Abstract
Pulmonary fibrosis is characterized by the accumulation of fibroblasts and myofibroblasts. These cells may accumulate from three potential sources: the expansion of resident lung fibroblasts, the process of epithelial-mesenchymal transition, or the recruitment and differentiation of circulating mesenchymal precursors known as fibrocytes. We have previously demonstrated that fibrocytes participate in lung fibrogenesis following administration of FITC to mice. We now demonstrate that leukotriene-deficient 5-LO−/− mice are protected from FITC-induced fibrosis. Both murine and human fibrocytes express both cysteinyl leukotriene receptor (CysLT) 1 and CysLT2. In addition, fibrocytes are capable of producing CysLTs and can be regulated via the autocrine or paracrine secretion of these lipid mediators. Exogenous administration of leukotriene (LT) D4, but not LTC4 induces proliferation of both murine and human fibrocytes in a dose-dependent manner. Consistent with this result, CysLT1 receptor antagonists are able to block the mitogenic effects of exogenous LTD4 on fibrocytes. Endogenous production of CysLTs contributes to basal fibrocyte proliferation, but does not alter fibrocyte responses to basic fibroblast growth factor. Although CysLTs can induce the migration of fibrocytes in vitro, they do not appear to be essential for fibrocyte recruitment to the lung in vivo, possibly due to compensatory chemokine-mediated recruitment signals. However, CysLTs do appear to regulate the proliferation of fibrocytes once they are recruited to the lung. These data provide mechanistic insight into the therapeutic benefit of leukotriene synthesis inhibitors and CysLT1 receptor antagonists in animal models of fibrosis.
Idiopathic pulmonary fibrosis (IPF)3 likely results from an abnormal healing response to injury of the alveolar surface in the lung (1). Development of the disease is characterized by alveolar epithelial cell injury, inflammatory cell accumulation, fibroblast hyperplasia, and collagen deposition. Ultimately, IPF results in loss of lung elasticity and reduction of the alveolar surface area, causing impairment of gas exchange and severe compromises in pulmonary function (2).
The pathogenesis of pulmonary fibrosis is not completely understood, but is thought to involve expansion of local lung fibroblasts as well as recruitment of fibrocytes to the lung (3–8). Fibrocytes are bone marrow-derived cells which share phenotypic and functional properties of both leukocytes and mesenchymal cells. They are characterized by the expression of CD45 or CD34 and collagen 1. They directly contribute to extracellular matrix generation and promote fibrotic responses through the synthesis of fibroblast products collagen 1, collagen 3, and fibronectin (3–9). Fibrocytes migrate to sites of injury in a diverse group of lung diseases where they play a crucial role in tissue remodeling and repair (6 – 8). At sites of tissue injury, fibrocytes synthesize extracellular matrix and express fibrogenic cytokines such as IL-1β, TNF-α, CCL2, CXCL1, platelet-derived growth factor α, TGF-β1, and M-CSF (4). Fibrocyte recruitment is a complex biological phenomenon mediated by a variety of ligands and receptors. Fibrocytes have been shown to be recruited to the lungs by CXCR4, CCR7, and CCR2-mediated signals (6, 7). Interestingly, fibrocytes are recruited to both bleomycin- and FITC-treated lungs, and the severity of the disease correlates with the number of fibrocytes recruited (6, 7).
Eicosanoids are lipid mediators derived from arachidonic acid metabolism and they can promote or inhibit fibrotic lung disease by influencing mesenchymal cell activity. Leukotrienes (LTs) promote fibroblast chemotaxis, fibroblast proliferation, and collagen synthesis (10–12). PGs PGE2 and prostacyclin inhibit such profibrotic responses (13–15). Derangements of eicosanoid synthesis are present in fibrotic lungs. IPF patients exhibit underproduction of PGE2 (16) and overproduction of LTs (12). Levels of both classes of LTs-cysteinyl LTs (CysLTs; LTC4, LTD4, and LTE4) and LTB4 are elevated in these patients. Alveolar macrophages (AMs) are a primary source of LT synthesis and contribute to increased production of LTs in IPF lung homogenates (12).
Animal models of fibrotic lung disease are also characterized by increased LT production following lung injury (17). Previously, we have demonstrated that mice that are genetically deficient in 5-lipoxygenase (5-LO), an enzyme necessary for LT generation, are protected from bleomycin-induced pulmonary fibrosis (17). In addition, treatment with the CysLT receptor 1 (CysLT1) antagonists, MK571 (18) or montelukast (19), also limit bleomycin-induced fibrotic changes. Strong evidence therefore indicates that LTs and fibrocytes both participate in pulmonary fibrosis. LTs exert direct effects on migration (20), proliferation (21), and matrix protein synthesis (22) by fibroblasts, but the effects of LTs on fibrocytes remain unknown. In this study, we present novel evidence for LT regulation of fibrocytes during fibrogenesis.
Materials and Methods
Mice
C57BL/6 and 5-LO−/− mice backcrossed to this background were purchased from The Jackson Laboratory. Due to limited availability of these mice, however, some experiments were performed with 5-LO−/− (129-Alox5tm1Fun/J) (23) and strain-matched (129SvEv) wild-type (WT) mice bred in the University of Michigan Unit for Laboratory Animal Medicine from breeders originally obtained from The Jackson Laboratory. Both female and male mice were used. Mice were studied between 2 and 5 mo of age. Animal protocols were approved by the University Committee on the Use and Care of Animals.
FITC model of pulmonary fibrosis
FITC inoculation was performed as previously described (24). Briefly, mice were anesthetized with ketamine and xylazine. The trachea was exposed and entered with a needle under direct visualization. FITC (28 mg; Sigma-Aldrich) was dissolved in 10 ml of sterile PBS, vortexed extensively, and sonicated for 30 s. This slurry was transferred to multiuse vials and vortexed extensively before each 50-μl aliquot was removed for intratracheal injection using a 23-gauge needle. Collagen accumulation in the lung was measured by hydroxyproline assay on day 21 after FITC treatment as we have previously described (6).
Reagents used
CysLT1 antagonists MK571 and Ly171883 were purchased from BIOMOL. LTD4 was purchased from Cayman Chemical. Abs against human CysLT1 (rabbit polyclonal, sc-25448) and murine CysLT2 (goat polyclonal, sc-27097) were purchased from Santa Cruz Biotechnology. Appropriate secondary Abs conjugated to peroxidase for Western blotting were purchased from Pierce.
Fibrocyte isolation
Murine lungs were perfused with 5 ml of normal saline and removed using aseptic conditions. Lungs were minced with scissors in DMEM complete medium containing 10% FCS. Lungs from a single animal were placed in 15 ml of medium in tissue culture flasks. Mesenchymal cells were allowed to grow out of the minced tissue and when cells reached 70% confluence they were passaged using trypsin digestion. Mesenchymal cells were grown for 14 days before being harvested by trypsin digestion. Cells were stained with anti-CD45 Abs coupled to magnetic beads (Miltenyi Biotec). Labeled cells were then sorted by binding the cell population to MS- or LS-positive selection columns using a SuperMACS apparatus (Miltenyi Biotec) according to manufacturer’s instructions. Cells were then washed extensively. CD45+ cells were retained on the column and can be removed by flushing the column with buffer once it is removed from the magnetic field. CD45− cells were collected in the original flow-through. For extra purity, CD45+ cells were sometimes reapplied to a second MS-positive selection column. The absolute number of lung fibrocytes was determined by counting the cells that were retained on the column by a hemocytometer. Immunohistochemical staining or flow cytometry staining on this population confirmed that these cells were CD45+CD13+col 1+.
For isolation of human fibrocytes, 20 ml of peripheral blood was collected in heparinized Vacutainers (BD Biosciences) from consenting normal volunteers. Whole blood was diluted 1/1 with 0.9% normal saline. Thirty milliliters of the diluted whole blood was layered onto 15 ml of Ficoll-Hypaque in a 50-ml conical tube and centrifuged at 1200 rpm for 45 min. The buffy coat layer was removed, washed three times in serum-free medium (SFM), and the cell pellet was then cultured in complete medium containing 20% FCS for 14 days. At this time point, adherent cells were >95% fibrocytes as determined by flow cytometry for CD45 and procollagen 1 expression. Fibrocytes were trypsinized and replated in SFM for proliferation assays or Western blot analysis. These experiments were approved by the University of Michigan Institutional Review Board.
Proliferation assay
Murine fibrocytes (2 × 105/ml) were plated in 96-well flat-bottom tissue culture dishes and were cultured for 8–32 h in SFM before the addition of 10 μCi of [3H]thymidine for an additional 16 h. Cells were harvested onto glass fiber filters using an automated cell harvester and filters were counted using a beta scintillation counter. In some experiments, fibrocytes from WT or 5-LO−/− mice were cultured in the presence or absence of LTD4 or LTC4 at indicated doses. Some experiments also included the CysLT1 antagonists, Ly171883 (1 μM) or MK571 (10 nM). Human fibrocytes were cultured at 2 × 105 cells/ml in SFM or SFM containing 10 nM LTD4.
Western blots
Cultured fibrocytes in 35-mm dishes were washed with ice-cold PBS and 200 −l of cold lysis buffer (1% w/w Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 0.15 M NaCl, 0.01 M NaH2PO4, 0.02 M EDTA, 0.05 M NaF, 0.002 M NA3VO4, and 1/100 dilution of Calbiochem Protease Cocktail Set II (Calbiochem-Novabiochem)) was added to each sample. Lysates were assayed for total protein concentration using the DC Protein Assay (Bio-Rad). Four micrograms of protein from each sample was then analyzed for expression of CysLT1, CysLT2, or β-actin using methods that have been described previously (25).
Chemotaxis assay
Lung mince cultures were grown for 14 days and serum-starved for 24 h before magnetic purification of CD45+ fibrocytes. Purified fibrocytes (1 × 106/ml) were plated in the top wells of Boyden chambers and migration (cells per high-powered field) was measured in response to 0.1% FBS (negative control), fibronectin (positive control, 100 μg/ml; Sigma-Aldrich) or LTD4 (10 nM; Cayman Chemical) through gelatin-coated 5- to 8-μm filters. Checkerboard analysis proved that the migration was directional.
Semiquantitative real-time PCR
Semiquantitative real-time RT-PCR was performed on an Applied Biosystems Prism 7000 thermocycler. Gene-specific primers and probes were designed using Primer Express software (PerkinElmer/PE Applied Biosystems). The sequences for all primers and probes are found in Table I. Briefly, the reaction mixture contained 250 ng of RNA, 12.5 μl of TaqMan 2× Universal PCR Master Mix, 0.625 μl of 40× MultiScribe and RNase Inhibitor Mix (Applied Biosystems and Roche), 250 nM FAM probe, and forward and reverse primers at 300 nM in a final volume of 25 μl. For each experiment, samples were run in triplicate. The average cycle threshold (CT) was determined for each sample, and relative gene expression was calculated using the comparative CT method (26), which assesses the difference in gene expression between the gene of interest and an internal standard gene (β-actin) for each sample to generate the ΔΔCT. The average of the control sample (AMs) was set to 1 for each experiment and the relative gene expression for each experimental sample was compared with that.
Table I.
Primers and probes for RT-PCR analysisa
| Description | Primer Sequence |
|---|---|
| CysLT1 forward primer | CTGAGGTACCAGATAGAGGCTGATC |
| CysLT1 reverse primer | CTTGGTGCCTTGGAGGTACATT |
| CysLT1 probe | TTCCTGCTTTGGCTTCTCAAGGGCTG |
| CysLT2 forward primer | TGCTGAGTGTGGTGCGTTTC |
| CysLT2 reverse primer | CCAGGCACTCCTGACACTGGTG |
| CysLT2 probe | ACAGTCCACCCCTTCCGGATGTTCC |
| β-Actin forward primer | CTGCCTGACGGCCAAGTC |
| β-Actin reverse primer | CAAGAAGGAAGGCTGGAAAAGAG |
| β-Actin probe | AACGAGCGGTTCCGATGCCCTG |
| BLT1 forward primer | ACGCACGGTCACCGCCCTGC |
| BLT1 reverse primer | CACCGCTAGGAAGGGCAGCAGG |
| BLT1 probe | CTGGACCGATCACTGGCAGTGG |
| BLT2 forward primer | GTAGTATGGAGCTTAGCGGGC |
| BLT2 reverse primer | AGGGTCTCCAGGCTCAGATG |
| BLT2 probe | GTGCTCGCCGTCCCGGCCGC |
CysLT1, CysLT2, and β-actin were analyzed by real-time PCR; BLT1 and BLT2 were analyzed by conventional RT-PCR followed by Southern blotting.
Expression of the LTB4 receptors, BLT1 and BLT2, were examined by 40 cycles of conventional RT-PCR using primers and probes listed in Table I. Southern blotting using internal probes confirmed the absence of a BLT1 or BLT2 signal in isolated fibrocytes. Neutrophils were used as a positive control for BLT1 and BLT2 amplification.
Collagenase digestions of whole lung
For some experiments, lungs were digested with collagenase on day 5 after FITC treatment according to a previously published protocol (27). Lungs were excised, minced, and enzymatically digested for 30 min using 15 ml/lung of digestion buffer (RPMI 1640, 5% FCS, antibiotics, and 1 mg/ml collagenase; Boehringer Mannheim) and 30 μg/ml DNase (Sigma-Aldrich). The cell suspension and undigested fragments were further dispersed by repeated passage through the bore of a 10-ml syringe without a needle. The total cell suspension was pelleted and any contaminating erythrocytes were eliminated by lysis in ice-cold NH4Cl buffer (0.829% NH4Cl, 0.1% KHCO3, and 0.0372% Na2 EDTA, pH 7.4). The pellet was resuspended in 5 ml of complete medium (RPMI 1640, 5% FCS, and 1% penicillin/streptomycin) and dispersed by 20 passages through a 5-ml syringe. The dispersed cells were filtered through a Nytex filter (Tetko) to remove clumps. The total volume was brought up to 10 ml with complete medium. An equal volume of 40% Percoll (Sigma-Aldrich) was added, and the cells were centrifuged at 3000 rpm for 30 min (room temperature) with no brake. The cell pellets were resuspended in complete medium and leukocytes were counted on a hemocytometer in the presence of trypan blue. Cells were >90% viable by trypan blue exclusion. Recovered leukocytes were analyzed by flow cytometry.
Bronchoalveolar lavage (BAL)
Alveolar cells were obtained via ex vivo lung lavage using a previously described protocol (28). Briefly, these cells were collected in lavage fluid consisting of complete medium (DMEM, 1% penicillin-streptomycin, 1% L-glutamine, 10% FCS, and 0.1% Fungizone) and 5 mM EDTA. The cells were enumerated by counting on a hemocytometer before use. In some experiments, alveolar cells were enriched by a 1-h adherence step in SFM before being cultured for 24 h in complete medium. The adherent fraction consists largely of AMs in untreated mice and of AMs, fibrocytes, and neutrophils after FITC treatment. In some cultures, 5 μM Ca2+ ionophore (A23187) was added for 1 h in SFM as a maximal stimulus for LT synthesis. In other experiments, BAL cell pellets were cultured for 14 days ex vivo.
Flow cytometry analysis
Cells obtained by collagenase digestion or BAL were incubated for 15 min on ice with Fc block (clone 24G2; BD Pharmingen) before surface staining with CD45-PerCP-Cy5.5 (BD Pharmingen) followed by fixation and permeabilization using the BD Pharmingen Cytofix/Cytoperm kit according to the manufacturer’s instructions. Cells were then blocked with goat IgG before staining for collagen 1 (rabbit anti-mouse; Accurate Chemical & Scientific) followed by a donkey anti-rabbit PE secondary Ab (Jackson ImmunoResearch Laboratories). Cells were analyzed on a flow cytometer (FACScan; BD Biosciences). Human fibrocytes were stained with anti-human CD45 FITC (BD Biosciences) and, following a blocking step, collagen was assessed using anti-human procollagen I Ab from Santa Cruz Biotechnology followed by a donkey anti-goat PE secondary Ab from Jackson ImmunoResearch Laboratories.
Assay of LTs
The measurements for CysLTs and LTB4 were performed on lung homogenates and alveolar cell and fibrocyte supernatants using enzyme immunoassay (EIA) kits obtained from Cayman Chemical according to the manufacturer’s instructions. The lower limit of detection was 10 pg/ml. Lipids were first extracted from lung homogenates using C18 SepPak cartridges according to our previously published protocol (17).
Statistical analysis
When analyzing three or more groups, statistical significance was measured by ANOVA. For comparison between data from two groups, data were analyzed by Student’s t test. A p < 0.05 was considered significant.
Results
FITC treatment stimulates CysLT production
To verify that FITC deposition resulted in CysLT release, we treated WT (C57BL/6) mice with FITC on day 0 and homogenized lungs on days 0, 3, and 7. Lipids were extracted from lung homogenates using C18 SepPak cartridges. Levels of CysLTs increased on days 3 and 7 after FITC treatment (Fig. 1A). We next measured the production of CysLTs from alveolar cells purified by BAL on days 0 and 7 after FITC treatment. Plastic-adherent cells from the BAL, which likely include AMs, fibrocytes, and neutrophils, were cultured for 1 h in the presence or absence of the calcium ionophore A23187 (Fig. 1B) to provide a maximal stimulus for arachidonic acid release and LT synthesis. Synthesis of CysLTs was significantly elevated in cells purified from FITC-treated mice ( p = 0.0002). Similar results were seen in FITC-treated 129SvEv mice. These results indicate that inflammatory cells likely contribute to increased lung CysLTs after FITC treatment.
FIGURE 1.
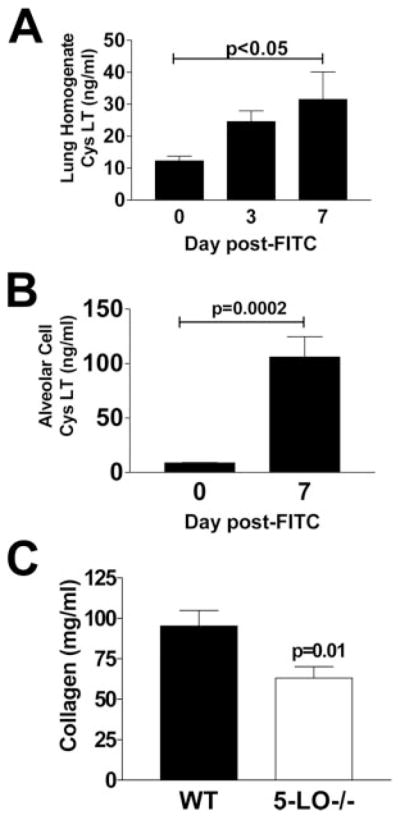
FITC deposition results in release of CysLTs. A, WT (C57BL/6) mice were injected with FITC on day 0. Lung homogenates were collected on days 0, 3, and 7 after FITC treatment. Lipids were extracted and levels of CysLTs were determined by specific EIA, n = 4–6/group, p < 0.05 by ANOVA at day 7. B, Mice were injected with FITC on day 0 and plastic-adherent BAL cells were harvested on day 0 or 7. These cells (which consist mostly of AMs but may contain some fibrocytes and neutrophils after FITC treatment) were cultured at 5 × 105/ml for 1 h in the presence of 5 μM A23187 and supernatants were analyzed for CysLTs via specific EIA, n = 4, p = 0.0002 by Student’s t test. C, WT (129SvEv) or 5-LO−/− mice were injected with FITC intratracheally on day 0. On day 21, mice were euthanized, lungs were removed, and collagen content was determined via hydroxyproline assay, n = 10, p = 0.01 by Student’s t test.
5-LO−/− mice are protected from FITC-induced fibrosis
Previous studies have demonstrated that 5-LO−/− mice are protected from bleomycin-induced lung fibrosis (17). To verify that 5-LO−/− mice were protected from FITC-induced fibrosis, we injected WT (129SvEv) or 5-LO−/− mice with FITC on day 0 and measured collagen accumulation in the lungs by hydroxyproline assay on day 21 after FITC treatment. Fig. 1C demonstrates that 5-LO−/− mice are significantly protected from FITC-induced fibrosis.
Fibrocytes express both the CysLT1 and CysLT2 receptors
We next investigated the expression of LT receptors on fibrocytes. Fibrocytes were purified from C57BL/6 mice and total mRNA was prepared. The mRNA was analyzed for expression of the two CysLT receptors, cysLT1 and cysLT2, by real-time RT-PCR using mRNA levels in AMs as a positive control. To directly compare the expression of CysLT1 and CysLT2 in fibrocytes, the expression of CysLT1 in AMs was set to 1 and then the expression of CysLT1 and CysLT2 on fibrocytes was compared with this value. As noted in Fig. 2A, the levels of CysLT1 mRNA were ~12-fold higher in fibrocytes compared with AMs. The levels of CysLT2 mRNA on fibrocytes were approximately one-half the level of CysLT1 mRNA. We next analyzed the expression of CysLT1 and CysLT2 protein in isolated murine AMs, fibrocytes, and fibroblasts by Western blot analysis (Fig. 2B). Despite the differences in mRNA levels noted above, the levels of CysLT1 protein appear similar in both fibrocytes and AMs. In contrast, CysLT1 is expressed at low levels in fibroblasts. CysLT2 protein levels in AMs and fibrocytes appear similar. Interestingly, the level of CysLT2 noted in fibroblasts is greater than the levels on either fibrocytes or AMs. CysLT1 and CysLT2 protein levels in fibrocytes isolated from human peripheral blood were detected at similar ratios to those found in cells from mice (Fig. 2C). The receptors for LTB4, BLT1, and BLT2, were analyzed by conventional RT-PCR followed by Southern blotting using internal probes, but were found to be undetectable in murine fibrocytes (data not shown). Thus, LT effects on fibrocytes are likely mediated via the CysLT1 and/or CysLT2 receptors.
FIGURE 2.
Murine and human fibrocytes express CysLT1 and CysLT2. A, Murine fibrocytes were purified from C57BL/6 lung digest cultures, and total RNA was prepared and analyzed for the expression of CysLT1 and CysLT2 by real-time RT-PCR. The expression level of each gene was first normalized to β-actin and next compared with the expression level of CysLT1 in AMs. This data are representative of two independent experiments. B, Murine fibrocytes and fibroblasts were purified from lung digest cultures. AMs were isolated from BAL. Four micrograms of protein from each lysate was run and analyzed by Western blotting with anti-human CysLT1 or anti-murine CysLT2 receptor Abs. Each lane represents cells from a single animal. Blots were stripped and probed for β-actin as a housekeeping control. C, Human fibrocytes were isolated from buffy coats of two normal volunteers. Western blotting was performed on 4 μg of cell lysates as above.
Fibrocytes produce CysLTs
Although it is known that leukocytes produce CysLTs, the ability of fibrocytes, which do maintain some leukocyte markers, to make LTs is unknown. Fibrocytes were cultured overnight in SFM and supernatants were collected for analysis of total CysLTs and LTB4 by specific EIA. Levels of CysLTs in overnight unstimulated cultures of fibrocytes were 57.2 ± 10.8 pg/ml for CysLTs (Fig. 3). However, when cells were treated with the Ca2+ ionophore A23187 for 1 h, which provides a maximal stimulus for arachidonic acid release and LT biosynthesis, levels of CysLTs measured in the fibrocyte cultures were 470 ± 63 pg/ml. For comparison, the levels of CysLTs produced by ionophore-treated fibroblast cultures were only 24.6 ± 15 pg/ml. Levels of LTB4 in the fibrocyte cultures were undetectable in the overnight unstimulated conditions and reached 25 ± 6 pg/ml in the ionophore-stimulated conditions (data not shown). Thus, fibrocytes retain the ability to produce LTs and thus may be responsive to CysLT signaling in either an autocrine or paracrine manner.
FIGURE 3.
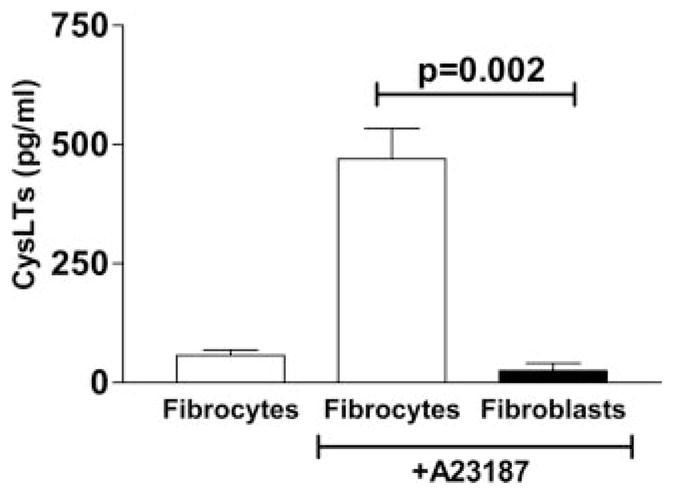
Fibrocytes produce LTs. Fibrocytes and fibroblasts were purified from WT (C57BL/6) mice and cultured at 105/ml overnight in SFM. The next morning, A23187, a calcium ionophore was added for 1 h at 5 μM. Supernatants were collected and analyzed for the production of CysLTs by specific EIA, n = 4. Ionophore-stimulated fibrocytes produced significantly more CysLTs than did fibroblasts, p = 0.002 by Student’s t test.
LTs enhance fibrocyte proliferation
We examined the effect of endogenous LTs on fibrocyte proliferation by comparing basal proliferative rates of fibrocytes from WT (C57BL/6) and 5-LO−/− mice over 48 h in complete medium. Fibrocytes from 5-LO−/− mice exhibited a diminished proliferative capacity (Fig. 4A). Thus, endogenous LT production can influence fibrocyte proliferation in vitro.
FIGURE 4.
LTs regulate fibrocyte proliferation. A, Fibrocytes were purified from lung mince cultures at day 14 from either WT (C57BL/6) or 5-LO−/− mice. Fibrocytes were cultured at 2 × 105/ml in complete medium for 48 h and proliferation was measured via [3H]thymidine incorporation over the final 16 h, n = 6, p = 0.0025 by Student’s t test. B, Fibrocytes were purified from WT (129SvEv) mice or strain-matched 5-LO−/− mice (C) and cultured at 2 × 105/ml in SFM with or without 1–100 nM LTD4 or LTC4 for 24 h. Proliferation was measured over the final 16 h as previously described, n = 6; *, p < 0.05 by ANOVA.
We next tested the influence of exogenous LTC4 and LTD4 on the proliferation of fibrocytes from WT (129SvEv, Fig. 4B) and 5-LO−/− mice (Fig. 4C). Exogenous addition of LTD4 increased fibrocyte proliferation of both WT and 5-LO−/− fibrocytes in a dose-dependent manner within 24 h. In contrast, the addition of LTC4 had little effect in either cell type. LTD4 is the most potent agonist of the CysLT1 receptor (29). The efficacy of LTD4 compared with LTC4 suggested that that these effects were being mediated via CysLT1 rather than CysLT2.
To verify receptor utilization, exogenous LTD4 was added to fibrocytes from WT (C57BL/6) mice in the presence or absence of CysLT1-specific receptor antagonists MK571 (Fig. 5A) and Ly171883 (Fig. 5B). Fibrocytes treated with 10 nM LTD4 proliferated significantly more than fibrocytes did in SFM. Treatment with the CysLT1 antagonists MK571 or Ly171883 abrogated this LTD4 enhancement of fibrocyte proliferation. The significant effects of the CysLT1 receptor antagonists to block all effects of exogenous LTD4 confirm that LTD4 signals proliferation of fibroblasts via the CysLT1 receptor.
FIGURE 5.
Exogenous LTD4 can augment fibrocyte proliferation in a CysLT1-dependent manner. Fibrocytes were purified from lung mince cultures of WT (C57BL/6) mice and cultured at 2 × 105/ml in SFM in the presence or absence of 10 nM LTD4 and either MK571 at 10 nM (A) or Ly171883 at 1 μM (B) for 48 h, n = 6. LTD4 was able to significantly stimulate fibrocyte proliferation in both experiments. MK571 and Ly171883 both significantly reduced LTD4-stimulated proliferation (p < 0.05 for both by ANOVA).
Endogenous CysLTs do not influence fibrocyte proliferation in response to basic fibroblast growth factor (bFGF)
To determine whether fibrocytes from WT (129SvEv) and 5-LO−/− mice would show differences in proliferation to a different stimulus, fibrocytes were isolated from lung digests of both genotypes and tested for proliferation in response to either SFM or 100 ng/ml bFGF over 48 h. This stimulus increased fibrocyte proliferation in WT cells from 563 ± 82 to 890 ± 168 cpm (n = 6). In cells from 5-LO−/− mice, bFGF stimulated proliferation from 450 ± 54 to 993 ± 321 cpm (n = 6, p < 0.05 for bFGF compared with SFM in each group, but p = NS between WT and 5-LO−/− cells plus bFGF). Thus, there was no significant difference in the response of fibrocytes from WT or 5-LO−/− mice in response to a different proliferative stimulus.
CysLTs do not enhance the differentiation of fibrocytes to fibroblasts in vitro
To determine whether CysLTs affect the rate of fibrocyte differentiation to fibroblasts, purified fibrocytes (>98% CD45+ on day 0) from WT (C57BL/6 mice) were cultured in the presence or absence of 10 nM LTD4 for 4 days. The percentage of CD45+ cells remaining in each culture was then determined by flow cytometry. The percentages of CD45+ cells remaining in the culture were similar for both treatments at day 4 (47.2% untreated vs 43.8% plus LTD4), indicating that LTD4 did not enhance the differentiation of CD45+ fibrocytes to CD45− fibroblasts.
LTs mediate fibrocyte chemotaxis in vitro
After identifying a role for LTs in fibrocyte proliferation, we next ascertained whether LTs affect the migration of fibrocytes. Using Boyden chamber assays, we demonstrated that LTD4 stimulates migration of fibrocytes from WT (C57BL/6) mice (Fig. 6). The ability of LTD4 to stimulate chemotaxis of fibrocytes was similar to the potency of fibronectin which was used as a positive control for mesenchymal cell chemotaxis.
FIGURE 6.
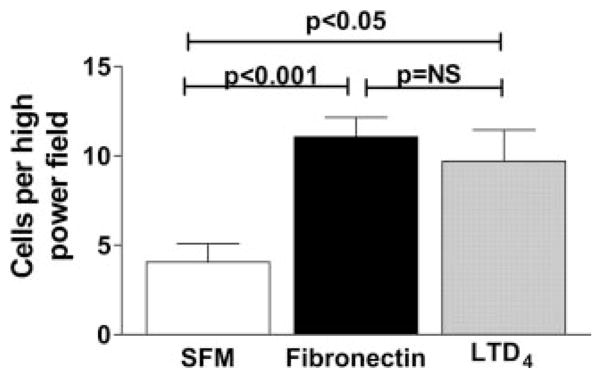
LTD4 stimulates fibrocyte chemotaxis in vitro. Fibrocytes were purified from lung mince cultures of WT (C57BL/6) mice. In brief, 1 × 106 serum-starved fibrocytes were plated in the top well of Boyden chambers and chemotaxis through 8-μm gelatin-coated filters was measured in response to fibronectin (100 mg/ml) or 10 nM LTD4. Ten high-powered fields were counted for each condition. LTD4 stimulation of chemotaxis of fibrocytes is equivalent to that of fibronectin and statistically increased over control (SFM alone), p < 0.05.
Fewer fibrocytes are cultured from the lungs of FITC-treated 5-LO−/− mice
We next analyzed the impact of LTs on fibrocyte accumulation by comparing fibrocyte numbers that could be cultured from the lungs in WT (129SvEv) and 5-LO−/− mice. We challenged both groups of mice with FITC and 5 days later performed a BAL. Cell pellets from the BAL were cultured for 14 days to allow leukocyte populations to die off and mesenchymal populations (fibrocytes) to expand. We found that the number of fibrocytes that can be cultured from 5-LO−/− mice is less than the number of fibrocytes that can be cultured from WT mice after FITC treatment (Fig. 7). These results were verified in WT and 5-LO−/− mice on the C57BL/6 background. In brief, 17 ± 0.5 × 104 fibrocytes were cultured from FITC-treated C57BL/6 mice, whereas only 8.6 ± 0.3 × 104 fibrocytes were cultured from 5-LO−/− mice after FITC treatment ( p = 0.0006). Because our previous results suggested that endogenous LTs could augment fibrocyte chemotaxis as well as proliferation in vitro, it was not clear whether these findings reflected a difference in the initial recruitment of fibrocytes to the lung or differences in the proliferative capacity of the fibrocytes during the 2-wk culture period.
FIGURE 7.
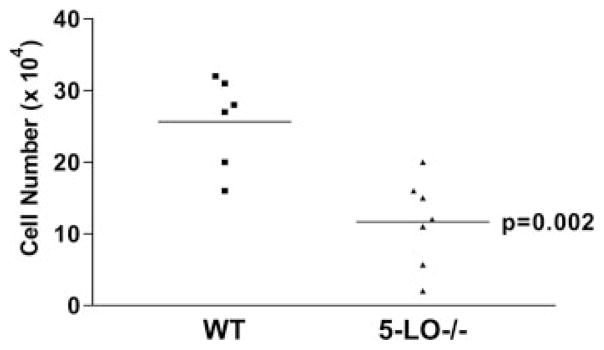
Fewer fibrocytes were cultured from the lungs of FITC-treated 5-LO−/− mice. WT (129SvEv) or 5-LO−/− mice were challenged with FITC on day 0. On day 5 after FITC treatment, BAL was performed and cell pellets were cultured for 14 days to enumerate fibrocytes. Fewer fibrocytes were cultured from the BAL of 5-LO−/− mice compared with WT mice, n = 6–7/group, p = 0.002 by Student’s t test.
LTs are not essential for fibrocyte recruitment in vivo
To analyze the initial recruitment of fibrocytes to the lung in response to FITC, we used flow cytometry to identify CD45+ collagen 1+ cells in either the BAL fluid after FITC treatment or in lung digests after FITC treatment. We compared the recruitment of fibrocytes in WT (C57BL/6) vs 5-LO−/− mice (Fig. 8) and also the recruitment of fibrocytes in WT mice treated with FITC in the presence or absence of the CysLT1 receptor antagonist, MK571 (data not shown). We have previously demonstrated that day 5 represents the peak time point for fibrocyte accumulation after FITC treatment (6). In the present study, we found that the number of fibrocytes recruited to the lung on day 5 after FITC treatment was not affected either by 5-LO genetic ablation or by MK571 treatment. Taken together, these results suggest that endogenous LTs influence proliferation rather than recruitment of fibrocytes.
FIGURE 8.
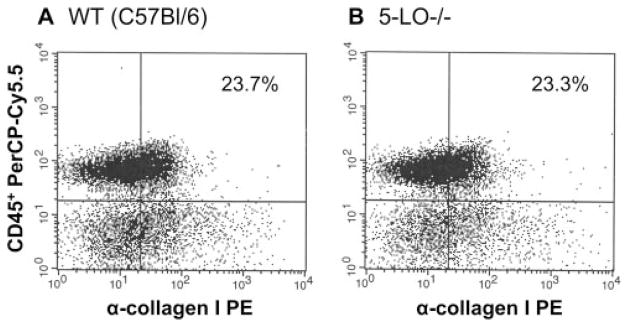
LTs are not necessary for fibrocyte recruitment in response to FITC in vivo. WT (C57BL/6) or 5-LO−/− mice were treated with FITC on day 0. On day 5 after FITC treatment, lungs were subjected to collagenase/DNase digest and cells were stained for expression of CD45 and collagen 1. Similar percentages of CD45+ col 1+ fibrocytes were noted in both groups. Similarly, absolute numbers were not different either. Similar results were obtained when we analyzed the recruitment of fibrocytes to WT mice treated with FITC in the presence or absence of MK571, analyzed BAL cells rather than lung digests, or analyzed WT and 5-LO−/− mice on the 129SvEv background. Thus, LTs do not appear to be essential for fibrocyte recruitment in vivo. Graphs are representative of n = 3 mice/group.
Human fibrocytes proliferate in response to LTD4
Our previous results indicated that human fibrocytes express both CysLT1 and CysLT2 (Fig. 2C). In an effort to determine whether circulating human fibrocytes proliferate in response to LTD4, peripheral blood was collected from five normal volunteers and fibrocytes were isolated. Fibrocytes were then plated in quadruplicate at 20,000 cells/well in SFM or SFM containing 10 nM LTD4. LTD4 significantly stimulated the proliferation of the fibrocytes from each subject ( p = 0.01 compared with SFM only for each). The means of the unstimulated and LTD4-stimulated proliferation rates for each subject are shown in Fig. 9. Thus, both murine and human fibrocytes proliferate in response to LTD4.
FIGURE 9.
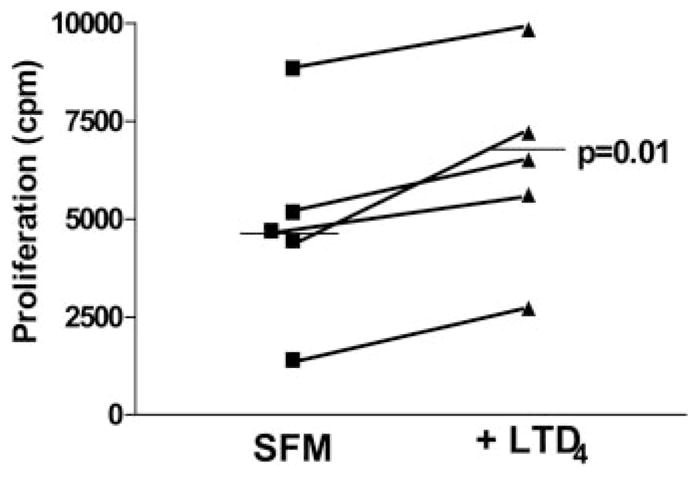
LTD4 stimulates human fibrocyte proliferation. Fibrocytes were isolated from buffy coats of n = 5 normal volunteers grown in the presence of complete medium with 20% FCS for 14 days before being trypsinized and plated at 2 × 105cells/ml in 96-well flat-bottom plates in the presence of SFM or SFM + 10 nM LTD4 for 48 h. Proliferation was measured by incorporation of [3H]thymidine over the final 16 h. Addition of LTD4 significantly stimulated the proliferation of fibrocytes from each subject (p = 0.01 by paired t test). The mean of each group is shown as the black horizontal line.
Discussion
Fibrocytes have been shown convincingly to be recruited to murine lungs in response to fibrotic injury (6, 7). Adoptive transfer of fibrocytes can augment the development of fibrosis in animal models (30). In addition, recently published data demonstrate that circulating fibrocytes are elevated in patients with IPF compared with normal volunteers (31). Thus, both murine and human studies suggest a critical role for fibrocytes in the development of fibrotic lung disease. As such, a better understanding of the factors which recruit and activate these cells is needed. Fibrocytes may be able to augment fibrosis through a variety of mechanisms. First, they contribute to collagen production directly. Second, they secrete profibrotic and proinflammatory factors which likely augment fibrotic responses. Third, they may differentiate into myofibroblasts to participate in lung contraction and extracellular matrix deposition. Increases in the total number of fibrocytes within the lung, either by direct chemotactic mechanisms or in situ proliferation, would likely augment fibrotic responses. Our studies are the first to demonstrate the ability of fibrocytes to make and respond to LTs.
The goal of this research was to test the hypothesis that LTs regulate fibrocytes in a manner that is critical to the development of pulmonary fibrosis. We began our studies by demonstrating that LT production was specifically stimulated by the instillation of FITC as it is by bleomycin (17) and that LTs play a causal role in FITC-induced fibrosis. Using this model, we showed that fibrocytes produce CysLTs and respond to them in an autocrine or a paracrine fashion by proliferating and migrating in vitro. We also demonstrate that bFGF is a fibrocyte mitogen. These are the first studies to provide evidence for any factor that regulates murine fibrocyte proliferation. One previous report demonstrates that addition of endothelin 1 or TGFβ1 to human circulating fibrocytes can stimulate BrdU incorporation over a period of 4 days (8). It is interesting to note that in other systems, endothelin 1 and TGFβ1 have been noted to up-regulate CysLT production (32, 33). Thus, it is possible that CysLTs are common effector molecules for fibrocyte proliferation, but they do not enhance fibrocyte differentiation into fibroblasts in vitro. Our in vivo studies show that the initial recruitment of fibrocytes is not inhibited in the absence of LTs. Because fibrocytes can be recruited to sites of FITC-induced injury by CCR2, CXCR4, and CCR7-mediated signals (6, 7), we speculate that factors such as CCL12 or stromal cell-derived factor 1 may be responsible for fibrocyte recruitment in the absence of LTs. As a result, the fact that fewer fibrocytes can be cultured from BAL of 5-LO−/− mice after FITC-induced injury likely reflects reduced proliferation rates. Given these findings, we propose that protection from fibrosis exhibited by 5-LO−/− mice (Ref. 17 and this study) or mice treated with CysLT1 antagonists (18, 19) likely results, at least in part, from diminished in situ proliferation of fibrocytes in the challenged lungs.
Fibrocytes are cells that express both mesenchymal and leukocyte markers. Our studies demonstrate that fibrocytes, like leukocytes, retain the ability to produce LTs. This is the first description of eicosanoid production by this novel cell type. The ability of fibrocytes to produce LTs would be expected to augment the chemotaxis of both leukocytes and resident fibroblasts. Our studies confirm that resident lung fibroblasts express both CysLT1 and CysLT2 receptors. In addition, previous reports have demonstrated that CysLTs can augment proliferation of resident lung fibroblasts (10–12). The secretion of LTB4 may serve as a potent neutrophil chemotactic molecule (34). The role that neutrophils play in fibrotic responses is unclear, but certainly the release of reactive oxygen species and neutrophil elastase may contribute to local tissue injury (35). The fact that CysLTs are elevated in fibrotic lungs is well documented in both murine and human studies (12, 17) and in the mouse, CysLTs are the predominant LT generated in the lung (17). Thus, it is likely that the recruitment of fibrocytes to the lung via any chemotactic stimulus would result in the local expansion of these cells via CysLT-mediated proliferation. Since fibrocytes have also been shown to be recruited to airways in the setting of asthma (8), the presence of CysLTs in this disease would also be expected to enhance the local proliferation of fibrocytes and this may be an important aspect of the airway remodeling that occurs in this disease setting. In this regard, it is important to note that human fibrocytes also proliferate in response to LTD4.
Our results demonstrate that LTD4 is a more potent mitogen for fibrocytes than LTC4. Additionally, CysLT1 receptor antagonists block the effects of LTD4 on fibrocyte proliferation. These results suggest that LTD4 mediates its effects on fibrocytes solely via the CysLT1 receptor. We speculate that the proliferative effects of LTD4 on fibrocytes are important to the pathogenesis of lung fibrosis. We used two different CysLT1 receptor antagonists in these studies, MK571 and Ly171883. MK571 has previously been shown to be a competitive inhibitor of the multi-drug resistance-associated protein ATP-binding cassette transporter, which can regulate CysLT secretion, but only at doses above 1 μM (36). Similarly, Ly171883 has been shown to be both a PPAR-α (37) and peroxisome proliferator-activated receptor γ (38) ligand, but again at doses 10–10,000-fold higher than used in our studies. Thus, we believe the findings that both of these inhibitors blocked LTD4-induced proliferation when used at current doses strongly support a role for CysLT1-mediated proliferative effects.
5-LO−/− mice are protected from lung fibrosis induced by both bleomycin (17) and FITC at day 21 postchallenge. Our studies provide mechanistic insight into these observations and suggest that 5-LO−/− mice are protected because LTD4-induced mitogenic effects on fibrocytes mediated via CysLT1 are lacking in the 5-LO−/− mice. Our results are consistent with previous studies which demonstrated that dosing of mice with either of the CysLT1 receptor antagonists MK571 (18) or montelukast (19) limited bleomycin-induced lung injury and fibrosis in mice. Additionally, our findings are consistent with the observation that cPLA2−/− mice have reduced levels of LTs in their lungs in response to bleomycin challenge and are protected from fibrosis (39). LTC4 synthase-deficient mice are similarly protected from bleomycin-induced fibrosis (40). However, our findings are not consistent with those of Beller et al. (40), who demonstrated that CysLT1−/− mice showed evidence of septal thickening and deposition of reticular fibers that was enhanced compared with WT mice at day 12 after bleomycin treatment. Nor are our observations consistent with the findings from this same group (41) that CysLT2−/− mice were protected from bleomycin-induced fibrosis on day 12. Based on our findings of CysLT2 on resident fibroblasts, we would anticipate that CysLT2−/− mice might show some protection from fibrosis. However, it is not clear why the reported results in the CysLT1−/− mice are different from the results using CysLT1 antagonists in vivo (18, 19) or our results in the 5-LO−/− mice, but this may reflect differences in the time points of assessment (day 12 vs day 21), model systems used, or methodology (Ashcroft scores vs hydroxyproline assays).
There remains a critical need to identify new modes of therapy for fibrotic lung disorders. Our present findings provide a new rationale for using LT inhibitors or CysLT1 receptor antagonists in antifibrotic therapy. One potential advantage of targeting long-term therapy to blockade of the CysLT1 pathway rather than blockade of all LT production may be the ability to deliver antifibrotic therapy without diminishing the ability of LTB4 to participate in innate immune actions on AMs and polymorphonuclear neutrophils.
Footnotes
This work was supported by National Institutes of Health Grants HL071586 (to B.B.M.) and P50HL56402 (to B.B.M., G.B.T., and M.P.G.), a Career Investigator Award (to B.B.M.) from the American Lung Association of Michigan, and a research grant from the Martin Edward Galvin Fund for Fibrosis Research.
Abbreviations used in this paper: IPF, idiopathic pulmonary fibrosis; LT, leukotriene; AM, alveolar macrophage; CysLT, cysteinyl leukotriene; WT, wild type; SFM, serum-free medium; CT, cycle threshold; BAL, bronchoalveolar lavage; bFGF, basic fibroblast growth factor.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Kuhn C. Pathology. In: Phan S, Thrall R, editors. Pulmonary Fibrosis. Dekker; New York: 1995. pp. 59–83. [Google Scholar]
- 2.Gottleib DJ, Snider GL. Lung function in pulmonary fibrosis. In: Phan S, Thrall R, editors. Pulmonary Fibrosis. Vol. 85 Dekker; New York: 1995. [Google Scholar]
- 3.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 4.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 5.Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep. 2000;2:501–505. doi: 10.1007/s11926-000-0027-5. [DOI] [PubMed] [Google Scholar]
- 6.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M, Sun G, Stacey M, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;170:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 9.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 10.Ozaki O, Hayashi H, Tani K, Ogushi F, Yasuoka U, Ogura T. Neutrophil chemotactic factor in the respiratory tract of patients with chronic airway diseases or idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1992;145:85–91. doi: 10.1164/ajrccm/145.1.85. [DOI] [PubMed] [Google Scholar]
- 11.Wardlaw A, Hay H, Cromwell O, Collins J, Kay A. Leukotrienes, LTC4 and LTB4, in bronchoalveolar lavage in bronchial asthma and other respiratory diseases. J Allergy Clin Immunol. 1989;84:19–26. doi: 10.1016/0091-6749(89)90173-5. [DOI] [PubMed] [Google Scholar]
- 12.Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Golden M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97:1827–1836. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bitterman P, Wewers N, Rennard S, Adelberg S, Cyrstal R. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986;77:700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias J, Rossman M, Zurier R, Daniele R. Human alveolar macrophage inhibition of lung fibroblast growth: a prostaglandin-dependent process. Am Rev Respir Dis. 1985;131:94–99. doi: 10.1164/arrd.1985.131.1.94. [DOI] [PubMed] [Google Scholar]
- 15.Korn J, Halushka P, Leroy E. Mononuclear cell modulation of connective tissue function: suppression of fibroblast growth by stimulation of endogenous prostaglandin production. J Clin Invest. 1980;65:543–554. doi: 10.1172/JCI109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilborn J, Crofford L, Burdick M, Kunkel S, Strieter R, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest. 1995;95:1861–1868. doi: 10.1172/JCI117866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters-Golden M, Bailie M, Marshall T, Wilke C, Phan SH, Toews G, Moore B. Protection from pulmonary fibrosis in leukotriene-deficient mice. Am J Respir Crit Care Med. 2002;165:229–235. doi: 10.1164/ajrccm.165.2.2104050. [DOI] [PubMed] [Google Scholar]
- 18.Failla M, Genovese T, Mazzon E, Gili E, Muia C, Sortino M, Crimi N, Caputi AP, Cuzzocrea S, Vancheri C. Pharmacological inhibition of leukotrienes in an animal model of bleomycin-induced acute lung injury. Respir Res. 2006;7:137. doi: 10.1186/1465-9921-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumo T, Kondo M, Nagai A. Cysteinyl-leukotriene 1 receptor antagonist attenuates bleomycin-induced pulmonary fibrosis in mice. Life Sci. 2007;80:1882–1886. doi: 10.1016/j.lfs.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Mensing H, Czarnetzki B. Leukotriene B4 induces in vitro fibroblast chemotaxis. J Invest Dermatol. 1984;82:9–12. doi: 10.1111/1523-1747.ep12258678. [DOI] [PubMed] [Google Scholar]
- 21.Baud L, Perez J, Denis M, Ardaillou R. Modulation of fibroblast proliferation by sulfidopeptide leukotrienes: effect of indomethacin. J Immunol. 1987;138:1190–1195. [PubMed] [Google Scholar]
- 22.Phan SH, McGarry B, Loeffler K, Kunkel S. Binding of leukotriene C4 to rat lung fibroblasts and stimulation of collagen synthesis in vitro. Biochemistry. 1988;27:2846–2853. doi: 10.1021/bi00408a028. [DOI] [PubMed] [Google Scholar]
- 23.Chen XS, Sheller JR, Johnson EN, Funk CD. Role of leukotrienes revealed by targeted disruption of the 5-lipoxygenase gene. Nature. 1994;372:179–182. doi: 10.1038/372179a0. [DOI] [PubMed] [Google Scholar]
- 24.Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 25.Thomas PE, Peters-Golden M, White ES, Thannickal VJ, Moore BB. PGE2 inhibition of TGF-β1-induced myofibroblast differentiation is Smad-independent but involves cell shape and adhesion-dependent signaling. Am J Physiol. 2007;293:L417–L428. doi: 10.1152/ajplung.00489.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink L, Seeger W, Ermert L, Hanze J, Stahl U, Grimminger F, Kummer W, Bohle RM. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4:1329–1333. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]
- 27.Huffnagle GB, Strieter RM, Standiford TJ, Mcdonald RA, Burdick MD, Kunkel SL, Toews GB. The role of monocyte chemotactic protein-1 (Mcp-1) in the recruitment of monocytes and CD4+ T-cells during a pulmonary cryptococcus-neoformans infection. J Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 28.Petersgolden M, Thebert P. Inhibition by methylprednisolone of zymosan-induced leukotriene synthesis in alveolar macrophages. Am Rev Respir Dis. 1987;135:1020–1026. doi: 10.1164/arrd.1987.135.5.1020. [DOI] [PubMed] [Google Scholar]
- 29.Evans JF. Cysteinyl leukotriene receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:587–597. doi: 10.1016/s0090-6980(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 30.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35:175–181. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehrad B, Burdick M, Zisman D, Keane P, Belpario J, Strieter R. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 32.Faisy C, Naline E, Diehl JL, Emonds-Alt X, Chinet T, Advenier C. In vitro sensitization of human bronchus by β2-adrenergic agonists. Am J Physiol. 2002;283:L1033–L1042. doi: 10.1152/ajplung.00063.2002. [DOI] [PubMed] [Google Scholar]
- 33.James AJ, Penrose JF, Cazaly AM, Holgate ST, Sampson AP. Human bronchial fibroblasts express the 5-lipoxygenase pathway. Respir Res. 2006;7:102. doi: 10.1186/1465-9921-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buret A, Dunkley M, Clancy R, Cripps A. Effector mechanisms of intestinally induced immunity to Pseudomonas aeruginosa in the rat lung: role of neutrophils and leukotriene B4. Infect Immun. 1993;61:671–679. doi: 10.1128/iai.61.2.671-679.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeiher BG, Matsuoka S, Kawabata K, Repine JE. Neutrophil elastase and acute lung injury: prospects for sivelestat and other neutrophil elastase inhibitors as therapeutics. Crit Care Med. 2002;30:S281–S287. doi: 10.1097/00003246-200205001-00018. [DOI] [PubMed] [Google Scholar]
- 36.Shen H, Paul S, Breuninger LM, Ciaccio PJ, Laing NM, Helt M, Tew KD, Kruh GD. Cellular and in vitro transport of glutathione conjugates by MRP. Biochemistry. 1996;35:5719–5725. doi: 10.1021/bi960098n. [DOI] [PubMed] [Google Scholar]
- 37.Downie MM, Sanders DA, Maier LM, Stock DM, Kealey T. Peroxisome proliferator-activated receptor and farnesoid X receptor ligands differentially regulate sebaceous differentiation in human sebaceous gland organ cultures in vitro. Br J Dermatol. 2004;151:766–775. doi: 10.1111/j.1365-2133.2004.06171.x. [DOI] [PubMed] [Google Scholar]
- 38.Yamakawa K, Hosoi M, Koyama H, Tanaka S, Fukumoto S, Morii H, Nishizawa Y. Peroxisome proliferator-activated receptor-γ agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;271:571–574. doi: 10.1006/bbrc.2000.2665. [DOI] [PubMed] [Google Scholar]
- 39.Nagase T, Uozumi N, Ishii S, Kita Y, Yamamoto H, Ohga E, Ouchi Y, Shimizu T. A pivotal role of cytosolic phospholipase A2 in bleomycin-induced pulmonary fibrosis. Nat Med. 2002;8:480–484. doi: 10.1038/nm0502-480. [DOI] [PubMed] [Google Scholar]
- 40.Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci USA. 2004;101:3047–3052. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beller TC, Maekawa A, Friend DS, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of the cysteinyl leukotriene 2 receptor in increased vascular permeability and in bleomycin-induced pulmonary fibrosis in mice. J Biol Chem. 2004;279:46129–46134. doi: 10.1074/jbc.M407057200. [DOI] [PubMed] [Google Scholar]





