of DP thymocytes. CD4+ T cells underwent expansion, existing in a highly activated state and producing excessive IL-4; this phenomenon induced B cell hyper-activation and production of excessive IgE and IgG1. These data demonstrate that after three weeks of tamoxifen injection, the phenotype of ERCreLATf/m mice closely resembles that of LATY136F mice.
In order to more closely investigate the effect of mutating the LATY136 site in mature T cells on the breakdown of peripheral tolerance, LAT−/− mice were reconstituted with peripheral ERCreLATf/m T cells. This experimental design eliminated aspects of the LATY136F phenotype related to impaired pre-TCR signaling and the partial block from the DN3 to the DN4 stage of thymocyte development, as well as issues associated with defective positive and negative selection. After tamoxifen treatment, LAT−/− mice that were reconstituted with peripheral ERCreLATf/m T cells exhibited signs of a lymphoproliferative disorder similar to that in LATY136F mice. The development of this lymphoproliferative syndrome in our experimental model, in spite of normal thymocyte development and selection processes, suggested that the Y136F mutation in mature T cells alone is sufficient for the development of the lymphoproliferative disorder in LATY136F mice.
Even though negative selection is indeed impaired in LATY136F thymocytes, it might not contribute significantly to the development of the autoimmune disorder in these mice. It is possible that a breakdown in peripheral tolerance is the predominant cause of this disease. Our previous studies show that CD4+CD25+ T cells are absent in LATY136F mice. Adoptive transfer of wildtype CD4+CD25+ T cells into LATY136F neonates rescued these mice from the autoimmune syndrome, demonstrating a role for these cells in the control of autoimmunity (19). While these data showed a defect in Treg cell development, another study suggested that a small population of Foxp3+ cells are actually present in LATY136F mice (20). In our model, ERCreLATf/m Treg cells developed normally before deletion of the wildtype allele. After deletion, regulatory T cells expressing the LATY136F mutant were able to survive in the periphery. In fact, the total number of ERCreLATf/m CD4+Foxp3+ regulatory T cells in lymph nodes was approximately 3 times that of the ERCreLATf/+ control. Our in vitro suppression assays demonstrated that ERCreLATf/m CD4+CD25+ regulatory T cells lacked the ability to control the proliferation of wildtype CD4+CD25− cells. These data suggested that the LAT-PLC-γ1 interaction is essential for the suppressive function of regulatory T cells.
Our data in vivo and in vitro indicated that ERCreLATf/m Treg cells were not functional despite that they expressed Foxp3. However, our previous study has shown that ectopic expression of Foxp3 into the LATY136F T cells restores their suppressive capability. While these results appear to be contradictory, this can be easily explained by the differences of Foxp3 expression in these cells. In ERCreLATf/m Treg cells, Foxp3 expression was similar or slightly lower than that in WT Treg cells (Figure 3e and 5a). In our retroviral experiments, the expression of ectopic Foxp3 in the LATY136F T cells should be much higher than the endogenous one. Studies done by Ramsdell's group show that even CD8+ T cells with overexpression of Foxp3 have suppressive function(27). Thus, it is not surprising that overexpression of Foxp3 by retroviral transduction restores their suppressive function. Our current data suggested that expression of Foxp3 at the endogenous level in Treg cells is not sufficient for their suppressive function and normal signaling through LAT is also required.
Many studies have been done to elucidate the mechanisms that enable regulatory T cells to suppress the proliferation of effector T cells. Although it is well known that regulatory T cells are able to produce suppressive cytokines, such as IL-10 and TGF-β, there are conflicting reports on the importance of these cytokines in Treg cell function. While some in vitro data do not support a role for these cytokines in the suppressive function of Treg cells (28, 29), several in vivo studies have revealed their importance in dampening inflammation caused by infectious agents, allergies, and environmental components (30-34). However, studies using IL10flox/flox × Foxp3YFP-CRE mice demonstrate that IL-10 produced by Treg cells is not necessary for the regulation of systemic autoimmunity (33). TGF-β is another suppressive cytokine that may be important in Treg cell function. In some models, such as IBD and type I diabetes, TGF-β is produced by Treg cells but in other models, this suppressive cytokine is produced by different cell types (34-37). Studies done using an autoimmune gastritis model show that TGF-β is not essential for Treg cell function (38, 39). Our data showed that the LAT-PLC-γ1 interaction is important in production of IL-10 and TGF-β by Treg cells in response to TCR stimulation. Addition of IL-10 in the suppression assay did not rescue the function of ERCreLATf/m Treg cells in vitro (data not shown), suggesting that the impaired production of these cytokines is less likely the reason for the defective function of these Treg cells. Given these conflicting data pertaining to the role of TGF-β and IL-10 in Treg cell-mediated suppression, it is still possible that these cytokines may contribute the suppressive function of Treg cells in vivo.
ERCreLATf/m CD4 T cells have a defect in the ability to flux calcium in response to TCR stimulation. Recent studies demonstrated the necessity of a robust calcium flux for the suppressive function of Treg cells (40). Therefore, it is possible that the impaired function of ERCreLATf/m Treg cells can be explained by the importance of the LAT-PLC-γ1 interaction in TCR-mediated calcium signaling. Even though the defects in TCR-mediated proliferation and IL-2 production by ERCreLATf/m T cells could be corrected by stimulation with PMA and ionomycin (Figure 2), which bypasses TCR proximal signaling, ERCreLATf/m Treg cells pre-activated with PMA and ionomycin for four hours still failed to suppress the proliferation of responder cells (data not shown). Thus, it is possible that continuous activation of Treg cells by engagement with APCs is required for efficient suppression or that the LAT-PLC-γ1 interaction is required for maintenance of Treg program and function, which could not be simply corrected by stimulation with PMA and ionomycin. Another mechanism through which Treg cells may induce the suppression of conventional T cells is termed “IL-2 sink.” This process is described as the ability of Treg cells to absorb IL-2 from the surrounding environment via the IL-2Rα (CD25), thus depriving effector T cells of this cytokine which drives proliferation and differentiation. Bim, a proapoptotic factor, is upregulated in effector T cells as a result of this IL-2 starvation, inducing CD4+CD25− T cells to undergo apoptosis (41). Treg cells in ERCreLATf/m mice after tamoxifen treatment express normal levels of CD25. In addition, ERCreLATf/m T cells can expand in vitro in the presence of IL-2, indicating that CD25-mediated signaling is normal. This suggests that “IL-2 sink” is unlikely to be a mechanism of suppression in our experimental system.
Studies by Wing et al demonstrated that while the expression of CTLA-4 on regulatory T cells is not necessary for Treg cell development and survival, it is essential for both their in vivo and in vitro suppressive capabilities (23). In these studies, mice with CTLA-4 specifically deleted in Treg cells (CKO) develop a lymphoproliferative syndrome similar to the condition observed here. While wildtype Treg cells are able to prevent the upregulation of CD80 and CD86, DCs cultured with CKO Treg cells still expressed high levels of these cell surface molecules (23, 42, 43). This demonstrates that CTLA-4 is required to thwart DC activation and subsequently diminish the activation of conventional T cells through the CD28 coreceptor molecule (23). It has also been shown that high expression of CTLA-4 on regulatory T cells correlates to their ability to increase the activity of indoleamine 2,3-dioxygenase (IDO) in dendritic cells (43, 44). IDO is an immunosuppressive enzyme that has been linked to the suppression of effector cell proliferation (44, 45). The activation of IDO initiates tryptophan catabolism, leading to the production of pro-apoptotic catabolites known as kynurenines, and the subsequent regulation of effector cells. Our data reveal a decrease in the protein expression of CTLA-4 in ERCreLATf/m Treg cells. Whether this reduction in CTLA-4 expression in Treg cells is indeed the cause of the observed functional defect remains to be determined. While it is known that Treg cells need to be activated via the TCR for their suppressive activity, it is less clear what signals downstream of the TCR regulate Treg cell function. Our experimental system will allow us to decipher how TCR signaling regulates Treg cell suppression and will also enable us to dissect the molecular mechanisms that lead to the development of autoimmunity.
Acknowledgments
This work was supported by National Institutes of Heath grants AI048674 and AI056156. W.Z. is a scholar of Leukemia and Lymphoma Society.
Footnotes
Add-in-proof: During the preparation of this manuscript, a similar study using mice in which the floxed LAT can be deleted upon injection of diptheria toxin also showed development of the autoimmunity after deletion of WT LAT (46).
References
- 1.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 2.Weber JR, Orstavik S, Torgersen KM, Danbolt NC, Berg SF, Ryan JC, Tasken K, Imboden JB, Vaage JT. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. The Journal of experimental medicine. 1998;187:1157–1161. doi: 10.1084/jem.187.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Irvin BJ, Trible RP, Abraham RT, Samelson LE. Functional analysis of LAT in TCR-mediated signaling pathways using a LAT-deficient Jurkat cell line. International immunology. 1999;11:943–950. doi: 10.1093/intimm/11.6.943. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. The Journal of biological chemistry. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 5.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCgamma1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhu M, Janssen E, Zhang W. Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J Immunol. 2003;170:325–333. doi: 10.4049/jimmunol.170.1.325. [DOI] [PubMed] [Google Scholar]
- 7.Sommers CL, Menon RK, Grinberg A, Zhang W, Samelson LE, Love PE. Knock-in mutation of the distal four tyrosines of linker for activation of T cells blocks murine T cell development. The Journal of experimental medicine. 2001;194:135–142. doi: 10.1084/jem.194.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J, Weiss A. Identification of the minimal tyrosine residues required for linker for activation of T cell function. The Journal of biological chemistry. 2001;276:29588–29595. doi: 10.1074/jbc.M102221200. [DOI] [PubMed] [Google Scholar]
- 9.Buday L, Egan SE, Rodriguez Viciana P, Cantrell DA, Downward J. A complex of Grb2 adaptor protein, Sos exchange factor, and a 36-kDa membrane-bound tyrosine phosphoprotein is implicated in ras activation in T cells. The Journal of biological chemistry. 1994;269:9019–9023. [PubMed] [Google Scholar]
- 10.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Molecular and cellular biology. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaraman T, Ondrias K, Ondriasova E, Marks AR. Science. Vol. 272. New York, N.Y: 1996. Regulation of the inositol 1,4,5-trisphosphate receptor by tyrosine phosphorylation; pp. 1492–1494. [DOI] [PubMed] [Google Scholar]
- 12.Putney JW, Jr, Bird GS. The signal for capacitative calcium entry. Cell. 1993;75:199–201. doi: 10.1016/0092-8674(93)80061-i. [DOI] [PubMed] [Google Scholar]
- 13.Vig M, Kinet JP. Calcium signaling in immune cells. Nature immunology. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sommers CL, Park CS, Lee J, Feng C, Fuller CL, Grinberg A, Hildebrand JA, Lacana E, Menon RK, Shores EW, Samelson LE, Love PE. Science. Vol. 296. New York, N.Y: 2002. A LAT mutation that inhibits T cell development yet induces lymphoproliferation; pp. 2040–2043. [DOI] [PubMed] [Google Scholar]
- 15.Von Boehmer H. Control of T-cell development by the pre-T and alpha beta T-cell receptor. Annals of the New York Academy of Sciences. 1995;766:52–61. doi: 10.1111/j.1749-6632.1995.tb26648.x. [DOI] [PubMed] [Google Scholar]
- 16.Aguado E, Richelme S, Nunez-Cruz S, Miazek A, Mura AM, Richelme M, Guo XJ, Sainty D, He HT, Malissen B, Malissen M. Science. Vol. 296. New York, N.Y: 2002. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor; pp. 2036–2040. [DOI] [PubMed] [Google Scholar]
- 17.Archambaud C, Sansoni A, Mingueneau M, Devilard E, Delsol G, Malissen B, Malissen M. STAT6 deletion converts the Th2 inflammatory pathology afflicting Lat(Y136F) mice into a lymphoproliferative disorder involving Th1 and CD8 effector T cells. J Immunol. 2009;182:2680–2689. doi: 10.4049/jimmunol.0803257. [DOI] [PubMed] [Google Scholar]
- 18.Sommers CL, Lee J, Steiner KL, Gurson JM, Depersis CL, El-Khoury D, Fuller CL, Shores EW, Love PE, Samelson LE. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. The Journal of experimental medicine. 2005;201:1125–1134. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koonpaew S, Shen S, Flowers L, Zhang W. LAT-mediated signaling in CD4+CD25+ regulatory T cell development. The Journal of experimental medicine. 2006;203:119–129. doi: 10.1084/jem.20050903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Kissenpfennig A, Mingueneau M, Richelme S, Perrin P, Chevrier S, Genton C, Lucas B, DiSanto JP, Acha-Orbea H, Malissen B, Malissen M. Th2 lymphoproliferative disorder of LatY136F mutant mice unfolds independently of TCR-MHC engagement and is insensitive to the action of Foxp3+ regulatory T cells. J Immunol. 2008;180:1565–1575. doi: 10.4049/jimmunol.180.3.1565. [DOI] [PubMed] [Google Scholar]
- 21.Shen S, Zhu M, Lau J, Chuck M, Zhang W. The essential role of LAT in thymocyte development during transition from the double-positive to single-positive stage. J Immunol. 2009;182:5596–5604. doi: 10.4049/jimmunol.0803170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro-Shelef M, Lin KI, Savitsky D, Liao J, Calame K. Blimp-1 is required for maintenance of long-lived plasma cells in the bone marrow. The Journal of experimental medicine. 2005;202:1471–1476. doi: 10.1084/jem.20051611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. Science. Vol. 322. New York, N.Y: 2008. CTLA-4 control over Foxp3+ regulatory T cell function; pp. 271–275. [DOI] [PubMed] [Google Scholar]
- 24.Friedline RH, Brown DS, Nguyen H, Kornfeld H, Lee J, Zhang Y, Appleby M, Der SD, Kang J, Chambers CA. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. The Journal of experimental medicine. 2009;206:421–434. doi: 10.1084/jem.20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roose JP, Mollenauer M, Gupta VA, Stone J, Weiss A. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Molecular and cellular biology. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winston LA, Hunter T. Intracellular signalling: putting JAKs on the kinase MAP. Curr Biol. 1996;6:668–671. doi: 10.1016/s0960-9822(09)00445-x. [DOI] [PubMed] [Google Scholar]
- 27.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. International immunology. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. The Journal of experimental medicine. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 31.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. The Journal of experimental medicine. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. The Journal of experimental medicine. 2003;197:111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nature medicine. 2003;9:1202–1208. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 36.Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, Maizels RM. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. The Journal of experimental medicine. 2005;202:1199–1212. doi: 10.1084/jem.20042572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kullberg MC, Hay V, Cheever AW, Mamura M, Sher A, Letterio JJ, Shevach EM, Piccirillo CA. TGF-beta1 production by CD4+ CD25+ regulatory T cells is not essential for suppression of intestinal inflammation. European journal of immunology. 2005;35:2886–2895. doi: 10.1002/eji.200526106. [DOI] [PubMed] [Google Scholar]
- 38.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. The Journal of experimental medicine. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nature immunology. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nature immunology. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature immunology. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 42.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. European journal of immunology. 2000;30:1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 43.Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology. 2006;118:240–249. doi: 10.1111/j.1365-2567.2006.02362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nature immunology. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 45.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Science. Vol. 297. New York, N.Y: 2002. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase; pp. 1867–1870. [DOI] [PubMed] [Google Scholar]
- 46.Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, Archambaud C, Wang Y, Perrin P, Bertosio E, Sansoni A, Richelme S, Locksley RM, Aguado E, Malissen M, Malissen B. Loss of the LAT Adaptor Converts Antigen-Responsive T Cells into Pathogenic Effectors that Function Independently of the T Cell Receptor. Immunity. 2009 doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]