Abstract
Stem cells hold significant promise for regeneration of tissue defects and disease-modifying therapies. Although numerous promising stem cell approaches are advancing in clinical trials, intraoperative stem cell therapies offer more immediate hope by integrating an autologous cell source with a well-established surgical intervention in a single procedure. Herein, the major developments in intraoperative stem cell approaches, from in vivo models to clinical studies, are reviewed, and the potential regenerative mechanisms and the roles of different cell populations in the regeneration process are discussed. Although intraoperative stem cell therapies have been shown to be safe and effective for several indications, there are still critical challenges to be tackled prior to adoption into the standard surgical armamentarium.
Keywords: mesenchymal stem cell, clinical research, autologous, cell isolation
INTRODUCTION
Regenerative medicine promises to restore structure and function to damaged tissues and organs. Approaches utilizing exogenous cell sources typically harness stem cells or progenitor cells and are currently being tested in hundreds of cell therapy clinical trials. These trials include cells derived from both autologous and allogeneic sources. In particular, intraoperative cell therapies, which integrate autologous cell-based therapy with surgical interventions into a single procedure, offer enormous hope for the near future, and some approaches have already reached clinical fruition. The intraoperative cell therapy process typically includes tissue harvesting and processing to obtain the desired cell product, surgical intervention depending on the clinical application, and cell delivery (see Figure 1). Intraoperative cell therapy benefits from the accessibility and safety of using the patient’s own cells, which do not trigger an immune response, and from the many relevant cell types that can be harvested using minimally invasive techniques. This therapy also circumvents many of the limitations of exogenous cell therapy by avoiding in vitro cell manipulation and costly cell expansion, the need forGood Manufacturing Practice facilities, the need to hire personnelwith cell culture training, the potential for contamination, and a second procedure (at a different time point) to harvest the cells. It may be beneficial to avoid cell culture to limit phenotype changes that can occur when cells are removed from their native microenvironment for an extended time frame. Additionally, strategies performed entirely within the operating room (without culture expansion) may reduce the wait time to surgery. Importantly, the US Food and Drug Administration, the European Medicines Agency, and other regulatory authorities generally consider adult cell products as biological products that can be divided into two categories: minimally manipulated biological products (e.g., autologous blood products, including platelet-rich plasma or platelet concentrate, and allogeneic blood products, such as bone marrow or umbilical cord blood), and manipulated biological products such as culture-expanded mesenchymal stem cells (MSCs). Certain intraoperative cell approaches fit under the minimally manipulated biological product category, in which extensive clinical trials are not required, thus accelerating potential translation to the clinic. The primary focus of this review is to present an overview of autologous cell therapy approaches in which cell products are harvested, minimally manipulated, and delivered to the patient on the same day.
Figure 1.
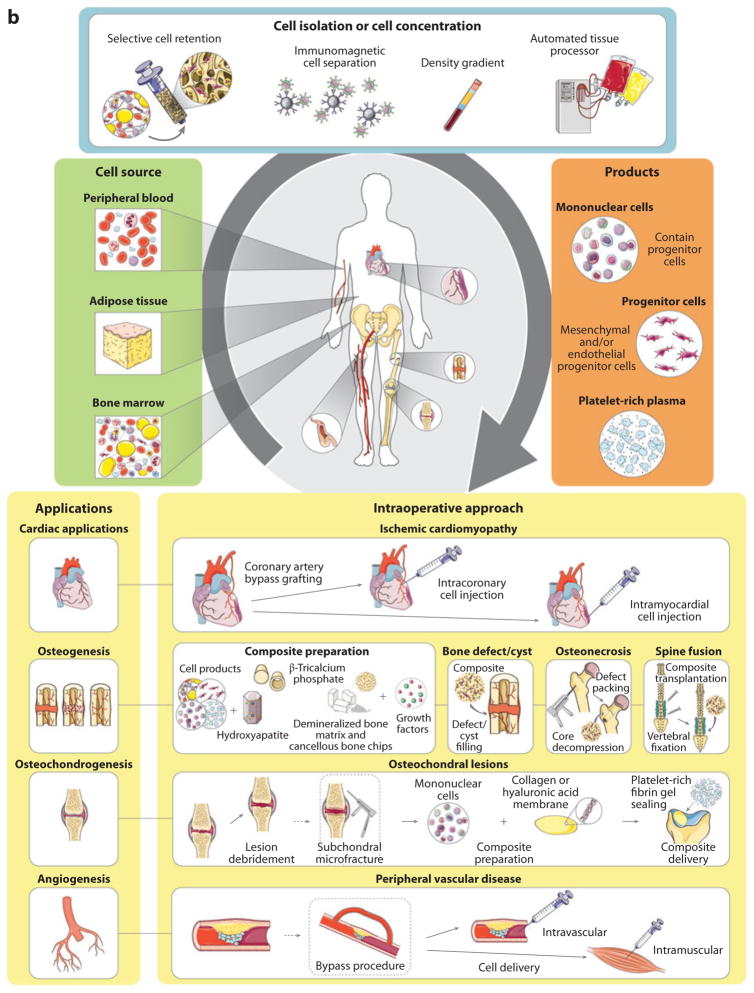
Intraoperative stem cell therapy. (a) The intraoperative cell therapy process typically includes tissue harvesting and processing to obtain the desired cell product, and an intraoperative cell delivery strategy that depends on the clinical application. (b) The intraoperative cell therapy process starts with autologous tissue harvesting. Tissues, including peripheral blood, adipose tissue, and bone marrow, can be used as sources of stem cells (green box). The tissue can then be processed using multiple methods (blue box) to obtain the desired cell product (orange box). The stem cell therapy can be applied as an adjunctive treatment in combination with surgery or an interventional treatment (yellow boxes). Figure was produced using Servier Medical Art (http://www.servier.com/servier-medical-art).
INTRAOPERATIVE STEM CELL THERAPY
To date, conventional intraoperative stem cell approaches have been rather simplistic, typically utilizing whole bone marrow without a cell concentration strategy or specific methods to deliver the cells or to control their function in vivo. The field is rapidly evolving toward achieving greater control over the cell composition, phenotype, and function in vivo by harnessing bioengineering approaches. These approaches include the concentration and selection of stem cell or progenitor populations, along with the incorporation of biomaterials including scaffolds or matrices with appropriate chemical and physical properties to promote rapid attachment of specific cell types or to direct cell fate in vivo. Table 1 summarizes published studies about intraoperative stem cell therapies, including those describing preclinical models, case reports, and clinical trials to treat a wide array of acute and chronic conditions. In the following sections, we describe intraoperative approaches that have been developed for several clinical applications, focusing on the technical procedures and clinical outcomes.
Table 1.
Examples of clinical applications of intraoperative cell therapies
| Application | Example | General surgical procedure | Outcome | Biomaterials | Cell population | References |
|---|---|---|---|---|---|---|
| Angiogenesis | Peripheral vascular disease | Bone marrow aspiration/peripheral blood collection Cell isolation/concentration Intramuscular/intravascular cell delivery |
Increased blood perfusion Limb salvage Wound healing Improvements in rest pain, pain-free walking time |
– | MNCs MNCs and platelets |
42, 45–56 |
| Angiogenesis/myogenesis | Ischemic cardiomyopathy | Bone marrow aspiration Cell isolation/concentration Coronary artery bypass grafting Intramyocardial/intracoronary cell injection |
Enhanced local perfusion Improved contractility on infarct areas Increase in left ventricular ejection fraction |
– | CD133+ cells MNCs |
59–66 |
| Osteochondrogenesis | Osteochondral lesions | Bone marrow aspiration/peripheral blood collection Cell isolation/concentration Lesion preparation (lesion debridement and/or subchondral microfracture) Cellular composite preparation Composite delivery Platelet-rich fibrin gel sealing |
Osteochondral regeneration in various degrees of remodeling although not complete hyaline cartilage formation | Collagen powder Hyaluronic acid membrane |
MNCs MNCs and platelets |
32–34, 39 |
| Osteogenesis | Bone defect healing | Bone marrow aspiration Cell concentration Graft preparation Defect filling |
Bone regeneration | Demineralized bone matrix Cancellous bone chips Hydroxyapatite β-TCP Collagen powder BMPs |
BM cells MNCs, RBCs, and platelets MNCs and platelets |
4–9, 14, 15, 22, 30, 113 |
| Alveolar bone regeneration for cleft alveolus | Bone marrow aspiration Cell concentration Composite preparation Packing into alveolar cleft |
Cleft lip reconstruction | BM cells and platelets | 19, 21 | ||
| Bone cyst treatment | Bone marrow aspiration Composite preparation (demineralized bone matrix gel and bone marrow aspirate mixing) Bone cyst filling |
Cyst expansion halting Cyst ossification |
BM cells | 10, 11 | ||
| Osteonecrosis | Bone marrow aspiration Cell concentration Core decompression Composite preparation Defect packing with graft material |
Pain reduction Delayed progression of the disease Necrotic lesion regression |
BM cells MNCs |
23, 25–29 | ||
| Spine fusion | Bone marrow aspiration Cell concentration Composite preparation Vertebral fixation Composite transplantation |
Successful spinal fusion | BM cells | 17, 18, 20, 114 |
Abbreviations: β-TCP, beta-tricalcium phosphate; BM, bone marrow; BMP, bone morphogenetic protein; MNC, mononuclear cell; RBC, red blood cell.
Osteogenesis
Natural bone grafting materials have evolved over the past two centuries to include autologous or allogeneic grafts of cortical bone, corticocancellous bone, cancellous bone, and demineralized bone matrix (1). The first intraoperative bone autograft was performed in Germany in 1820, yet this procedure did not become common clinical practice until F.H. Albee summarized his experience with 3,000 autologous bone grafting procedures in 1915 (2). Autologous cancellous bone that includes bone chips, a heterogeneous population of cells, and several growth factors has become the gold standard for osseous regeneration and reconstructive procedures that produce the most successful and predictable clinical results. This success has been attributed partially to the presence of cells within the graft (3). The history of and progress with bone autografts have been extensively reviewed elsewhere (3). Here we focus on the use of intraoperatively aspirated and delivered marrow stromal cells.
The first intraoperative cell therapies to promote osteogenic regeneration utilized bone marrow that was aspirated via a needle and then injected percutaneously into bone defects (4–8). Compared with performing a bone autograft harvested from the iliac crest, injecting autologous marrow is less invasive and results in fewer complications at the donor and recipient sites (6, 9). Percutaneous injections of whole bone marrow mixed with demineralized bone powder were also delivered to bone cysts to successfully halt the expansion phase and promote cyst ossification (10, 11). Although autologous bone marrow has been used to augment osteogenic wound healing for decades, the exact mechanism mediating this response is not clear, given that isolated bone marrow is extremely heterogeneous (i.e., is composed of multiple cell types) and contains an array of cytokines, extracellular matrix components, and often small pieces of bone.
A possible correlation between effectiveness of bone marrow and cell concentration (12) prompted investigation into methods to sort cells prior to transplantation. For example, subpopulations obtained by density gradient separation of bone marrow showed increased osteogenesis (13). Furthermore, in the late 1980s, Connolly et al. (14) proposed a method to enhance the osteogenic potential of an injectable bone marrow preparation through concentration of bone marrow nucleated cells by centrifugation. The concentrated bone marrow significantly increased osteogenesis, resulting in enhanced regeneration as assessed radiographically. An important clinical study by Hernigou et al. (15) evaluated the correlation between the number of bone marrow–derived progenitor cells (determined by fibroblast colony-forming units) and the extent of bone healing in a tibial shaft nonunion. The investigators observed a threshold for the total number of progenitor cells present in concentrated bone marrow (equivalent to a cell concentration of 1,000 progenitor cells per cubic centimeter) required for the success of the treatment (union of the tibial shaft fracture). As an alternative to centrifugation to concentrate cells for osteogenic applications, work from George Muschler’s (16) laboratory in Cleveland demonstrated the feasibility of selective cell retention (SCR) technology (see Figure 2) for rapid isolation and concentration (3–4×) of connective tissue progenitor cells within the operating room, from fresh bone marrow aspirates and vertebral bodies. Through the use of this process, bone graft performance was enhanced. SCR is technically simple and fast: The porous, biocompatible, and implantable substrate, which has surface properties that promote rapid attachment of connective tissue progenitor cells, is loaded into a syringe, and the bone marrow is passed through the matrix samples at a low flow rate to obtain composite graft enriched in progenitor cells. Muschler et al. (17) examined cancellous bone as the matrix and the impact of a bone marrow clot (bone marrow aspirate harvest without heparin) in animal models for spine fusion. The clinical outcome was assessed using the union score (degree of union, from 0% to 100% complete fusion of both facet joints and the entire lamina), quantitative computed tomography (to determine the bone volume, bone density, and cross-sectional area of the fusion mass), and mechanical testing (load displacement curves were generated to calculate stiffness, maximum load, displacement failure, and total energy failure). The results show that the combination of the enriched cellular bone graft and bone marrow clot permits one to achieve significantly improved clinical outcomes than those of either technique alone (enriched bone graft or bone graft plus bone marrow clot). The authors present several hypotheses for the positive effect of the bone marrow clot on the graft efficacy: The fibrin clot may provide mechanical stability and act as a scaffold for the transplanted and endogenous cells; the degranulation of platelets provides a supplement of osteogenic cytokines, namely, platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factors (FGFs), and transforming growth factor β (TGF-β); and the fibrinolytic activity of the bone marrow clot may serve as a source of angiogenic factors.
Figure 2.
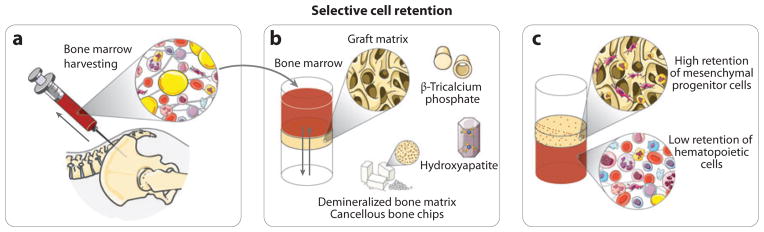
Selective cell retention. After harvesting, (a) the bone marrow is drawn through (b) an implantable graft matrix. This process facilitates matrix exposure to a high volume of bone marrow in a short time. The process is repeated until graft saturation is achieved. Several porous materials can be used as a graft matrix, e.g., demineralized bone matrix, cancellous bone chips, hydroxyapatite, and β-tricalcium phosphate. A higher fraction of mesenchymal progenitor cells are retained within the graft, whereas the retention of hematopoietic cells is significantly lower (c). After enrichment the graft is ready for implantation, although postprocessing procedures such as addition of whole bone marrow or platelet-rich plasma can be performed to improve graft handling properties and to improve the therapeutic potential of the graft. Figure was produced using Servier Medical Art (http://www.servier.com/servier-medical-art).
Muschler et al. (18) also explored demineralized cortical bone as an alternative matrix for SCR, using the same animal model for spinal fusion and the same methods to evaluate the outcome. Augmentation of cell delivery with demineralized cortical bone powder and cancellous bone matrix has been extensively explored for intraoperative osteogenic applications, given their successful track record for promoting new bone growth. Ideally, materials should be osteoconductive (i.e., they should present chemical and physical surface properties that promote osteogenic cell attachment and migration) and osteoinductive (i.e., they should stimulate osteogenic differentiation). β-Tricalcium phosphate (β-TCP), another material that has been extensively studied as a scaffold for tissue engineering of bone, was also evaluated as a matrix for intraoperative stem cell therapy settings—namely, for alveolar bone regeneration for cleft alveolus and also for spine fusion (19– 21). In vitro assays have shown that MSCs can adhere to the porous structure of β-TCP within 2 h of combination and can sustain cell proliferation, making them a good candidate for intraoperative applications (20). The efficacy of SCR was also tested using a mixture of demineralized bone and cancellous chips clotted with platelet-rich plasma in a canine critical-size segmental femoral defect model (22). At 16 weeks, 100% response was obtained, which is equivalent to the results of the standard autograft method to repair critical-size defects. A preliminary clinical report describes the short-term (2-year) results of the intraoperative approach to treat three patients with extensive secondary osteonecrosis of the femoral condyles (23).This treatment, which combined decompression and debridement of the necrotic lesion and the commercial SCR system, facilitated recovery of knee function. The SCR system was introduced in 2003 as Cellect® by DePuy Biologics (a Johnson & Johnson company), in combination with bone graft substitutes, as a minimally invasive therapy for spinal fusion that removed the need for bone graft harvesting. In general, the SCR system shows results that are clinically comparable with those of bone autograft, without the morbidity observed at the graft site. Challenges posed by this technology include a lack of control over the transplanted cells (this challenge applies to most exogenous cell-based therapies) and difficulty obtaining the quantities of cells that can be achieved through in vitro culture expansion (24).
In addition to the Cellect system, several automated blood cell processors are used for intraoperative concentration of blood or bone marrow to treat, for example, osteonecrosis of the femoral head (25–28). These studies follow surgical protocols similar to those of the SCR system, described in detail in the paper by Hernigou et al. (29). Briefly, the bone marrow is harvested with the patient under general anesthesia, and the aspirated bone marrow is concentrated and injected into the femoral head after core decompression. The surgical treatment was considered safe and effective for symptomatic hips with osteonecrosis but without collapse (the clinical success drops from as high as 90% to lower than 50% when the procedure is performed after joint collapse). After successful treatment, the patients experienced reduction in pain and other joint symptoms, delayed progression of the disease, and necrotic lesion regression.
Importantly, osteoconductive and osteoinductive implants containing therapeutic agents without an exogenous cell source can successfully promote new bone formation for the treatment of fractures and for spinal fusion procedures. For example, the OP-1™ product, recently acquired from Stryker by Olympus Biotech Corporation, is an osteoconductive and osteoinductive bone graft material comprising recombinant human bone morphogenetic protein-7 (rhBMP-7) and type I bovine collagen that has been used in approximately 40,000 patients. The product was approved by the US Food and Drug Administration in 2001 for revision of posterolateral lumbar spine fusion and for the treatment of long bone nonunion fractures; it is also approved in Australia, Canada, Switzerland, and the European Union. The product is prepared in the operating room by combining collagen and BMP-7 powders with sterile saline, and it is believed to activate and recruit stem and progenitor cells upon implantation. A similar mechanism has been proposed for bone morphogenetic protein-2 (BMP-2), which is supplied via a collagen sponge in the Infuse® bone graft (Medtronic) that was first approved in 2002 for use in anterior lumbar fusion surgery on skeletally mature patients. Interestingly, some studies have examined improvement of these strategies through intraoperative addition of autologous whole bone marrow. For example, through the use of a canine femoral defect model (30), the delivery of an osteoinductive material (OP-1) with bone marrow was examined. Although the addition of bone marrow promoted increased bone formation in the center of the defect, no overall functional differences were observed between the OP-1 implant plus bone marrow aspirate group and the OP-1 implant plus clotted blood control group. Perhaps the results would have been different had selective populations of cells been used in place of whole bone marrow. Whole bone marrow without prior concentration may promote hypoxia and nutrient deprivation in the defect because bone marrow is metabolically demanding owing to its high cell content. Therefore, it may be of interest to examine supplementing OP-1 or the Infuse bone graft with concentrated progenitor cells. Furthermore, growth factor therapy such as recombinant BMP-2 is under great scrutiny, given the recent analysis showing that risks for adverse events, including risks for ectopic bone formation, osteolysis, pain, new malignancy, implant displacement, subsidence, infection, urogenital events, and retrograde ejaculation, are 10– 50 times higher than the original estimates (31). These risks may shift future bone regenerative strategies to those that do not require the addition of recombinant growth factors.
Osteochondrogenesis
Large osteochondral lesions do not spontaneously repair; thus, interventions to restore cartilage structure and function are required. Intraoperative approaches were developed for osteochondral repair to avoid the need for two surgeries (associated with cell expansion strategies) and to address challenges posed by the expansion of chondrocytes, which have limited regenerative potential. In one example, bone marrow–derived mononuclear cells that were collected and separated intraoperatively were mixed with autologous fibrin gel and delivered by injection to stimulate chondral regeneration within a full-thickness rabbit articular cartilage defect model (32). Treatment using autologous bone marrow–derived mononuclear cells promoted superior cartilage repair when compared with treatment using autologous peripheral blood–derived mononuclear cells mixed with autologous fibrin gel, or fibrin gel alone. Interestingly, although several studies have shown that fibrin gel has potential as a scaffold for cartilage, the results obtained with the fibrin gel alone were inferior to those of the repair obtained with no treatment. It is critical to consider that fibrin composition can dramatically impact cell invasion by endogenous cells and bone formation. Recently, Giannini et al. (33) developed a one-step intraoperative arthroscopic repair technique using bone marrow–derived cells to repair talar osteochondral lesions and validated the application of this procedure to osteochondral lesions of the knee (34). The surgical procedure begins with bone marrow aspiration with the patient under general or spinal anesthesia. After bone marrow collection, the buffy coat is concentrated using Harvest Technologies’ SmartPReP BMAC™ system. In parallel, surgeons start the arthroplasty, which includes lesion debridement, composite preparation (mixing the buffy coat with collagen powder or combining it with a hyaluronic acid membrane) and delivery, and composite stabilization using autologous platelet-rich fibrin gel. Patients experienced improvement of joint function with satisfactory graft integration and varying degrees of tissue remodeling, as indicated by the presence of proteoglycans and type II collagen, hyaline cartilage markers. Nonetheless, by the end of the follow-up period (24 months postoperatively), the histologic evaluation did not show complete hyaline cartilage. The short follow-up period, the small number of patients (48 for the ankle and 20 for the knee), and the limited number of biopsy specimens limit the conclusions that can be drawn from these studies. However, these studies represent a step toward osteochondral regeneration using intraoperative cell approaches.
Another single-step surgical procedure for the treatment of osteochondral lesions, proposed by Benthien & Behrens (35–37), is termed Autologous Matrix-Induced Chondrogenesis (AMIC®), reviewed by Steinwachs et al. (38). It combines subchondral microfracture with the fixation of a type I/III collagen membrane with fibrin glue or sutures. To increase the number of stem cells in the osteochondral defect, the standard AMIC technique (which relies on recruitment of MSCs stimulated through microfracture) was combined with the intraoperative delivery of concentrated bone marrow (39). The combined surgical procedure encompasses bone marrow harvesting, bone marrow concentration using Biomet’s MarrowStim™ concentration kit, lesion debridement, microfracturing, collagen membrane preparation, placement of the concentrated bone marrow, and stabilization of the membrane using fibrin glue. Although the addition of bone marrow has not been conclusively demonstrated to be advantageous, increased numbers of MSCs were observed in the iliac crest bone marrow compared with numbers in blood induced by the microfracture procedure. The cartilage repair of osteochondral lesions may be related to the survival of a small number of progenitor cells that are delivered into the defect, promoting the early-stage regeneration process. This hypothesis is supported by the evidence of bone marrow–derived MSC survival, up to 24 weeks after transplantation into an osteochondral defect (40). Although bone marrow stimulation techniques such as microfracturing may increase the local level of cytokines that promote cartilage repair and may induce progenitor cells to migrate into the lesion, the microfracture-derived progenitor cells are too few to induce hyaline cartilage formation by themselves. Therefore, the delivery of intraoperatively isolated cells may be important for increasing the number of relevant cells in the defect and increasing their chance of survival.
Angiogenesis
Peripheral vascular disease (PVD) often results in reduced or blocked blood flow in the lower extremities, which can lead to critical limb ischemia that is associated with rest pain and tissue necrosis (ulcerations and gangrene). For some patients, revascularization is required to restore tissue perfusion. However, direct intervention to restore blood flow (e.g., through bypass surgery) is not always an option because the blockages are localized in distal small arteries. Therefore, treatments that promote angiogenesis have become a therapeutic focus for these patients. A comprehensive review on therapeutic angiogenesis for PVD using autologous bone marrow cell transplantation can be found in the paper by Matoba & Matsubara (41). The therapeutic application of bone marrow mononuclear cells harvested and sorted intraoperatively for the treatment of critical limb ischemia patients was first reported in 2002 (42). This pilot study was motivated by evidence that bone marrow mononuclear cells could support collateral vessel formation in animal models (43, 44). Patients with bilateral leg ischemia received intramuscular injection of intraoperatively isolated and sorted bone marrow mononuclear cells (harvested from the iliac crest) in one leg and peripheral blood mononuclear cells in the other leg, as a control. Ankle-brachial index (the ratio between the blood pressure at the ankle and the blood pressure in the arm), rest pain, transcutaneous oxygen pressure, and pain-free walking time were significantly improved in the legs injected with bone marrow mononuclear cells. Additional studies have validated these results and have included additional assessment measures including the perfusion blood flow increase through perfusion scintigraphy, determination of endothelial dysfunction improvement, evaluation of wound healing, and examination of follow-up improvement in symptoms and clinical function (45–54). Furthermore, implantation of intraoperative bone marrow–derived mononuclear cells into ischemic limbs significantly contributed to reduced numbers of major amputations (42, 45, 47–53). Another report describes a similar study using granulocyte colony-stimulating factor, administered each of the five days prior to the operation, to mobilize peripheral blood mononuclear cells (55). Distal intramuscular injection of bone marrow buffy coat was also proposed as an adjunctive intraoperative cell treatment to be performed in combination with bypass surgery and/or interventional treatment (56). Although several cell-based therapies to promote angiogenesis involve an infusion of unfractionated mononuclear cells that contains stem and progenitor cells with the capability to differentiate into multiple lineages, endothelial differentiation appears to be favored (43, 44).
Although intramuscular injection is the main cell delivery route for PVD therapy, some cell delivery strategies combine intramuscular injection and intraarterial injection. This combination allows stem cells to reach targeted vessels and thus increases the concentration of stem cells in ischemic muscle regions that are still perfused (48, 56).
A fully automated system (the Tissue Genesis Cell Isolation System™) to isolate stem cells from autologous adipose tissue harvested by liposuction is being explored in an ongoing phase I trial for treatment of PVD (57). After the tissue is processed in the operating room, the obtained stem cells are seeded onto the inner surface of standard prosthetic grafts to promote graft endothelialization and avoid clot formation. The first patients treated with this method regained sensation in the leg and toes and started to walk without debilitating pain (58).
Cardiac Applications
It is compelling to consider intraoperative stem cell therapy for the treatment of ischemic myocardium on the basis of the potential for transplanted cells to inhibit apoptosis and scar formation, to promote connective tissue remodeling and production of extracellular matrix, to trigger endogenous stem cell homing, to modulate inflammation, and to promote angiogenesis with the hope of regenerating healthy myocardial tissue. Intraoperative administration of bone marrow cells, via intramyocardial or intracoronary injection, has been applied following coronary artery bypass grafting (CABG), for example, to enhance angiogenesis and myocardial repair. This technique can be augmented with the application of autologous fibrin glue immediately after cell injection to avoid loss of cells due to regurgitation (59). Although the majority of the clinical trials combining CABG and autologous stem cell transplantation have employed whole bone marrow–derived mononuclear cells (59–63), other studies used CD133+ cells sorted intraoperatively through immunomagnetic cell separation systems (64–66). An intraoperative method to isolate bone marrow–derived CD34+ cells for cardiac therapy was also proposed: It involves rapid erythrocyte depletion using hydroxyethyl starch and low-speed centrifugation, followed by immunomagnetic CD34+ cell sorting (67). In general, intraoperative isolation and application of cells have promoted revascularization, resulting in enhanced myocardial perfusion, and contractility of infarct areas, leading to improvement in left ventricular ejection fraction (59, 65). Although injection of bone marrow–derived MSCs into peri-infarct areas may improve contractility and although injection of cells into the central zone of the myocardial infarction can promote angiogenesis, myogenic differentiation, and reverse ischemic remodeling (68), administration of bone marrow–derived cells directly into nonviable scarred myocardium does not appear to improve contractile function (62, 68). Therefore, when considering the cell delivery strategy for the regeneration of damaged heart tissue, it may be important to consider multiple sites of injection, including viable, peri-infarct areas and the central zone of the myocardial infarction (68).
REGENERATIVE MECHANISMS
Discerning the mechanisms that mediate the therapeutic effects of intraoperative stem cell therapies is a daunting task, and current data are insufficient to draw substantial conclusions. This is not surprising, given that the majority of the intraoperative stem cell studies focus on the assessment of safety and feasibility and thus, for the most part, neglect the investigation into mechanisms responsible for the observed outcomes. Potential mechanisms include the following: (a) Stem or progenitor cells within the transplant replenish progenitor cells in the host; (b) cells in the transplant differentiate and produce de novo tissue; (c) surviving (or dying) transplanted cells secrete trophic, angiogenic, or immunomodulatory factors that provide signals to local endogenous cells via paracrine signaling or to distant cells through endocrine mechanisms (which may result in mobilization and homing of distant host cells). It is also possible that transplanted cells may fuse with host cells (in a process known as cell fusion). The elucidation of mechanisms mediating functional improvements is complicated by the heterogeneous composition of many of the intraoperatively infused cell populations (e.g., bone marrow), where certain cell types may serve a dominant role or multiple cell types may work together synergistically. Specifically, given that many intraoperative cell therapy approaches employ heterogeneous cell populations that may include stem or progenitor cells, it is possible that positive outcomes may not be mediated by the stem or progenitor cells. Also, given that cells are isolated and processed within the operating room, there is likely considerable diversity in how cells are obtained, processed, and infused (despite efforts to standardize procedures with well-designed kits and devices), and this diversity may impact mode of action and outcome. Furthermore, one must not forget that, by virtue, intraoperative cell therapies involve isolation of an autologous cell source; thus, there can be significant differences in relative cell numbers and phenotypes, which may be a function of age, sex, race, body mass index, ancestry/genetics, diet, and environment. The mechanism likely also depends on the specific application (e.g., osteogenesis versus angiogenesis versus reduction of scar formation) and delivery method (e.g., local injection, systemic infusion, or delivery in combination with a biomaterial). Thus, many questions remain unanswered, and new techniques will likely be required to elucidate in vivo biology related to how transplanted cells mediate therapeutic responses (69). It is also likely that strategies to control the fate and function of cells following transplantation [e.g., by engineering exogenous cells within the operating room (70)] will provide an axis to maximize the therapeutic effect by harnessing specific mechanisms. These strategies should help reduce variability of the response.
CELL SOURCES
Adult stem and progenitor cells can be intraoperatively harvested from several tissues including bone marrow, adipose tissue, and peripheral blood. Bone marrow is the most common source, most likely because it can be easily accessed in a rapid manner and because several tools are available to harvest marrow (from bone marrow transplantation). Bone marrow contains hematopoietic cells and mesenchymal cells, in addition to other cells types that may play a role in promoting tissue regeneration. However, one of the main limitations of intraoperative stem cell therapy approaches is the limited quantity of harvested source material and the low yield of cell isolation protocols, which typically yield stem cells at a frequency per mononucleated cell of 1 in 10,000 to 1 in 100,000 (71–74). To circumvent these challenges and to avoid the highly invasive procedure of bone marrow harvesting that causes pain at the site of aspiration, alternative sources from which to isolate autologous stem cells should be considered. For example, bone dusts, usually discarded as waste during spinal fusion, were proven to be a source of stem cells (75). Adipose tissue is a promising alternative source of cells that have multilineage differentiation potential, and it can be collected through a less invasive method and in larger quantities than bone marrow, with minimal morbidity upon harvest (76).
Several studies have established a comparative analysis among stem cells derived from different sources (76–79). The study developed by Peng et al. (76) included immunophenotypic analysis of stem cells isolated from rat bone marrow and adipose tissue, and it revealed a greater percentage of CD44+, CD73+, and CD90+ cells in adipose tissue. Moreover, greater proliferation capacity in cells isolated from adipose tissue is suggested by a higher and constant growth rate throughout 10 generations, a lower population-doubling time, the highest proportion of cell population in S phase, and higher telomerase activity. These results are supported by other published studies that focus on the assessment of different adult stem cell sources (77, 79). Adipose tissue could be considered a good alternative to bone marrow for intraoperative applications on the basis of stem cell abundance, frequency, and expansion potential. Although some patients might have limited adipose tissue for autologous settings, owing to the high frequency of adipose tissue–derived MSCs (their occurrence is 100 to 300 times higher than in bone marrow, and the number of stem cells that can be isolated per unit volume of lipoaspirate is approximately 10-fold greater than that from bone marrow), small adipose tissue reservoirs might be sufficient for stem cell isolation (77, 80–82). The number of required stem cells also depends on the clinical applications. For example, freshly isolated adipose tissue–derived stem cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate small focal cartilage defects; however, for larger osteochondral defects, subcutaneous adipose tissue would be advisable (83). When harvesting adipose tissue for intraoperative stem cell therapy, one must also consider that the tissue harvesting site and the surgical procedure have an effect on the yield of stem cells. For instance, adipose tissue harvest from the abdomen through resection or tumescent liposuction yields more stem cells compared with ultrasound-assisted liposuction and the adipose tissue harvested from the hip/thigh region (81, 84). In the review by Hoogendoorn et al. (85) about the status of cell-based treatments for intervertebral disc regeneration, the authors propose a concept for a one-step procedure using adipose tissue–derived stem cells for regenerative treatment of mildly degenerated intervertebral discs and for spinal fusion of severe intervertebral disc degeneration. The adipose tissue should be harvested by minimally invasive techniques and should be processed by enzymatic digestion and centrifugal enrichment to obtain stem cells within a single surgical procedure lasting less than 2 h. For spinal fusions, the authors suggest short-term ex vivo triggering of osteogenesis by 15 min exposure to BMP-2 (86), mixing stem cells with a porous calcium phosphate scaffold, and implanting a bioresorbable polymer cage filled with the scaffold seeded with triggered stem cells. This one-step procedure for spinal fusion is described in detail elsewhere (87). For the regenerative treatment of mildly degenerated intervertebral discs, adipose tissue–derived stem cells, after cell isolation, could be transplanted by injection into the intervertebral disc after appropriate ex vivo cell triggering and cell carrier seeding. Case reports and phase I to III clinical trials using autologous adipose tissue–derived stem cells in a variety of fields are reviewed and discussed by Gimble et al. (88).
POTENTIAL ROLES OF INTRAOPERATIVELY PROCESSED AND TRANSPLANTED CELL PRODUCTS
Given that many intraoperative cell therapy approaches take advantage of the heterogeneous cell populations contained in the bone marrow, there remains controversy about the specific subpopulations responsible for the different regeneration mechanisms and about the role of transplanted cells versus endogenous cells. The majority of intraoperative cell therapies used to promote angiogenesis for PVD patients infuse unfractionated mononuclear cells, and some authors suggest that the synergetic effect of different cell populations on the promotion of angiogenesis in PVD seems to involve bone marrow–derived CD34+ cells that express receptors for basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and angiopoietin-2. These authors also suggest that the CD34− fraction is responsible for secreting the angiogenic factors (bFGF and VEGF) and angiopoietin-2, which stimulates CD34+ to form collateral vessels and promotes the maturation and maintenance of the newly formed vessels (42, 45). The buffy coat contains high numbers of CD34+ and CD133+ cells, a significant subset of which also coexpress vascular endothelial growth factor receptor 2 (VEGFR-2), and CD34+CD133+VEGFR-2+ cells were defined by Peichev et al. (89) as functional endothelial progenitor cells. However, Case et al. (90) claim that the CD34+ fraction of mononuclear cells (derived from granulocyte colony-stimulating factor–mobilized peripheral blood and umbilical cord blood) that also coexpresses CD133 and VEGFR is positive for CD45 (>99%). The authors also state that this cell fraction (CD34+CD45+) is a distinct and primitive hematopoietic subpopulation, as opposed to a population of endothelial progenitor cells, and therefore cannot form new blood vessels. However, the CD34+CD45− cell fraction was enriched in endothelial colony–forming cells. This finding illustrates the importance of defining precise biological potential of different stem cell subpopulations to design more effective stem cell therapies.
The promotion of angiogenesis by the injection of bone marrow–derived cells into ischemic myocardial areas seems to play a critical role in the improvement of regional cardiac performance. The injection of bone marrow–derived mononuclear cells contributes to improved myocardial perfusion owing to an increase in the myocardial microvessel density. The myocardial perfusion improvement seems to be positively correlated with the number of CD34+ cells transplanted (60). The majority of studies fail to show direct evidence of cell fate after transplantation. For instance, there is no evidence that the therapeutic effects can be attributed to the differentiation of the transplanted cells into cardiomyocytes. In a study by Ang et al. (62), bone marrow–derived mononuclear cells were injected into scarred myocardium to prevent the effects of functional improvement in viable myocardium following revascularization. Therefore, the functional assessment was focused only on revascularizing chronically scarred, nonviable myocardium. This way, any difference in function could not be attributed to differences in perfusion. The injection of bone marrow–derived cells did not improve the contractile function, suggesting that the bone marrow–derived cells do not contribute to myocardial regeneration. Nonetheless, these results should not be extrapolated to other clinical settings, such as acute myocardial infarction or chronically ischemic but viable myocardium. The absence of myocardial regeneration may be due to the failure of cell engraftment in chronically scarred and nonviable tissue.
Several osteogenic intraoperative cell therapies also involve the application of unfractionated bone marrow cells or bone marrow–derived mononuclear cells. By contrast, SCR is based on the concept that connective tissue progenitors are retained in the graft composite with significantly greater frequency than other bone marrow–derived cells. Connective tissue progenitors are defined by colony-forming units that express alkaline phosphatase (73, 74, 91). In addition to alkaline phosphatase expression, connective tissue progenitors can be identified through other markers such as STRO-1 (92) and activated leukocyte–cell adhesion molecule (93). Although connective tissue progenitors are an obvious choice for osteogenic applications, other selected cell types and cytokines play a key role in the establishment of optimal conditions for bone regeneration. Therefore, intraoperative cell therapies for osteochondrogenic applications also include the delivery of relevant cytokines for tissue regeneration, which can be achieved through autologous platelet products. The delivery of platelet products, such as platelet-rich plasma or platelet-rich fibrin gel, contributes to the stimulation of progenitor cells, and these platelet products act as a chemoattractant owing to the release of growth factors by the α granules, including PDGF, TGF-β1, TGF-β2, platelet-derived EGF, VEGF, insulin growth factor 1, and platelet factor 4 (94–97). Furthermore, platelet-rich plasma in combination with thrombin also increases the handling properties of the final grafts for osteogenic applications owing to its clotting effect (22).
METHODS USED TO INTRAOPERATIVELY ISOLATE OR CONCENTRATE CELL PRODUCTS
Current and proposed intraoperative cell therapies include the delivery of mononuclear cells derived from the bone marrow, mesenchymal and endothelial progenitor cells derived from the bone marrow or from the adipose tissue, the whole bone marrow cell population, and platelet-rich plasma. These cell populations/products can be obtained intraoperatively through several methods, summarized in Table 2: density gradient, automated cell processor, immune-based cell sorting, and SCR.
Table 2.
Methods to isolate/concentrate cell populations used in intraoperative settings and related commercial products
| Method | Brief description | Product, company | Reference(s): Method | Reference(s): Intraoperative |
|---|---|---|---|---|
| Selective cell retention | Bone graft products are used to selectively retain progenitor cells from whole bone marrow or other blood products, increasing the cell concentration that will be delivered. The bone graft products are loaded into a syringe, and the bone marrow is passed through the matrix samples several times via a low flow rate. | Cellect®, DePuy | 115 | 17, 18, 20, 22 23 |
| Immune-based cell sorting | Immune-based cell sorting, through magnetic activated cell sorting, is used to isolate specific cells on the basis of their surface antigens (64, 66, 67, 101), including positive selection of a target cell population or negative selection through removal of unwanted cell populations. | CliniMACS®, Miltenyi Biotec Isolex®, Baxter | – | 64, 66, 101 67 |
| Density gradient | Cell separation can be achieved on the basis of the density and size differences of different cell populations. This can be accomplished through simple centrifugation, optimized centrifugation protocols, and centrifugation using density media. The cell population recovered depends on the density medium, centrifugation protocol, and fraction collected. | Density media: | 98 | 14, 19, 21, 50, 52, 54, 61, 62 |
| Ficoll-Paque™ and Percoll™, GE Healthcare | – | – | ||
| LymphoPrep™, Axis-Shield | – | – | ||
| Some devices were developed to improve the handling of bone marrow and other blood products, such as platelet-rich plasma, through density gradient cell isolation protocols in order to avoid contamination and to provide more consistent results. | Devices: | – | – | |
| GenesisCS, EmCyte Corporation | – | 48 | ||
| MarrowStim™ Concentration Kit, Biomet | 116 | 39 | ||
| SmartPReP, Harvest Technologies | – | 33, 34, 52, 56 | ||
| Automated blood processor | Cellular products are separated from blood and related products using fully automated and closed units, on the basis of the density gradient principle. | CS-3000 Plus Blood Cell Separator, Baxter | 117–119 | 42 |
| Sepax®, Biosafe | 117–120 | 56 | ||
| COBE®, CaridianBCT | – | 15, 20, 26–29, 55, 63, 113 | ||
| AS blood cell separator, Fresenius | – | 50, 51 |
The most traditional method to obtain mononuclear cells from bone marrow is the density gradient. Complementary strategies, such as red blood cell lysis and dextran sedimentation (98), are used to optimize cell isolation, but the extremely low yield continues to be a limitation and hinders the development of intraoperative approaches. Furthermore, during density gradient media separation processes, cells are exposed to potential toxic agents and require the use of a centrifuge. Autologous platelet-rich plasma can also be processed from peripheral blood or from bone marrow using density gradient techniques, representing a safe and cost-effective method to obtain growth factors (21). The handling properties of platelet-rich plasma can be improved if it is processed into a platelet gel, through the embedding of concentrated platelets within a network of polymerized fibrin. Several commercially available products serve this purpose, such as Platelex® and Vivostat PRF® (99, 100).
Automated cell processors perform washing and tissue digestion, concentration of the buffy coat with or without a sedimentation agent such as hydroxyethyl starch, volume reduction by plasma depletion, and platelet-rich plasma processing, and they have been used in intraoperative settings to process cells from bone marrow, peripheral blood, and adipose tissue. Automated cell processors provide the benefit of superior operational reliability and efficiency, reduced contamination risk, and standardization of cell processing protocols.
Immune-based cell sorting, through magnetic activated cell sorting, is used to isolate specific cell subpopulations on the basis of their surface antigens, such as CD34+ or CD133+ cells for angiogenic applications (64, 66, 67, 101). Immune-based cell sorting is limited to homogeneous cell subpopulations that have a well-defined panel of markers. However, the use of highly homogeneous stem cell populations for intraoperative approaches may hinder an efficient regeneration. The experimental evidence supports the idea of a delicate and complex balance between different putative mechanisms. Although highly homogenous stem cell populations allow the design of a more controlled and defined therapeutic technique, discarding other cellular components may disrupt the equilibrium between important regeneration processes involving different cell types and related trophic signaling. The isolation of mesenchymal progenitor cells by immune-based methods is restricted by the fact that the antigenic profile displayed by these cells in not unique. Thus, mesenchymal progenitor cells cannot be associated with a particular cell phenotype, and there is no consensus about what antibodies should be used to isolate them. Strategies focusing on methods to achieve one-step isolation of relevant cell populations are crucial to the success of intraoperative stem cell therapies.
In the field of orthopedics, protocols have been constructed on the basis of SCR on appropriate substrates that promote rapid attachment of connective tissue progenitors. Substrates such as cancellous bone graft, porous hydroxyapatite, and fibrin microbeads are used to selectively concentrate and retain mesenchymal progenitor cells from whole bone marrow (17, 18, 20, 22, 102). However, in some cases, the isolation time frame is not aligned with the intraoperative requirements, and the materials used to date are not ideal because they are significantly limited with respect to mechanical properties, availability, and risk of disease transmission. Therefore, there exists great opportunity to optimize and expand the utility and scope of these intraoperative SCR approaches.
CHALLENGES AND NEXT STEPS
To leverage the developments achieved in the field of cell-based therapies, a few topics should be considered carefully, and further improvement and progress with respect to these issues would greatly enhance the impact of intraoperative cell therapies. The limited number of cells available for delivery without previous cell expansion can limit the potential of intraoperative cell therapies. Therefore, the development of strategies to increase the cell isolation yield during the intraoperative time frame is highly recommended. A method termed systematic evolution of ligands by exponential enrichment has shown promising isolation results when used to target MSCs from whole bone marrow (103). This method uses aptamers, such as single-stranded DNA or RNA molecules, with high affinity and specificity for the membrane proteins of the target cells. The aptamers function as molecular probes for molecular signatures of cells that do not present a panel of highly specific surface markers (104). Although current reports of intraoperative cell therapy do not explore adipose tissue as a progenitor cell source, several authors propose methods for intraoperative generation of autologous cell therapies based on lipoaspirate cells. Cell isolation from adipose tissue can be optimized by appropriate selection of enzymatic treatment, washing and centrifugation steps, and the availability of clinical-grade devices for cell isolation from adipose tissue (for example, Celution® by Cytori Therapeutics and Tissue Genesis Cell Isolation System™ by Tissue Genesis) (105, 106). However, methods that require extensive manipulation of cells in the operating room, such as the use of enzymes, may lead to prolonged regulatory approval processes. Furthermore, efforts are under way to develop adipose cell constructs using different materials to promote rapid attachment of adipose stromal vascular fraction cells that are known to include multipotent and progenitor cells. Biodegradable polymeric scaffolds, macroporous poly(L-lactide-cocaprolactone), and porous natural type I/III collagen were proposed for a one-step surgical procedure for cartilage and bone tissue engineering applications. These techniques promoted cell attachment after 5–10 min, and a great portion of colony-forming unit fibroblasts within the stromal vascular fraction preferentially adhered to the polymeric scaffolds (107). Stromal vascular fraction cells can also be embedded in fibrin hydrogels through the method proposed by Bensaïd et al. (108). The hydrogels allow an instant seeding of the cells and can be used as a coating for bone-substitute materials in the intraoperative engineering of osteogenic grafts (109).
Strategies to incorporate chemical and physical cues to direct cell fate in vivo are critical for the success of intraoperative cell therapies. Additional cues may be crucial for promoting cell proliferation and survival and thus to helping reach a sufficient number of cells for a positive outcome. Such a strategy could be designed as a two-step stimulation, first to promote cell expansion in order to reach an optimal cell concentration and then to direct cell function toward desired regeneration mechanisms.
The use of unfractionated cell populations in intraoperative cell therapies leads to poorly defined cell products owing to their heterogeneity and lack of understanding of their in vivo fate and function. This heterogeneity also hinders the generalization of findings from different groups because differences in patients, processing conditions, cell source, and other clinical parameters have a high impact on cell phenotype. A comprehensive characterization of the different cell populations and their properties represents a necessary condition for further development of efficient intraoperative cell therapies. Accurate in vivo cell tracking of endogenous and implanted cells is crucial for defining cell populations and their therapeutic mechanisms in order to design effective strategies in terms of cell isolation, choice of adequate cell populations, cell delivery, treatment timing, and control of cell fate in vivo. Although cell heterogeneity represents a challenge to further elucidating regenerative mechanisms, it is a key aspect of the cells’ diverse therapeutic effects.
The variability from patient to patient in terms of number of progenitor cells also poses a challenge for the intraoperative technique itself (15). Currently, the number of progenitor cells is determined through in vitro assays requiring cell culture, such as colony-forming unit fibroblast assays; therefore, the number of progenitor cells delivered intraoperatively is determined retrospectively. There is a need to evaluate intraoperatively the number of stem and progenitor cells that were collected and that will be delivered, increasing the complexity level of this approach. If we consider the patient variability in terms of the number of cells with different phenotypes, the concept of cell sorting is appealing because it allows the standardization of the appropriate number of cells, presenting a well-defined phenotype, to be delivered. However, this standardization requires the elucidation of specific signatures of relevant cell subpopulations.
Cell delivery strategies have a great impact on the outcome of cell therapies. Delivery methods should promote cell survival and direct cell function to maximize the positive outcome. A recent report proposes the development of an intraoperative cell therapy approach for autologous vascular graft coating, which would promote the recellularization of decellularized homografts or xenografts to minimize postimplantation complications (101). The strategy focuses on the delivery of bone marrow–derived CD133+ cells embedded in autologous fibrin, via spray administration, using the commercially available system Vivostat®. This concept takes advantage of the promising properties of fibrin as an extracellular matrix for angiogenesis: Fibrin is able to support the self-organization process of bone marrow–derived mononuclear cells to produce vascular structures within a vascular 3D fibrin matrices (110), and fibrin tubular structures can withstand circumferential strain due to collagen deposition of interstitial cells seeded on the constructs, leading to an increase of the tensile strength (111). Nonetheless, because transplanted fibrin matrix is degraded by fibrinolytic processes prior to complete cell engraftment, fibrin chemical stabilization or other alternatives must be developed to improve the stability of the coating during the engraftment period.
Limitations in the design of many intraoperative clinical studies seriously hinder the interpretation of the clinical data, compromising the ability to draw valid conclusions. Frequent study limitations include the small number of patients, lack of controls (due to practical and ethical issues), patient inclusion criteria (the patients who agree to participate in studies for novel therapies usually have already undergone several unsuccessful treatments, so they represent the worst-case scenario when starting the intraoperative cell therapy), and absence of long-term follow-up. Randomized double-blind controlled trials are clearly required to validate the most promising intraoperative approaches. Despite these challenges, there are great opportunities to translate basic research and preliminary clinical studies into widely available clinical treatments and thus push even further the progress already made in this field.
SUMMARY
In recent years, intraoperative cell therapy has emerged as an important and exciting approach that can potentially treat many medical conditions. Intraoperative approaches, using multiple cell types, have already been shown to be safe and effective for multiple indications. Furthermore, many approaches are being developed to increase the therapeutic impact, optimize the desired outcome, and overcome current limitations—namely, low yield of cell isolation methods and lack of control of cell fate and function after implantation. The development of these approaches depends on the identification of relevant cell subpopulations and on the understanding of regenerative mechanisms through different perspectives ranging from basic biology to general pathology. Cell therapy brings together a multitude of disciplines: biology, chemistry, biomaterials science, medicine, and engineering, among others. Therefore, an effort should be made to develop collaborative studies that take advantage of the promising developments in each field. For example, the review by Discher et al. (112) highlights the importance of integrative approaches to better control stem function, such as the use of cytokine combinations, material systems including extracellular matrix components, and biomechanical interactions, as well as a systems biology approach to better predict stem cell behavior in vivo. Although there is significant opportunity to improve and expand the utility of existing intraoperative stem cell therapies, elucidating the main regenerative mechanisms that mediate favorable therapeutic response and conducting more controlled clinical studies should remain a priority.
Acknowledgments
The authors thank Vikram Juneja and Vijay Kumar for helpful feedback and refinements to this review. M.B.C. acknowledges support from Fundação para a Ciência e a Tecnologia, Portugal (SFRH/BD/33723/2009), through the MIT-Portugal Program’s Bioengineering Systems Focus Area. This work was supported by National Institutes of Health grants HL095722 andHL097172 to J.M.K.
Footnotes
DISCLOSURE STATEMENT
J.M.K. is a co-owner of Megacell Therapeutics, a company that has an option to license IP generated by J.M.K. J.M.K. may benefit financially if the IP is licensed and further validated. J.M.K.’s interests were reviewed and are subject to a management plan overseen by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
LITERATURE CITED
- 1.Joneschild E, Urbaniak JR. Biology of the vascularized fibular graft. In: Lieberman JR, Friedlaender GE, editors. Bone Generation and Repair: Biology and Clinical Applications. New York: Humana; 2005. pp. 93–112. [Google Scholar]
- 2.de Boer HH. The history of bone grafts. Clin Orthop Relat Res. 1988;226:292–98. [PubMed] [Google Scholar]
- 3.Fujishiro T, Kobayashi H, Bauer TW. Autograft bone. In: Pietrzak WS, editor. Musculoskeletal Tissue Regeneration. New York: Humana; 2008. pp. 65–79. [Google Scholar]
- 4.Paley D, Young MC, Wiley AM, Fornasier VL, Jackson RW. Percutaneous bone marrow grafting of fractures and bony defects. An experimental study in rabbits. Clin Orthop Relat Res. 1986;208:300–12. [PubMed] [Google Scholar]
- 5.Healey JH, Zimmerman PA, McDonnell JM, Lane JM. Percutaneous bone marrow grafting of delayed union and nonunion in cancer patients. Clin Orthop Relat Res. 1990;256:280–85. [PubMed] [Google Scholar]
- 6.Connolly JF, Guse R, Tiedeman J, Dehne R. Autologous marrow injection as a substitute for operative grafting of tibial nonunions. Clin Orthop Relat Res. 1991;266:259–70. [PubMed] [Google Scholar]
- 7.Garg NK, Gaur S, Sharma S. Percutaneous autogenous bone marrow grafting in 20 cases of ununited fracture. Acta Orthop Scand. 1993;64:671–72. doi: 10.3109/17453679308994595. [DOI] [PubMed] [Google Scholar]
- 8.Siwach RC, Sangwan SS, Singh R, Goel A. Role of percutaneous bone marrow grafting in delayed unions, non-unions and poor regenerates. Indian J Med Sci. 2001;55:326–36. [PubMed] [Google Scholar]
- 9.Connolly JF. Injectable bone marrow preparations to stimulate osteogenic repair. Clin Orthop Relat Res. 1995;313:8–18. [PubMed] [Google Scholar]
- 10.Rougraff BT, Kling TJ. Treatment of active unicameral bone cysts with percutaneous injection of demineralized bone matrix and autogenous bone marrow. J Bone Jt Surg Am. 2002;84:921–29. doi: 10.2106/00004623-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Docquier P-L, Delloye C. Treatment of aneurysmal bone cysts by introduction of demineralized bone and autogenous bone marrow. J Bone Jt Surg Am. 2005;87:2253–58. doi: 10.2106/JBJS.D.02540. [DOI] [PubMed] [Google Scholar]
- 12.Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–59. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 13.Budenz RW, Bernard GW. Osteogenesis and leukopoiesis within diffusion-chamber implants of isolated bone marrow subpopulations. Am J Anat. 1980;159:455–74. doi: 10.1002/aja.1001590409. [DOI] [PubMed] [Google Scholar]
- 14.Connolly J, Guse R, Lippiello L, Dehne R. Development of an osteogenic bone-marrow preparation. J Bone Jt Surg Am. 1989;71:684–91. [PubMed] [Google Scholar]
- 15.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Jt Surg Am. 2005;87:1430–37. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 16.McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Jt Surg Am. 2005;87A:2655–61. doi: 10.2106/JBJS.E.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muschler GF, Nitto H, Matsukura Y, Boehm C, Valdevit A, et al. Spine fusion using cell matrix composites enriched in bone marrow–derived cells. Clin Orthop Relat Res. 2003;407:102–18. doi: 10.1097/00003086-200302000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muschler GF, Matsukura Y, Nitto H, Boehm CA, Valdevit AD, et al. Selective retention of bone marrow–derived cells to enhance spinal fusion. Clin Orthop Relat Res. 2005;432:242–51. doi: 10.1097/01.blo.0000149812.32857.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oyama T, Nishimoto S, Takeda M. Alveolar bone regeneration utilizing β-TCP and platelet-rich plasma (PRP) derived from bone marrow aspirate. Ann Plast Surg. 2005;54:222–23. doi: 10.1097/01.sap.0000150837.84954.d1. [DOI] [PubMed] [Google Scholar]
- 20.Gan Y, Dai K, Zhang P, Tang T, Zhu Z, Lu J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials. 2008;29:3973–82. doi: 10.1016/j.biomaterials.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Nishimoto S, Oyama T, Matsuda K. Simultaneous concentration of platelets and marrow cells: a simple and useful technique to obtain source cells and growth factors for regenerative medicine. Wound Repair Regen. 2007;15:156–62. doi: 10.1111/j.1524-475X.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 22.Brodke D, Pedrozo HA, Kapur TA, Attawia M, Kraus KH, et al. Bone grafts prepared with selective cell retention technology heal canine segmental defects as effectively as autograft. J Orthop Res. 2006;24:857–66. doi: 10.1002/jor.20094. [DOI] [PubMed] [Google Scholar]
- 23.Lee K, Goodman SB. Cell therapy for secondary osteonecrosis of the femoral condyles using the Cellect DBM System: a preliminary report. J Arthroplasty. 2009;24:43–48. doi: 10.1016/j.arth.2008.01.133. [DOI] [PubMed] [Google Scholar]
- 24.Kraus KH, Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet Surg. 2006;35:232–42. doi: 10.1111/j.1532-950X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 25.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Gangji V, Hauzeur J-P, Matos C, De Maertelaer V, Toungouz M, Lambermont M. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells: a pilot study. J Bone Jt Surg Am. 2004;86:1153–60. doi: 10.2106/00004623-200406000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Gangji V, Hauzeur J-P. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. J Bone Jt Surg Am. 2005;87:106–12. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 28.Hernigou P, Poignard A, Zilber S, Rouard H. Cell therapy of hip osteonecrosis with autologous bone marrow grafting. Indian J Orthop. 2009;43:40–45. doi: 10.4103/0019-5413.45322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernigou P, Manicom O, Poignard A, Nogier A, Filippini P, De Abreu L. Core decompression with marrow stem cells. Oper Tech Orthop. 2004;14:68–74. [Google Scholar]
- 30.Takigami H, Kumagai K, Latson L, Togawa D, Bauer T, et al. Bone formation following OP-1 implantation is improved by addition of autogenous bone marrow cells in a canine femur defect model. J Orthop Res. 2007;25:1333–42. doi: 10.1002/jor.20411. [DOI] [PubMed] [Google Scholar]
- 31.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11:471–91. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Chang F, Ishii T, Yanai T, Mishima H, Akaogi H, et al. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res. 2008;26:18–26. doi: 10.1002/jor.20470. [DOI] [PubMed] [Google Scholar]
- 33.Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow–derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467:3307–20. doi: 10.1007/s11999-009-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Jt Surg Am. 2010;92:2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 35.Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): combining microfracturing and a collagen I/III matrix for articular cartilage resurfacing. Cartilage. 2010;1:65–68. doi: 10.1177/1947603509360044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC): a one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg. 2010;76:260–63. [PubMed] [Google Scholar]
- 37.Benthien J, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1316–19. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 38.Steinwachs MR, Guggi T, Kreuz PC. Marrow stimulation techniques. Injury. 2008;39:26–31. doi: 10.1016/j.injury.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 39.de Girolamo L, Bertolini G, Cervellin M, Sozzi G, Volpi P. Treatment of chondral defects of the knee with one stepmatrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury. 2010;41:1172–77. doi: 10.1016/j.injury.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Oshima Y, Watanabe N, Matsuda K-i, Takai S, Kawata M, Kubo T. Behavior of transplanted bone marrow–derived GFP mesenchymal cells in osteochondral defect as a simulation of autologous transplantation. J Histochem Cytochem. 2005;53:207–16. doi: 10.1369/jhc.4A6280.2005. [DOI] [PubMed] [Google Scholar]
- 41.Matoba S, Matsubara H. Therapeutic angiogenesis for peripheral artery diseases by autologous bone marrow cell transplantation. Curr Pharm Des. 2009;15:2769–77. doi: 10.2174/138161209788923840. [DOI] [PubMed] [Google Scholar]
- 42.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 43.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K-i, et al. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 44.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–52. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 45.Miyamoto M, Yasutake M, Takano H, Takagi H, Takagi G, et al. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transplant. 2004;13:429–37. doi: 10.3727/000000004783983837. [DOI] [PubMed] [Google Scholar]
- 46.Higashi Y, Kimura M, Hara K, Noma K, Jitsuiki D, et al. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109:1215–18. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- 47.Kohlman-Trigoboff D, Lawson JH, Murphy MP. Stem cell use in a patient with an ischemic foot ulcer: a case study. J Vasc Nurs. 2006;24:56–61. doi: 10.1016/j.jvn.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Franz RW, Parks A, Shah KJ, Hankins T, Hartman JF, Wright ML. Use of autologous bone marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. J Vasc Surg. 2009;50:1378–90. doi: 10.1016/j.jvs.2009.07.113. [DOI] [PubMed] [Google Scholar]
- 49.Napoli C, Farzati B, Sica V, Iannuzzi E, Coppola G, et al. Beneficial effects of autologous bone marrow cell infusion and antioxidants/L-arginine in patients with chronic critical limb ischemia. Eur J Cardiovasc Prev Rehabil. 2008;15:709–18. doi: 10.1097/HJR.0b013e3283193a0f. [DOI] [PubMed] [Google Scholar]
- 50.Hernández P, Cortina L, Artaza H, Pol N, Lam RM, et al. Autologous bone-marrow mononuclear cell implantation in patients with severe lower limb ischaemia: a comparison of using blood cell separator and Ficoll density gradient centrifugation. Atherosclerosis. 2007;194:e52–56. doi: 10.1016/j.atherosclerosis.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 51.Koshikawa M, Shimodaira S, Yoshioka T, Kasai H, Watanabe N, et al. Therapeutic angiogenesis by bone marrow implantation for critical hand ischemia in patients with peripheral arterial disease: a pilot study. Curr Med Res Opin. 2006;22:793–98. doi: 10.1185/030079906X1000078. [DOI] [PubMed] [Google Scholar]
- 52.Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18:371–80. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 53.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–18. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 54.Kamata Y, Takahashi Y, Iwamoto M, Matsui K, Murakami Y, et al. Local implantation of autologous mononuclear cells from bone marrow and peripheral blood for treatment of ischaemic digits in patients with connective tissue diseases. Rheumatology. 2007;46:882–84. doi: 10.1093/rheumatology/kel436. [DOI] [PubMed] [Google Scholar]
- 55.Huang P, Li S, Han M, Xiao Z, Yang R, Han ZC. Autologous transplantation of granulocyte colony–stimulating factor–mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care. 2005;28:2155–60. doi: 10.2337/diacare.28.9.2155. [DOI] [PubMed] [Google Scholar]
- 56.Kolvenbach R, Kreissig C, Cagiannos C, Afifi R, Schmaltz E. Intraoperative adjunctive stem cell treatment in patients with critical limb ischemia using a novel point-of-care device. Ann Vasc Surg. 2010;24:367–72. doi: 10.1016/j.avsg.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 57.US Natl. Inst. Health. Feasibility study of the TGI adipose-derived stromal cell (ASC)-coated ePTFE vascular graft (TGI-PVG-IDE) 2011 http://clinicaltrials.gov/ct2/show/NCT01305863.
- 58.Univ. Louisville. UofL vascular surgeons perform first prosthetic bypass graft procedure using patient’s own stem cells with ‘point-of-care’ technology. 2011 http://louisville.edu/medschool/news-archive/uofl-vascular-surgeons-perform-first-prosthetic-bypass-graft-procedure-using-patient2019sown-stem-cells-with-2018point-of-care2019-technology.
- 59.Mocini D, Staibano M, Mele L, Giannantoni P, Menichella G, et al. Autologous bone marrow mononuclear cell transplantation in patients undergoing coronary artery bypass grafting. Am Heart J. 2006;151:192–97. doi: 10.1016/j.ahj.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Yaoita H, Takase S, Maruyama Y, Sato Y, Satokawa H, et al. Scintigraphic assessment of the effects of bone marrow–derived mononuclear cell transplantation combined with off-pump coronary artery bypass surgery in patients with ischemic heart disease. J Nucl Med. 2005;46:1610–17. [PubMed] [Google Scholar]
- 61.Zhao Q, Sun Y, Xia L, Chen A, Wang Z. Randomized study of mononuclear bone marrow cell transplantation in patients with coronary surgery. Ann Thorac Surg. 2008;86:1833–40. doi: 10.1016/j.athoracsur.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 62.Ang K-L, Chin D, Leyva F, Foley P, Kubal C, et al. Randomized, controlled trial of intramuscular or intracoronary injection of autologous bone marrow cells into scarred myocardium during CABG versus CABG alone. Nat Clin Pract Cardiovasc Med. 2008;5:663–70. doi: 10.1038/ncpcardio1321. [DOI] [PubMed] [Google Scholar]
- 63.Li T-S, Hamano K, Hirata K, Kobayashi T, Nishida M. The safety and feasibility of the local implantation of autologous bone marrow cells for ischemic heart disease. J Card Surg. 2003;18:S69–75. doi: 10.1046/j.1540-8191.18.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 64.Ghodsizad A, Klein H-M, Borowski A, Stoldt V, Feifel N, et al. Intraoperative isolation and processing of BM-derived stem cells. Cytotherapy. 2004;6:523–26. doi: 10.1080/14653240410005014. [DOI] [PubMed] [Google Scholar]
- 65.Stamm C, Kleine HD, Westphal B, Petzsch M, Kittner C, et al. CABG and bone marrow stem cell transplantation after myocardial infarction. Thorac Cardiovasc Surg. 2004;52:152–58. doi: 10.1055/s-2004-817981. [DOI] [PubMed] [Google Scholar]
- 66.Klein HM, Assmann A, Lichtenberg A, Heke M. Intraoperative CD133+ cell transplantation during coronary artery bypass grafting in ischemic cardiomyopathy. MMCTS. 2010;2010:3947. doi: 10.1510/mmcts.2009.003947. [DOI] [PubMed] [Google Scholar]
- 67.Donnenberg AD, Donnenberg VS, Griffin DL, Moore LR, Tekinturhan F, Kormos RL. Intraoperative preparation of autologous bone marrow–derived CD34-enriched cellular products for cardiac therapy. Cytotherapy. 2011;13(4):441–48. doi: 10.3109/14653249.2010.529888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin P, Wang E, Wang Y-h, Huang W, Kuang W, et al. Central zone of myocardial infarction: a neglected target area for heart cell therapy. J Cell Mol Med. 2011;16:636–47. doi: 10.1111/j.1582-4934.2011.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao W, Schafer S, Choi J, Yamanaka YJ, Lombardi ML, et al. Cell-surface sensors for real-time probing of cellular environments. Nat Nanotechnol. 2011;6:524–31. doi: 10.1038/nnano.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarkar D, Vemula PK, Zhao W, Gupta A, Karnik R, Karp JM. Engineered mesenchymal stem cells with self-assembled vesicles for systemic cell targeting. Biomaterials. 2010;31:5266–74. doi: 10.1016/j.biomaterials.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campagnoli C, Roberts IAG, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 72.Baksh D, Davies JE, Zandstra PW. Adult human bone marrow–derived mesenchymal progenitor cells are capable of adhesion-independent survival and expansion. Exp Hematol. 2003;31:723–32. doi: 10.1016/s0301-472x(03)00106-1. [DOI] [PubMed] [Google Scholar]
- 73.Muschler GF, Nitto H, Boehm CA, Easley KA. Age- and gender-related changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J Orthop Res. 2001;19:117–25. doi: 10.1016/S0736-0266(00)00010-3. [DOI] [PubMed] [Google Scholar]
- 74.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Jt Surg Am. 1997;79:1699–709. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 75.Ichiyanagi T, Anabuki K, Nishijima Y, Ono H. Isolation of mesenchymal stem cells from bone marrow wastes of spinal fusion procedure (TLIF) for low back pain patients and preparation of bone dusts for transplantable autologous bone graft with a serum glue. Biosci Trends. 2010;4:110–18. [PubMed] [Google Scholar]
- 76.Peng L, Jia Z, Yin X, Zhang X, Liu Y, et al. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008;17:761–74. doi: 10.1089/scd.2007.0217. [DOI] [PubMed] [Google Scholar]
- 77.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 78.Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–62. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 79.Rebelatto CK, Aguiar AM, Moretao MP, Senegaglia AC, Hansen P, et al. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med. 2008;233:901–13. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 80.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 81.Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, et al. Adipose tissue–derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8:166–77. doi: 10.1080/14653240600621125. [DOI] [PubMed] [Google Scholar]
- 82.Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an under appreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–54. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 83.Jurgens WJFM, van Dijk A, Zandieh Doulabi B, Niessen FB, Ritt MJPF, et al. Freshly isolated stromal cells from the infrapatellar fat pad are suitable for a one-step surgical procedure to regenerate cartilage tissue. Cytotherapy. 2009;11:1052–64. doi: 10.3109/14653240903219122. [DOI] [PubMed] [Google Scholar]
- 84.Jurgens WJFM, Oedayrajsingh-Varma MJ, Helder MN, Zandieh Doulabi B, Schouten TE, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415–26. doi: 10.1007/s00441-007-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoogendoorn RJW, Lu ZF, Kroeze RJ, Bank RA, Wuisman PIJM, Helder MN. Adipose stem cells for intervertebral disc regeneration: current status and concepts for the future. J Cell Mol Med. 2008;12:2205–16. doi: 10.1111/j.1582-4934.2008.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knippenberg M, Helder MN, Zandieh Doulabi B, Wuisman PIJM, Klein-Nulend J. Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells. Biochem Biophys Res Commun. 2006;342:902–8. doi: 10.1016/j.bbrc.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 87.Helder MN, Knippenberg M, Klein-Nulend J, Wuisman PIJM. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng. 2007;13:1799–808. doi: 10.1089/ten.2006.0165. [DOI] [PubMed] [Google Scholar]
- 88.Gimble J, Guilak F, Bunnell B. Clinical and preclinical translation of cell-based therapies using adipose tissue–derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–58. [PubMed] [Google Scholar]
- 90.Case J, Mead LE, Bessler WK, Prater D, White HA, et al. Human CD34+AC133+VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–18. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 91.Majors AK, Boehm CA, Nitto H, Midura RJ, Muschler GF. Characterization of human bone marrow stromal cells with respect to osteoblastic differentiation. J Orthop Res. 1997;15:546–57. doi: 10.1002/jor.1100150410. [DOI] [PubMed] [Google Scholar]
- 92.Simmons P, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 93.Bruder SP, Ricalton NS, Boynton RE, Connolly TJ, Jaiswal N, et al. Mesenchymal stem cell surface antigen SB-10 corresponds to activated leukocyte cell adhesion molecule and is involved in osteogenic differentiation. J Bone Miner Res. 1998;13:655–63. doi: 10.1359/jbmr.1998.13.4.655. [DOI] [PubMed] [Google Scholar]
- 94.Oprea WE, Karp JM, Hosseini MM, Davies JE. Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac Surg. 2003;14:292–300. doi: 10.1097/00001665-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Langer HF, Gawaz M. Platelets in regenerative medicine. Basic Res Cardiol. 2008;103:299–307. doi: 10.1007/s00395-008-0721-4. [DOI] [PubMed] [Google Scholar]
- 96.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–8. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 97.Kark LR, Karp JM, Davies JE. Platelet releasate increases the proliferation and migration of bone marrow–derived cells cultured under osteogenic conditions. Clin Oral Implants Res. 2006;17:321–27. doi: 10.1111/j.1600-0501.2005.01189.x. [DOI] [PubMed] [Google Scholar]
- 98.Peterbauer-Scherb A, van Griensven M, Meinl A, Gabriel C, Redl H, Wolbank S. Isolation of pig bone marrow mesenchymal stem cells suitable for one-step procedures in chondrogenic regeneration. J Tissue Eng Regen Med. 2010;4:485–90. doi: 10.1002/term.262. [DOI] [PubMed] [Google Scholar]
- 99.Leitner GC, Gruber R, Neumüller J, Wagner A, Kloimstein P, et al. Platelet content and growth factor release in platelet-rich plasma: a comparison of four different systems. Vox Sang. 2006;91:135–39. doi: 10.1111/j.1423-0410.2006.00815.x. [DOI] [PubMed] [Google Scholar]
- 100.Mazzucco L, Balbo V, Cattana E, Borzini P. Platelet-rich plasma and platelet gel preparation using Plateltex®. Vox Sang. 2008;94:202–8. doi: 10.1111/j.1423-0410.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 101.Kaminski A, Klopsch C, Mark P, Yerebakan C, Donndorf P, et al. Autologous valve replacement— CD133+ stem cell-plus-fibrin composite-based sprayed cell seeding for intraoperative heart valve tissue engineering. Tissue Eng Part C. 2010;17:299–309. doi: 10.1089/ten.TEC.2010.0051. [DOI] [PubMed] [Google Scholar]
- 102.Zangi L, Rivkin R, Kassis I, Levdansky L, Marx G, Gorodetsky R. High-yield isolation, expansion, and differentiation of rat bone marrow–derived mesenchymal stem cells with fibrin microbeads. Tissue Eng. 2006;12:2343–54. doi: 10.1089/ten.2006.12.2343. [DOI] [PubMed] [Google Scholar]
- 103.Guo K-T, Schäfer R, Paul A, Gerber A, Ziemer G, Wendel HP. A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells. 2006;24:2220–31. doi: 10.1634/stemcells.2006-0015. [DOI] [PubMed] [Google Scholar]
- 104.Guo K-T, Ziemer G, Paul A, Wendel HP. CELL-SELEX: novel perspectives of aptamer-based therapeutics. Int J Mol Sci. 2008;9:668–78. doi: 10.3390/ijms9040668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, et al. Characterization of adipose tissue–derived cells isolated with the Celution™ system. Cytotherapy. 2008;10:417–26. doi: 10.1080/14653240801982979. [DOI] [PubMed] [Google Scholar]
- 106.Hicok KC, Hedrick MH. Automated isolation and processing of adipose-derived stem and regenerative cells. Methods Mol Biol. 2011;702:87–105. doi: 10.1007/978-1-61737-960-4_8. [DOI] [PubMed] [Google Scholar]
- 107.Jurgens WJ, Kroeze RJ, Bank RA, Ritt MJPF, Helder MN. Rapid attachment of adipose stromal cells on resorbable polymeric scaffolds facilitates the one-step surgical procedure for cartilage and bone tissue engineering purposes. J Orthop Res. 2011;29:853–60. doi: 10.1002/jor.21314. [DOI] [PubMed] [Google Scholar]
- 108.Bensaïd W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 109.Müller AM, Mehrkens A, Schäfer DJ, Jaquiery C, Güven S, et al. Towards an intraoperative engineering of osteogenic and vasculogenic grafts from the stromal vascular fraction of human adipose tissue. Eur Cells Mater. 2010;19:127–35. doi: 10.22203/ecm.v019a13. [DOI] [PubMed] [Google Scholar]
- 110.Rüger BM, Breuss J, Hollemann D, Yanagida G, Fischer MB, et al. Vascular morphogenesis by adult bone marrow progenitor cells in three-dimensional fibrin matrices. Differentiation. 2008;76:772–83. doi: 10.1111/j.1432-0436.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 111.Syedain ZH, Weinberg JS, Tranquillo RT. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci USA. 2008;105:6537– 42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–77. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hernigou P, Mathieu G, Poignard A, Manicom O, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions: surgical technique. J Bone Jt Surg Am. 2006;88:322–27. doi: 10.2106/JBJS.F.00203. [DOI] [PubMed] [Google Scholar]
- 114.Gupta MC, Theerajunyaporn T, Maitra S, Schmidt MB, Holy CE, et al. Efficacy of mesenchymal stem cell enriched grafts in an ovine posterolateral lumbar spine model. Spine. 2007;32:720–26. doi: 10.1097/01.brs.0000258863.40984.32. [DOI] [PubMed] [Google Scholar]
- 115.Jacobsen K, Szczepanowski K, Al-Zube LA, Kim J, Lin SS. The role of intraoperative bone marrow aspirate stem cell concentration as a bone grafting technique. Tech Foot Ankle Surg. 2008;7:84–89. [Google Scholar]
- 116.Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, et al. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16:1059–69. [PubMed] [Google Scholar]
- 117.Mehta J, Singhal S, Gordon L, Tallman M, Williams S, et al. COBE Spectra is superior to Fenwal CS-3000 Plus for collection of hematopoietic stem cells. Bone Marrow Transplant. 2002;29:563–67. doi: 10.1038/sj.bmt.1703520. [DOI] [PubMed] [Google Scholar]
- 118.Ford CD, Lehman C, Strupp A, Kelley L. Comparison of CD34+ cell collection efficiency on the COBE Spectra and Fenwal CS-3000 plus. J Clin Apheresis. 2002;17:17–20. doi: 10.1002/jca.10011. [DOI] [PubMed] [Google Scholar]
- 119.Dzieczkowski JS, McGonigal M, Cook J, Sugrue M, Andersen J, Anderson KC. A comparison of peripheral blood stem cell apheresis using the Fenwal CS-3000 Plus and COBE Spectra. Transfus Sci. 1995;16:71–77. doi: 10.1016/0955-3886(94)00061-n. [DOI] [PubMed] [Google Scholar]
- 120.Aktas M, Radke T, Strauer B, Wernet P, Kogler G. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy. 2008;10:203–11. doi: 10.1080/14653240701851324. [DOI] [PubMed] [Google Scholar]