Abstract
Low levels of the adipocyte hormone leptin are considered to be the key signal contributing to inhibited gonadotrophin-releasing hormone (GnRH) release and reproductive acyclicity during negative energy balance. Hypoleptinaemia-induced inhibition of GnRH may be initiated with upstream inhibition of the secretagogue kisspeptin (Kiss1) because GnRH neurones do not express leptin receptors. The present study aimed to determine whether eliminating the hypoleptinaemia associated with caloric restriction (CR), by restoring leptin to normal basal levels, could reverse the suppression of the reproductive neuroendocrine axis. Fifty percent CR resulted in significant suppression of anteroventral periventricular Kiss1 mRNA, arcuate nucleus (ARH) Kiss1 and neurokinin B (NKB) mRNA levels and serum luteinising hormone (LH). Restoring leptin to normal basal levels did not restore Kiss1 or NKB mRNA or LH levels. Surprisingly, leptin did not activate expression of phosphorylated signal-transducer and activator of transcription-3 in ARC Kiss1 neurones, indicating that these neurones may not relay leptin signalling to GnRH neurones. Previous work in fasting models showing restoration of LH used a pharmacological dose of leptin. Therefore, in a 48-h fast study, replacement of leptin to pharmacological levels was compared with replacement of leptin to normal basal levels. Maintaining leptin at normal basal levels during the fast did not prevent inhibition of LH. By contrast, pharmacological levels of leptin did maintain LH at control values. These results suggest that, although leptin may be a permissive signal for reproductive function, hypoleptinaemia is unlikely to be the critical signal responsible for ARC Kiss1 and LH inhibition during negative energy balance.
Keywords: leptin, negative energy balance, kisspeptin, neurokinin B, gonadotrophin-releasing hormone, luteinising hormone, caloric restriction
Negative energy balance is the metabolic state in which energy input is insufficient for energy output. In mammals, negative energy balance results in a halting of cyclic reproductive function that is considered to be triggered by decreased release of gonadotrophin-releasing hormone (GnRH), which then results in lowered levels of luteinising hormone (LH) (1–3). However, the signal mediating this inhibition of GnRH release is still unclear.
Leptin is considered to be a key metabolic signal conveying energy reserve to the brain (4,5). Leptin is produced in adipocytes, and during negative energy balance, decreases in fat mass result in lowered levels of circulating leptin (6). Importantly, leptin has been implicated in stimulating LH release (7–10); therefore, it has been hypothesised that low levels of leptin during negative energy balance are critical for lowered GnRH release. Indeed, many studies have found that giving exogenous leptin during a 48-h fast maintains normal LH release (7,11–13). However, GnRH neurones do not appear to express leptin receptors (10,14) and it has long been hypothesised that an intermediate cell population likely mediates the effects of leptin on GnRH.
The GnRH secretagogue kisspeptin (Kiss1) is critical to our understanding of GnRH regulation because Kiss1 populations in the anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) have been implicated in positive and negative steroid feedback, respectively (15–18). ARC Kiss1 cells are of particular interest because they also express two other neuropeptides, neurokinin B (NKB) and dynorphin (DYN) across many species (19–24), and are referred to as KNDy neurones. NKB is also considered to be stimulatory for GnRH release (21,25,26), and may act autosynaptically to stimulate Kiss1 release (22,24). ARC KNDy cells were found to express leptin receptors in the mouse, and leptin has been demonstrated to stimulate Kiss1 (27,28), making them a likely candidate for the intermediate cells involved in the regulation of GnRH by leptin (27,29–33). Both AVPV Kiss1 and ARC Kiss1/NKB mRNA levels appear to be inhibited in some models of negative energy balance (19,28,34–38), suggesting that decreases in Kiss1 or NKB could translate to decreased stimulation of GnRH release. Taken together, the above findings have led to the updated hypothesis that low leptin levels during negative energy balance may drive the inhibition of Kiss1, and possibly NKB, which, in turn, leads to less stimulation of GnRH release.
To date, the effects of leptin on LH release during negative energy balance have primarily been studied at pharmacological doses. A recent study in our laboratory using the lactation model of negative energy balance demonstrated that eliminating hypoleptinaemia by restoring leptin to normal basal levels did not relieve inhibition of ARC Kiss1, NKB or LH (36). Importantly, when animals exit negative energy balance, leptin levels rise but only to levels observed in conditions of normal energy balance (36). Therefore, although high concentrations of leptin may be capable of stimulating Kiss1 and LH (7,12,13,28), the natural restoration of leptin levels after negative energy balance may not be a sufficient signal alone to relieve reproductive inhibition.
It is possible that, in our lactation study (36), leptin restoration to normal basal levels did not restore LH because its effects were masked by continued inhibitory signals specific to the suckling stimulus (39). The first goal of the present study was to determine whether AVPV Kiss1 and ARC KNDy mRNA levels were reduced with long-term caloric restriction (CR) and whether eliminating hypoleptinaemia was capable of restoring these reproductive neuropeptides or serum LH in this model lacking the suckling stimulus. The second goal was to compare the relative effectiveness of maintaining leptin at normal basal levels or at pharmacological levels in preventing the inhibition of LH secretion in animals fasted for 48 h. The 48-h fast was chosen for this comparative study because pharmacological leptin doses have been previously shown to be effective at maintaining LH in this model.
Materials and methods
Animals and tissue collection
Adult female Wistar rats (Simonsen, Gilroy, CA, USA), weighing between 200 and 220 g, were used in all studies. Animals were singly housed and maintained under a 12 : 12 h light/dark cycle (lights on 06.00 h) and allowed water ad lib. All protocols were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee and conducted in accordance with NIH Guidelines for Care and Use of Laboratory Animals.
At tissue collection, animals were briefly anaesthetised under isoflurane and decapitated. Trunk blood was collected and the brain was rapidly removed. A 1-mm coronal slice was made at the level of the optic chiasm, and a 2-mm2 punch was made from this slice corresponding to the AVPV and rapidly frozen. The remainder of the brain was further blocked with a caudal boundary of the mammillary bodies and lateral boundaries of the temporal sulci. This brain block was mounted ventral side up for vibratome sectioning in Kreb’s solution and the bottom 600 μm of the brain pertaining to the ARC was removed and rapidly frozen, as described previously (36). In addition, the uterus was dissected and weighed at the time of tissue collection. The remaining carcasses were then frozen until dual-energy X-ray absorptiometry (DEXA) was performed at a later time.
Experiment 1: 40% caloric restriction
All animals were ovariectomised (OVX) under a mixture of isoflurane/oxygen gas anaesthesia and during the same surgery implanted s.c. with silastic capsules (10 mm in length/100 g of body weight) containing either 30 μg/ml 17β-oestradiol dissolved in oil or oil alone. This method and dose of oestradiol replacement has been previously shown to result in low physiological levels of oestradiol corresponding to those observed during dioestrus (40) or negative energy balance (41). Importantly, this low dose of oestradiol does not significantly blunt the OVX-induced LH rise unless also combined with progesterone treatment (40); therefore, LH levels remain high and differences between groups can be more easily detected. Animals were given 5 mg/kg of the analgesic carprofen s.c. for recovery. The animals receiving oestradiol were split into three groups (Table 1): (i) control animals that were ad lib fed [OVX + oestradiol (E), n = 8]; (ii) animals on a 40% CR receiving 40% less calories than the OVX + E group for 14 days (OVX + E CR, n = 8); and (iii) animals on 40% CR also receiving leptin for the final 48 h of CR (OVX + E CR + L, n = 8). To assess the requirement of low levels of oestradiol for LH inhibition during CR, we included OVX control ad lib fed animals without oestradiol replacement (OVX, n = 8), as well as OVX animals weight matched (OVX CR*, n = 8) to the CR group with oestradiol replacement (OVX + E CR) to determine whether a primary action of oestradiol is required beyond its known effects on metabolism and body weight (Table 1). CR began 4 days after OVX and implantation of silastic implants to allow recovery from surgery before food intake manipulations.
Table 1.
Experimental Group Descriptions.
| Experiment | Group name |
Steroids | Food intake |
Leptin treatment |
|---|---|---|---|---|
| 40% CR | OVX + E | OVX + E | Ad lib | None |
| OVX + E | CR OVX + E | 40% CR for 14 days |
None | |
| OVX + E CR + L |
OVX + E | 40% CR for 14 days |
500 ng/h for last 48 h |
|
| OVX | OVX | Ad lib | None | |
| OVX CR* | OVX | CR to weight-match ‘OVX + E CR’ group |
None | |
| 50% CR | CTRL | OVX + E | Ad lib | None |
| 50% CR | OVX + E | 50% CR for 14 days |
None | |
| 50% CR + L |
OVX + E | 50% CR for 14 days |
500 ng/h for last 72 h |
|
| 48-h fast | CTRL | OVX + E | Ad lib | None |
| F | OVX + E | 48 h fast | None | |
| F + Leptin | OVX + E | 48 h fast | 500 ng/h, for 48 h |
|
| F + High Leptin |
OVX + E | 48 h fast | 3 μg/g twice daily, for 48 h |
CR, caloric restriction; CTRL, control; F, fasted; L, leptin; E, oestradiol; OVX, ovariectomised.
The 40% CR for the OVX + E CR and OVX + E CR + L groups was calculated each day based on the average food intake of the OVX + E group from the previous day. Food intake for the OVX CR* group was adjusted daily so that animals lost a comparable amount of weight as the OVX + E CR group, thus controlling for the metabolic effects of oestradiol. Animals on CR received food at 07.00 h. Leptin treatment for the OVX + E CR + L group was administered continuously through an osmotic minipump (Alzet, Cupertino, CA, USA) at a rate of 500 ng of recombinant rat leptin (400-21; Peprotech, Rocky Hill, NJ, USA) per hour; this dose restores leptin to normal basal levels, as previously described in our lactation studies (36). The biological efficacy of this leptin dose was demonstrated by its ability to induce phosphorylated signal-transducer and activator of transcription-3 (pSTAT3), a downstream effector of leptin-receptor activation, within 2 h after minipump implantation during a hypoleptinemic state (48-h fast; see also Supporting information, Fig. S1). The four remaining groups received minipumps containing saline. Osomotic minipumps were incubated in a 37 °C water bath for 12–24 h before s.c. implantation under isoflurane/oxygen anaesthesia at 07.00 h on day 12 of CR. Tissue was collected on day 14 of CR beginning at 07.00 h.
Experiment 2: 50% caloric restriction
There were three groups in the 50% CR experiments (Table 1): (i) OVX and oestradiol-replaced ad lib fed controls (CTRL, n = 8); (ii) OVX and oestradiolreplaced receiving 50% less calories than control for 14 days (50% CR, n = 8); and (iii) OVX and oestradiol-replaced animals on a 50% CR receiving leptin treatment (50% CR + L, n = 8). This experiment was carried out similarly to the 40% CR, with food intake and body weights measured and food allotment given daily at 07.00 h for each animal. Leptin treatment was also similar as described for the 40% CR study, except that it began on day 11 of CR so that animals were exposed to 72 h of leptin treatment. Tissue was once again collected at 07.00 h on day 14 of CR.
Experiment 3: 48-h fast
Animals were OVX and oestradiol replaced as described for the CR studies and split into four groups (Table 1): (i) ad lib fed controls (CTRL, n = 6); (ii) 48-h fast (F, n = 6); (iii) 48-h fast combined with minipump infusion of leptin as described for the CR studies (F + Leptin, n = 6); and (iv) 48-h fast combined with a pharmacological leptin treatment (F + High Leptin, n = 6). Fasting and leptin treatments began on the fourth day after OVX. The administration of leptin to achieve normal basal levels was the same as described for Experiments 1 and 2, and minipumps were implanted at 07.00 on the first day of the fast. The pharmacological leptin treatment was modified from the protocol used by Nagatani et al. (12). Animals in the F + High Leptin group were given two i.p. injections of 3 μg of leptin per gram of body weight at 07.00 h and 17.00 h each day of the fast. Animals in all other groups received i.p. saline injections. These different methods of leptin administration were used to replicate previously published results for both normal (36) and high (12) levels of leptin. Animals were euthanised and tissues were collected at 07.00 h on the third day of the experiment.
Experiment 4: leptin induction of pSTAT3 in ARC Kiss1 neurones
To determine whether leptin acts directly at ARC Kiss1 neurones, acute leptin injections were given followed by immunohistochemistry to investigate potential colocalisation of ARC Kiss1 and pSTAT3. Female rats were OVX, but not oestradiol replaced to keep ARC Kiss1 staining as high as possible and, 4 days later, given an acute i.p. injection of either leptin (1 μg/g of body weight; n = 4) or saline (n = 4) at 09.00. A high pharmacological dose of leptin was used to ensure maximum stimulation of pSTAT3. Forty-five minutes later animals were anaesthetised with tribromoethanol and perfused transcardially with saline and 4% paraformaldehyde. Brains were removed and kept in 4% paraformaldehyde overnight followed by 24 h in a 25% sucrose solution. Brains were then rapidly frozen and later cut into a one-in-six series of 20-μm sections using a sliding microtome.
RNA isolation and quantitative polymerase chain reaction (PCR) (Experiments 1–3)
RNA was isolated from ARCs and AVPVs using a Qiagen MiniPrep kit (Qiagen, Valencia, CA, USA), quantified with a Nanodrop Spectrophotomter (ND1000; Thermo Scientific, Wilmington, DE, USA) and treated with DNase (1 μg/μg RNA) before reverse transcription using random hexamer primers (Promega Corp., Madison, WI, USA). Quantitative PCR was carried out in 10-μl reactions consisting of 5 μl of Taqman universal PCR master mix, 2 μl of cDNA used at dilutions of 1 : 50 for ARC samples and 1 : 20 for AVPV samples, 300 nM of the primer and probe of interest, 80 nM of 18 s primers and 250 nM of the 18 s probe. Amplification was performed using the ABI/Prism 7700 sequences detector system (Applied Biosystems, Carlsbad, CA, USA) with 2 min at 50 °C, 10 min at 90 °C, and then 40 cycles each at 95 °C for 15 s followed by 60 °C for 60 s. The following primer probe sets were all purchased from Applied Biosystems: Kiss1 (Rn00710914_m1), NKB (Rn00569758_m1), Pro-DYN (PDYN). (Rn00571351_m1), agouti-related peptide (AgRP) (Rn01431702_g1), neuropeptide Y (NPY) (Rn01410146_m1), pro-opiomelanocortin (POMC) (Rn00595020_m1) and Suppressor of Cytokine Signaling 3 (SOCS3). (Rn00585674_s1).
The threshold for raw CT values for each gene of interest was adjusted to be in the exponential range of amplification. Standard curves on serial dilutions of pooled ARC cDNA were drawn on the basis of the log of the input RNA versus the critical threshold (CT) cycle. The efficiencies of these primers, as determined by the R2 value from standard curves, were all at or above 0.95. Once CT values were normalised for cDNA content using the line of best fit for the standard curve, CT values for genes of interest were normalised to 18 s CT values, which was the house-keeping gene used previously in the lactation study (36). Normalised CT values were then averaged across triplicates. Quantitative (q)PCR results were required to meet stringent criteria before they were included for analysis. Samples were excluded if: (i) at least two CT values did not fall within one logarithmic degree of each other; (ii) 18 s CT values were 3 or more logarithmic degrees away from the mean 18 s CT value; or (iii) normalised CT values were more than 2 SD away from the group mean.
Immunohistochemistry for Kiss1 and pSTAT3 (Experiment 4)
One series of tissue sections per animal was used for immunohistochemistry (IHC). Tissue prepared for pSTAT3/Kiss1 IHC was rinsed with potassium phosphate buffer and incubated in 1% NaOH/H2O2 solution for 20 min, followed by 10 min incubations in 3% glycine and 0.03% SDS. Tissue was then blocked in 2% normal donkey serum followed by incubation in the primary mouse anti-pSTAT3 antibody (dilution 1 : 2000; catalogue number 4113, Cell Signaling Technology, Beverly, MA, USA) for 1 h at room temperature followed by 24 h at 4 °C. For NiDAB detection, tissue was rinsed and incubated for an hour in either a biotinylated donkey anti-mouse or anti-rabbit antibody (dilution 1 : 600; catalogue number 715-065-150; Jackson Laboratories, Bar Harbor, ME, USA), followed by an half hour incubation in A/B solution (Vectostain Elite ABC kit; Vector Laboratories, Burlingame, CA, USA, dilution 1 : 222 for both the A and B solution). Tissue was then incubated in a NiDAB solution (SK 4100; Vector Laboratories) until adequate staining was observed. Tissue was then rinsed and incubated for an hour at room temperature, and 48 h at 4 °C in the rabbit anti-Kiss1 antibody (#564, a gift from Alan Caraty (Centre National de la Recherche Scientifique, Nouzilly, France). This antibody has been previously characterised and demonstrates highly specific Kiss1 detection in the ARC (42). After primary incubation, tissue was processed as above except DAB staining was used instead of NiDAB. All tissue was mounted and dehydrated through a series of increasing concentrations of ethanol, followed by xylene treatment and finally slides were coverslipped with Permount. For cell counts, the total number of Kiss1-immunoreactive (ir) cells and Kiss/pSTAT3-ir cells were counted in 5 s pertaining to the medial ARC for each animal.
Radioimmunoassay
Trunk blood was put on ice immediately after collection until it was spun at 2500 r.p.m. (4000g) for 25 min. Serum was collected from these samples and aliquoted for each radioimmunoassay (RIA) before storage at −20 °C. Leptin RIAs were performed by the Oregon National Primate Research Center Endocrine Services Laboratory using the leptin RIA kit with a lower detection threshold of 0.5 ng/ml (Linco Research, Inc., St Charles, MO, USA). All LH RIA assays were performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core and had a reported lower detection threshold of 0.4 ng/ml.
Statistical analysis
All analyses of group differences for qPCR, immunohistochemistry, RIA, DEXA and uterine weight results were performed by a one-way ANOVA with a Newman–Keul’s post-hoc test used for pairwise multiple comparisons. The group differences in the percent change in body weight for the 48-h fast study was also measured with this test. Repeated measures for daily differences in body weights were determined using a two-way ANOVA and Bonferroni post-hoc test. ARC Kiss1/pSTAT3 cell counts were analysed using a two-way ANOVA. All values are presented as the mean ± SE.
Results
Experiment 1: 40% caloric restriction
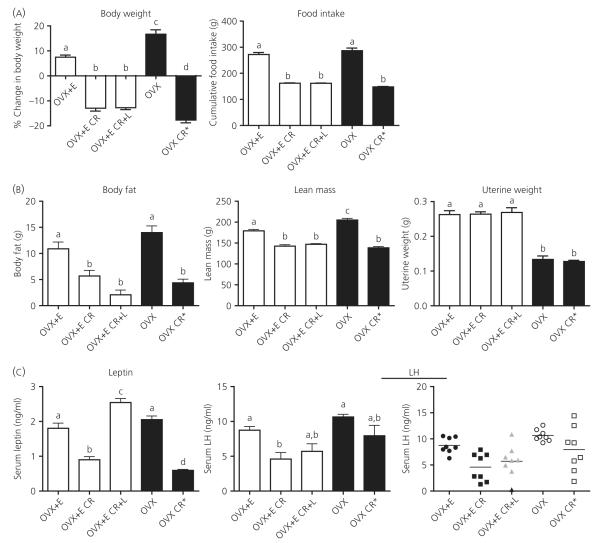
Low levels of oestradiol have previously been shown to be required for LH inhibition during a 48-h fast; therefore, to determine the possible necessity of oestradiol for LH inhibition during CR, we included OVX control animals without oestradiol replacement, as well as OVX animals weight-matched (OVX CR*) to the CR group with oestradiol replacement (OVX + E CR). Ad lib fed controls with low oestradiol (OVX + E) gained significantly less weight than those without oestradiol (OVX), despite similar levels of food intake (Fig. 1A; for daily body weight and food intake measurements, see Supporting information, Fig. S2). Consistent with these observed metabolic effects of oestradiol in the control groups, the OVX CR* group did receive slightly less food than the OVX + E CR group to achieve comparable loss in body weight (see Supporting information, Fig. S2); however, the cumulative food intake over the CR period was not significantly different (Fig. 1A). The total percent change in body weight was greater in the OVX CR* group compared to the OVX + E CR group; however, a two-way ANOVA to determine differences between changes in body weight on a daily basis (see Supporting information, Fig. S2) did not reveal significant differences between the two groups (P > 0.05). The OVX CR* and OVX + E CR groups had similar body compositions as determined by DEXA measurements, with both groups showing significant loss in both total body fat and lean mass (Fig. 1B). This comparable loss in both fat and lean mass, as well as a similar final loss in body weight, suggests that similar levels of negative energy balance were achieved regardless of steroid environment. Final uterine weights were significantly increased in groups receiving oestradiol (Fig. 1B), confirming the efficacy of the low oestradiol treatment.
Fig. 1.
Body composition and serum leptin and luteinising hormone (LH) levels in response to 40% caloric restriction (CR). (A) Change in body weight and cumulative food intake. Ovariectomised (OVX) + oestradiol (E), ad lib fed controls receiving a 2.5-cm silastic implant containing 30 μg/ml oestradiol; OVX + E CR, oestradiol replaced animals on 40% CR (compared to OVX + E group); OVX + E CR + L, oestradiol replaced animals on 40% CR implanted with an osmotic minipump delivering 500 ng of leptin per hour for the last 48 h of CR; OVX, ad lib fed controls not receiving oestradiol replacement; OVX CR*, animals without oestradiol that were CR to achieve comparable weight-loss as the OVX + E CR group. (B) Both body fat and lean mass were measured by dual-energy X-ray absorptiometry post mortem. Uteri were dissected and weighed at the time of euthanisation to verify effectiveness of oestradiol treatment. (C) Serum leptin and LH were measured by radioimmunoassay. LH results are presented both as a bar graph and scatterplot to demonstrate high variability in LH levels in all three CR groups (OVX + E CR, OVX + E CR + L, OVX CR*). Columns with different letters are significantly different (P < 0.05).
Leptin was significantly inhibited in the OVX + E CR and OVX CR* groups (Fig. 1C), with the OVX CR* group having lower leptin values compared to the OVX + E CR group. Leptin replacement resulted in serum levels slightly higher than those seen in controls; however, these leptin levels are still within the physiological range. Consistent with previous work, the low oestradiol treatment did not significantly blunt LH levels (40) because there was no difference in LH levels between the two control groups (Fig. 1C). LH levels were significantly inhibited in the OVX + E CR group compared to controls; however, LH levels were not significantly inhibited in the OVX CR* group (Fig. 1C), suggesting that oestradiol is required for CR-induced LH inhibition. Leptin replacement had a small effect on LH, resulting in levels not significantly different from the OVX + E or OVX + E CR group; however, a large amount of variability was observed in the LH values for the three CR groups (Fig. 1C).
Hypothalamic mRNA levels
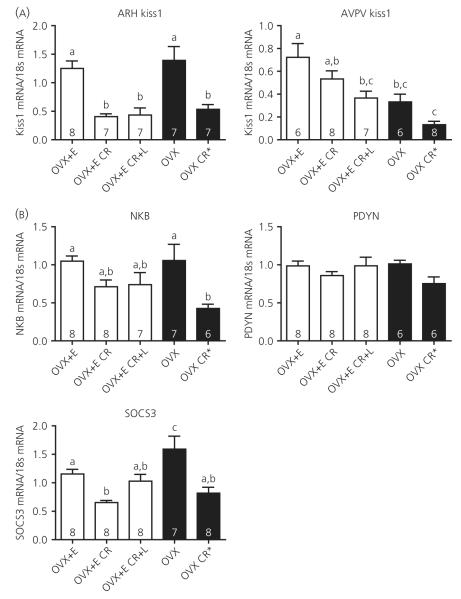
ARC Kiss1 mRNA was significantly suppressed with CR, regardless of oestradiol replacement, suggesting that, although oestradiol is required for CR-induced LH inhibition, it is not required for ARC Kiss1 inhibition (Fig. 2A). Restoration of leptin to normal basal levels had no effect on inhibited ARC Kiss1 levels. ARC Kiss1 mRNA levels were not significantly different between the OVX + E and OVX groups. AVPV Kiss1 mRNA was not significantly inhibited in the OVX + E CR group; however, levels were significantly lower in the OVX + E CR + L group, suggesting a possible combined effect of CR and leptin treatment (Fig. 2A). AVPV Kiss1 levels were significantly higher in the OVX + E group compared to OVX animals, consistent with the stimulatory effect of oestradiol on this nucleus and a previously reported higher level of sensitivity to oestradiol compared to the ARC Kiss1 population (43).
Fig. 2.
Hypothalamic mRNA expression in response to 40% caloric restriction (CR). (A) Kiss1 mRNA was measured by real-time polymerase chain reaction from microdissected arcuate nucleus (ARC) and anteroventral periventricular nucleus (AVPV) samples. (B) ARC reproductive mRNAs (NKB, neurokinin B; PDYN, Pro-DYN and Suppressor of Cytokine Signaling 3, SOCS3. mRNA were also measured from the microdissected ARCs. Columns with different letters are significantly different, P < 0.05; numbers inside histograms represent group size.
Despite significant suppression of ARC Kiss1 with CR, NKB mRNA was only partially inhibited in the OVX + E CR group, and leptin appeared to have no effect on NKB mRNA levels (Fig. 2B). Similar to the results in the lactation model (36), PDYN mRNA levels were not differentially regulated by negative energy balance or leptin treatment (Fig. 2B). SOCS3, a downstream signalling molecule of the leptin receptor, was significantly inhibited with CR and restoration of leptin to normal basal levels partially attenuated this inhibition (Fig. 2B). The negative energy balance condition was confirmed by the presence of increased ARC NPY and AgRP mRNA levels, although POMC mRNA levels were unchanged by CR (see Supporting information, Fig. S3A). Leptin had a small effect to attenuate the rises in AgRP and NPY levels, but had no affect on POMC levels.
Experiment 2: 50% caloric restriction
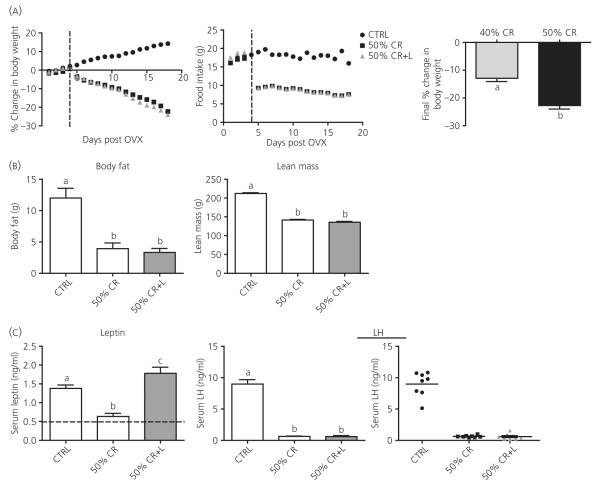
We hypothesised that the highly variable LH levels seen in the 40% CR groups were the result of animals being at a threshold of sufficient weight loss required for LH inhibition. To reduce the LH variability observed with 40% CR, and discern the true effects of leptin on LH, a more severe 50% CR was performed to induce more severe negative energy balance and weight loss. Because oestradiol appeared to be necessary for LH inhibition in 40% CR, only oestradiol replaced groups were included in this experiment. Fifty percent CR did result in a greater loss in body weight compared to the 40% CR (Fig. 3A), with animals losing a significant amount of both total body fat and lean mass (Fig. 3B). Leptin treatment was carried out for 72 h to determine whether more significant effects of leptin are observed with longer treatment. Despite the longer administration, leptin treatment could not attenuate CR-induced LH inhibition (Fig. 3C). By contrast to the variable LH levels in the 40% CR experiment (Fig. 1C), there was little LH variability in either the 50% CR or 50% CR + L group (Fig. 3C), suggesting there may be a threshold of sufficient weight loss required for LH inhibition during CR.
Fig. 3.
Body composition and serum leptin and luteinising hormone (LH) levels in response to 50% caloric restriction (CR). (A) Body weight was measured daily and is presented as the daily accumulative average of percent change in body weight. Food intake represents the average amount of food consumed per day. Ovariectomy and oestradiol silastic implantation were performed on day 0, and the dotted line marks the beginning of CR treatments on day 4. Leptin treatment had no additional effect on body weight in CR animals and changes in body weight were significantly different in CR groups compared to the control (CTRL) group beginning on day 5, 1 day after beginning CR. Right panel: Comparison of total weight loss for experimental groups across CR studies: 40% CR, ovariectomised (OVX) + oestradiol (E) CR group from Experiment 1; 50% CR, 50% CR group from Experiment 2. (B) Body fat and lean mass were determined by dual-energy X-ray absorptiometry post mortem. (C) Serum leptin and LH were measured by radioimmunoassay (RIA). Horizontal line in the leptin bar graph represents the lower threshold of detectability for the leptin RIA. LH data are presented both as bar graph and scatterplot. Columns with different letters are significantly different (P < 0.05).
Hypothalamic mRNA levels
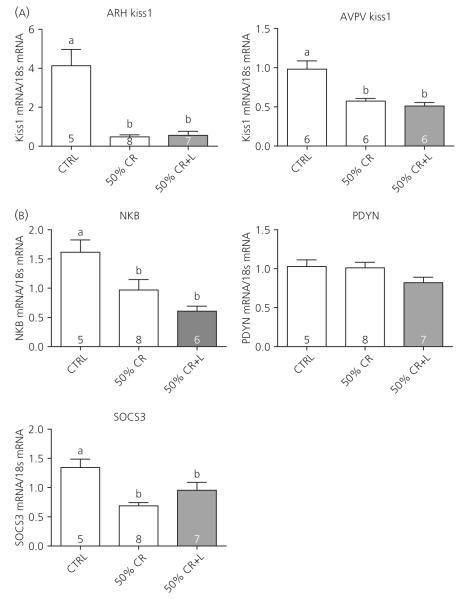
ARC Kiss1 mRNA was significantly suppressed by 50% CR, and leptin had no effect to restore ARC Kiss1 (Fig. 4A). Unlike the 40% CR, AVPV Kiss1 mRNA was also significantly suppressed by 50% CR and restoring leptin had no effect to restore AVPV Kiss1. NKB mRNA was also significantly suppressed by 50% CR, and leptin treatment had no effect to attenuate this inhibition (Fig. 4B). Once again, PDYN mRNA levels were similar across all groups, suggesting that PDYN does not appear to be regulated by CR or by leptin. Similar to the results with a 40% CR, leptin treatment partially restored the suppressed levels of ARC SOCS3 mRNA (Fig. 4B). Orexigenic AgRP and NPY mRNA levels were significantly increased by CR, and leptin partially reversed these increases. Similar to Experiment 1, POMC mRNA levels were unaffected by CR and leptin treatment (see Supporting information, Fig. S3B).
Fig. 4.
Hypothalamic mRNA expression in response to 50% caloric restriction (CR). (A) Kiss1 mRNA was measured by real-time polymerase chain reaction from microdissected arcuate nucleus (ARC) and anteroventral periventricular nucleus (AVPV) samples. (B) ARC reproductive mRNAs (NKB, neurokinin B; PDYN, Pro-DYN and Supressor of Cytokine Signaling 3 (SOCS3). mRNA were also measured from the microdissected ARCs. Columns with different letters are significantly different (P < 0.05); numbers inside histograms represent group size.
Experiment 3: 48-h fast
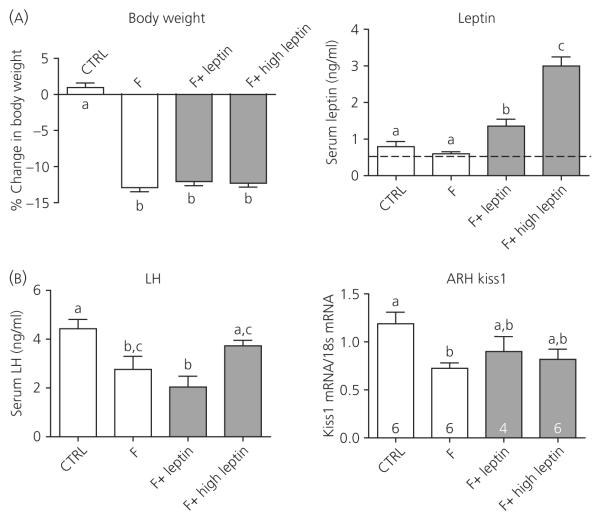
To determine whether the lack of the effects of leptin on LH observed in Experiments 1 and 2 was specific to the CR model, the leptin infusion regimen producing normal basal levels was given during a 48-h fast and compared with the previously reported effects of pharmacological doses (12). Control (CTRL) animals had a slight increase in body weight over the 48-h period, whereas all three fasted groups lost a comparable percentage of body weight (Fig. 5A). Leptin levels were not significantly inhibited by the 48-h fast; however, many samples in the fasted group (F) were below the detectable range; therefore, the reported leptin levels for this group are an overestimate of true levels (Fig. 5B). The leptin infusion (F + Leptin) once again resulted in leptin levels slightly higher than controls at the time of tissue collection (Fig. 5A). The pharmacological leptin treatment (F + High Leptin) resulted in significantly higher serum levels of leptin, even when measured 14 h after the last bolus injection (Fig. 5A).
Fig. 5.
Changes in body weight, serum leptin, serum luteinising hormone (LH), and arcuate nucleus (ARC) Kiss1 mRNA levels in response to the 48-h fast. (A) Change in body weight was calculated as the total % change in body weight over the 48 h period; CTRL, control; F, fasted; F + Leptin, fasted with 500 ng/g of body weight leptin treatment via osmotic minipump; F + High Leptin, fasted with twice daily injections of 3 μg/g of body weight leptin. Serum leptin was measured by radioimmunoassay (RIA) and the dotted line denotes the lower threshold of detectability for the leptin RIA. (B) Serum LH was also measured by RIA. Kiss1 mRNA was measured by real-time polymerase chain reaction from microdissected ARC samples. Columns with different letters are significantly different (P < 0.05).
Serum LH was significantly inhibited by the 48 h fast (Fig. 5B) and, consistent with previous results (7,12), the pharmacological dose of leptin did prevent LH inhibition. Unlike the pharmacological dose, maintaining leptin at normal basal levels was incapable of preventing the inhibition of LH. ARC Kiss1 mRNA was also significantly suppressed with a 48 h fast and, unexpectedly, both doses of leptin appeared to have a small effect to partially restore ARC Kiss1 mRNA levels (Fig. 5B).
Experiment 4: leptin induction of pSTAT3 in ARC Kiss1 neurones
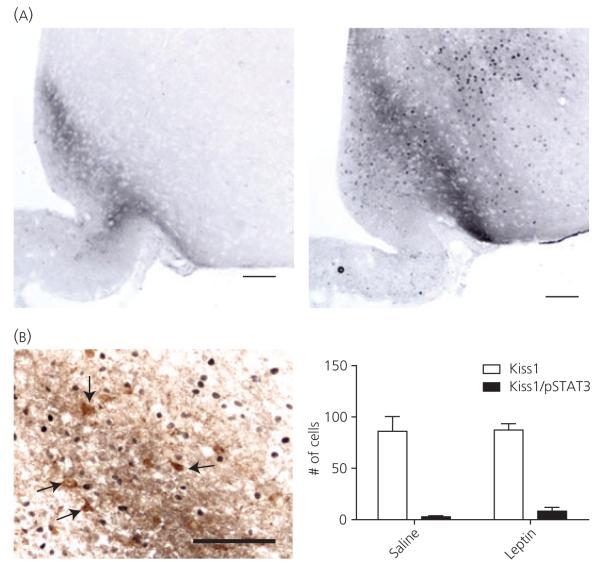
To determine whether leptin exerts a strong direct effect on ARC Kiss1 neurones, immunohistochemistry for ARC Kiss1 and pSTAT3 was performed in brains taken from animals receiving acute i.p. injections of either saline or leptin (1 μg/g body weight). Abundant pSTAT3-ir was observed in the hypothalamus ARC of leptin-injected animals (Fig. 6A), although few colocalised Kiss1/pSTAT3-ir cells were observed and there were no differences in the number of colocalised cells between saline and leptin-injected animals (Fig. 6B).
Fig. 6.
Phosphorylated signal-transducer and activator of transcription-3 (pSTAT3) and kisspeptin (Kiss1) immunohistochemistry following acute pharmacological leptin administration. (A) pSTAT3-immunoreactivity (ir) (NiDAB, dark brown nuclear staining) in the hypothalamus after saline (left) and leptin (right) treatment. (B) Arcuate nucleus (ARC) Kiss1-ir cells (DAB, brown staining, arrows) predominantly lacked pSTAT3-ir. The number of colocalised Kiss1/pSTAT3-ir cells was not different between saline and leptin-treated groups (right). Scale bar = 100 μm.
Discussion
The present study investigated whether abolishing hypoleptinaemia with a physiologically-relevant dose of leptin was capable of restoring GnRH/LH secretion during negative energy balance. The results obtained demonstrate that restoring leptin to normal basal values does not attenuate LH inhibition in a 50% CR. This dose of leptin was also incapable of maintaining LH levels during a 48-h fast. Therefore, although leptin may be a permissive signal for the suppression of cyclic reproductive function during negative energy balance, it appears unlikely that elimination of hypoleptinaemia is the critical driver of LH restoration. ARC Kiss1 is also suppressed with CR and fasting, and exogenous leptin could not restore ARC Kiss1 levels in the CR models, although there was a small effect of leptin in the fasting study.
There is a wealth of literature on the importance of leptin for normal reproductive function. Leptin is required for normal reproductive development because mutations in leptin or leptin receptors result in abnormal reproductive function (9,44,45), and exogenous leptin administration results in early onset of pubertal development (46). Studies across many species have also shown stimulation of LH by exogenous leptin under both normal and metabolically challenged conditions (7–10,47) and, critically, human studies suggest that leptin may be a viable treatment for women with exercise-induced hypothalamic amenorrhea (48).
Although the literature clearly points to an important role for leptin in reproductive function, recent studies have complicated the hypothesised role of leptin for negative energy balance-induced reproductive inhibition (49) found that when food restricted ewes are refed, LH parameters were restored before any increases in circulating leptin levels, suggesting that the elimination of hypoleptinaemia is not required for restoration of LH after negative energy balance. A recent study from our laboratory found that restoring leptin to normal basal levels had no effect to restore LH levels in the lactation model of negative energy balance (36). This dose of leptin, also used in the present study, resulted in normal basal leptin levels that are at least 50-fold lower than the levels reported with a standard pharmacological dose previously shown to attenuate LH inhibition (7). Indeed, the vast majority of studies demonstrating leptin-induced attenuation of LH inhibition have used similarly large pharmacological doses (7,10,12,13). However, it is important to acknowledge that although the administration of leptin by minipump in the present study resulted in serum levels in the normal physiological range, this administration cannot itself be termed ‘physiological’ because the diurnal leptin pattern was disrupted with continuous leptin infusion. Therefore, it remains possible that the effects of leptin in the present study were not observed as the result of a lack of diurnal rhythm.
Our previous work demonstrating a lack of effects of leptin upon negative energy balance-induced LH inhibition was performed in the lactation model (36), and we hypothesised that the effects of leptin in this model may have been masked by other inhibitory signals specific to lactation, such as the suckling stimulus (39). Therefore, the present study was designed to determine if leptin was ineffective during lactation as a result of the redundant inhibitory signals specific to this model or the lower dose of leptin administered. Seventy-two hours of exogenous leptin treatment, which restored leptin to normal basal levels, was incapable of restoring LH during 50% CR, suggesting the previously demonstrated lack of effects of leptin during lactation may not be a characteristic specific to this model. Additionally, although pharmacological leptin did prevent LH inhibition during a fast, consistent with previous results (7,12), maintaining leptin at normal basal levels did not. Leptin levels rise modestly when animals exit negative energy balance to return to normal basal levels and do not reach the very high levels observed with pharmacological doses (7,50). Therefore, although leptin appears to be required for normal reproductive development and can stimulate LH at pharmacological concentrations, hypoleptinaemia may not be the critical signal responsible for suppression of LH during negative energy balance.
In addition to LH inhibition, the present study has demonstrated that ARC Kiss1 is inhibited in both fasting and CR models of negative energy balance. Previous data describing fasting effects on ARC Kiss1 have been inconsistent, with data arguing both for and against inhibition of these cells (34,35,37,51,52). Given previous results suggesting a role for ARC Kiss1 in negative steroid feedback of GnRH release, the current results of ARC Kiss1 inhibition in the present study are consistent with previous work demonstrating a loss of pulsatile LH during a 48-h fast (12). AVPV Kiss1, which is considered to contribute to positive steroid feedback and the GnRH/LH surge, was only significantly inhibited in the 50% CR study, and not during fasting or the 40% CR experiments. An unexpected finding in the 40% CR study was the significant inhibition of AVPV Kiss1 in the presence of leptin. There are no obvious explanations for this result because inhibitory effects of leptin on AVPV Kiss1 have not been reported. AVPV Kiss1 is also suppressed during lactation (19), indicating this population may only be inhibited under severe conditions of negative energy balance. Interestingly, NKB and PDYN, which are coexpressed within the ARC KNDy neurones, were not consistently inhibited with negative energy balance, although NKB levels were inhibited with the more severe 50% CR, similar to lactation (19). More research is needed to understand how differential regulation of these three reproductive neuropeptides within the same KNDy cells may contribute to reproductive inhibition.
Similar to the LH results, restoring leptin to normal basal values was unable to attenuate inhibition of ARC Kiss1 or NKB mRNA or AVPV Kiss1 mRNA inhibition in the 50% CR experiment. Despite previous results showing leptin-receptor expression on ARC KNDy neurones in the mouse (27), the present study found a dramatic lack of ARC Kiss1/pSTAT3 colocalisation after acute treatment with a pharmacological dose of leptin, arguing against a strong direct regulatory relationship in the rat. Taken together with recent evidence observing a lack of leptin-receptor signalling in AVPV Kiss1 neurones (52), these findings suggest Kiss1 neurones are unlikely to be the cell population relaying leptin signalling to GnRH neurones. Pharmacological leptin treatment has been shown to stimulate Kiss1 levels (27,28) and, in the present study, leptin replacement to normal basal levels appeared to partially attenuate inhibition of ARC Kiss1 in the least severe model of negative energy balance (i.e. the 48-h fast model), suggesting there may be differential regulation of ARC Kiss1 by leptin depending on the model of negative energy balance.
Because of the lack of effects of leptin on Kiss1 mRNA and LH levels in the present study, it was important to demonstrate that the physiologically-relevant dose of leptin administered by minipump infusion was biologically active in the brain. To definitively answer this question, pSTAT3 staining was measured in fasted animals receiving either saline or the physiologically-relevant dose of leptin via osmotic minipump. Two hours of the physiologically-relevant leptin dose significantly increased pSTAT3 staining compared to animals receiving saline, confirming the biological activity of this dose in the brain. Although it is conceivable that leptin may be degraded at body temperature with longer minipump treatments such as those employed in Experiments 1–3, this appears unlikely given the abundance of studies showing significant effects of leptin after prolonged minipump administration for up to 2 weeks (53–59). In addition to pSTAT3 staining, ARC SOCS3 mRNA was also increased in the 40% CR and the 50% CR with the low leptin treatment, although this difference only reached statistical significance in the former model. Furthermore, this dose of leptin was used previously in our laboratory and shown to completely reverse the suppression of POMC in lactating rats (36), confirming that this dose is biologically relevant in the brain.
Unexpectedly, in the present study, POMC was not significantly inhibited in either CR model. This lack of POMC regulation with CR suggests that decreases in POMC may not be as strongly regulated with negative energy balance in females as has been previously reported for males (60–63), and therefore POMC inhibition is only observed in female rats with the severe hyperphagia and negative energy balance of lactation (36). Given the lack of POMC inhibition with CR, it was not surprising that leptin administration had no affect on POMC levels. Leptin infusion was also incapable of completely attenuating the large increases in NPY and AgRP in response to CR, consistent with previous work from our laboratory finding no effect of restoring leptin to normal basal levels on NPY and AgRP levels in lactating rats (36). Although this lack of leptin regulation on NPY and AgRP may appear controversial, it should be noted that previous effects of leptin on NPY and AgRP have been demonstrated with male mice using pharmacological doses (60,62,64). It appears likely that differences between the present study and previously reported leptin affects on NPY and AgRP are likely a result of either gender or doses of leptin.
Consistent with earlier fasting studies, our results found a requirement of oestradiol for negative energy balance-induced LH inhibition (12,65). As expected, the low dose of oestradiol administered alone did not blunt the OVX-induced LH rise (40); however, the oestradiol levels were clearly biologically active because significant effects on body weight, uterine weight and AVPV Kiss1 were observed. Interestingly, the results of the present study demonstrate that, although oestradiol is required for inhibition of LH, it is not required for of the inhibition of ARC Kiss1. In the 40% CR studies, ARC Kiss1 was uniformly suppressed in all CR groups with or without oestradiol treatment, whereas the LH values were widely variable, with some in the normal control range. Thus, it appears that suppression of ARC Kiss1 is not always tightly coupled to the suppression of LH secretion, lending support to the notion of Kiss1-independent regulation of LH secretion (66). The variable LH levels with a 14-day 40% CR suggest that there may be a threshold of sufficient weight loss required for LH inhibition. The uniform suppression of LH with 50% CR suggests all animals had achieved sufficient weight loss for LH inhibition. Importantly, it appears that once animals are in a severe enough state of negative energy balance, as demonstrated with the 50% CR, restoring leptin to normal basal levels has no effect to restore LH. These findings highlight the fact that many aspects of LH inhibition remain poorly understood, and further studies are needed to understand the multitude of signals contributing to LH inhibition.
The findings of the present study, coupled with our earlier studies of lactation (36), suggest that metabolic factors other than low leptin likely contribute to inhibition of reproductive pathways in models of negative energy balance. Previously studied candidates include ghrelin, insulin, glucose and NPY, amongst others (67,68). NPY and insulin do not appear be to critical players because insulin replacement during lactation did not restore reproductive function, and attenuation of elevated levels of NPY in both lactation and 50% CR were not accompanied with changes in LH (36). Ghrelin is also an interesting candidate for linking metabolic and reproductive function because ghrelin has been shown to be inhibitory to LH (69). However, although the elevated levels of ghrelin during fasting are consistent with a potential role in the inhibition of LH (70), during lactation, ghrelin levels are low (71) and exogenous ghrelin has no affect on LH (72), suggesting that it is unlikely to contribute to the suppression of LH. Clearly, much remains to be learned about the metabolic regulation of reproduction, although the results obtained in the present study argue against a critical role of hypoleptinaemia in the suppression of LH during negative energy balance because restoration of leptin to normal basal levels does not restore LH. Taken together with previous work clearly demonstrating a strong role for leptin in reproductive regulation, it is becoming clear that this pathway is more complex than previously hypothesised. Therefore, understanding the neurocircuitry involved in the inhibition of Kiss1 and GnRH release and the potential multitude of metabolic signals that could be involved in this process still remain two critical and unresolved questions in the field.
Supplementary Material
Acknowledgements
This study was supported by grants: HD014643, U54 HD018185, RR000163 and U54 HD028934 (to the University of Virginia). The authors have no conflicts of interest to declare.
Footnotes
Supporting information The following supplementary material is available:
This supplementary material can be found in the online article.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supplementary material supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Kile JP, Alexander BM, Moss GE, Hallford DM, Nett TM. Gonadotropin-releasing hormone overrides the negative effect of reduced dietary energy on gonadotropin synthesis and secretion in ewes. Endocrinology. 1991;128:843–849. doi: 10.1210/endo-128-2-843. [DOI] [PubMed] [Google Scholar]
- 2.Bergendahl M, Perheentupa A, Huhtaniemi I. Starvation-induced suppression of pituitary-testicular function in rats is reversed by pulsatile gonadotropin-releasing hormone substitution. Biol Reprod. 1991;44:413–419. doi: 10.1095/biolreprod44.3.413. [DOI] [PubMed] [Google Scholar]
- 3.Aloi JA, Bergendahl M, Iranmanesh A, Veldhuis JD. Pulsatile intravenous gonadotropin-releasing hormone administration averts fasting-induced hypogonadotropism and hypoandrogenemia in healthy, normal weight men. J Clin Endocrinol Metab. 1997;82:1543–1548. doi: 10.1210/jcem.82.5.3947. [DOI] [PubMed] [Google Scholar]
- 4.Elmquist JK, Flier JS. Neuroscience. The fat-brain axis enters a new dimension. Science. 2004;304:63–64. doi: 10.1126/science.1096746. [DOI] [PubMed] [Google Scholar]
- 5.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 6.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 7.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 8.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 9.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 10.Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham MJ, Clifton DK, Steiner RA. Leptin’s actions on the reproductive axis: perspectives and mechanisms. Biol Reprod. 1999;60:216–222. doi: 10.1095/biolreprod60.2.216. [DOI] [PubMed] [Google Scholar]
- 12.Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- 13.Donato J, Jr, Silva RJ, Sita LV, Lee S, Lee C, Lacchini S, Bittencourt JC, Franci CR, Canteras NS, Elias CF. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci. 2009;29:5240–5250. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150:2805–2812. doi: 10.1210/en.2008-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popa SM, Clifton DK, Steiner RA. The role of kisspeptins and GPR54 in the neuroendocrine regulation of reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- 16.Smith JT. Kisspeptin signalling in the brain: steroid regulation in the rodent and ewe. Brain Res Rev. 2008;57:288–298. doi: 10.1016/j.brainresrev.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Uenoyama Y, Tsukamura H, Maeda KI. Kisspeptin/metastin: a key molecule controlling two modes of gonadotrophin-releasing hormone/luteinising hormone release in female rats. J Neuroendocrinol. 2009;21:299–304. doi: 10.1111/j.1365-2826.2009.01853.x. [DOI] [PubMed] [Google Scholar]
- 18.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 19.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23:52–64. doi: 10.1111/j.1365-2826.2010.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 21.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151:4494–4503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 24.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neuro-sci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 26.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JT, Acohido BV, Clifton DK, Steiner RA. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J Neuroendocrinol. 2006;18:298–303. doi: 10.1111/j.1365-2826.2006.01417.x. [DOI] [PubMed] [Google Scholar]
- 28.Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocin-induced diabetic male rats. Diabetes. 2006;55:2602–2610. doi: 10.2337/db05-1584. [DOI] [PubMed] [Google Scholar]
- 29.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 30.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 31.Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 32.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 34.Castellano JM, Navarro VM, Fernandez-Fernandez R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 35.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. J Neuroendocrinol. 2008;20:1089–1097. doi: 10.1111/j.1365-2826.2008.01757.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Kirigiti MA, Grove KL, Smith MS. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology. 2009;150:4231–4240. doi: 10.1210/en.2009-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luque RM, Kineman RD, Tena-Sempere M. Regulation of hypothalamic expression of KiSS-1 and GPR54 genes by metabolic factors: analyses using mouse models and a cell line. Endocrinology. 2007;148:4601–4611. doi: 10.1210/en.2007-0500. [DOI] [PubMed] [Google Scholar]
- 38.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda KI, Tsukamura H. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148:2226–2232. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- 39.Brogan RS, Mitchell SE, Trayhurn P, Smith MS. Suppression of leptin during lactation: contribution of the suckling stimulus versus milk production. Endocrinology. 1999;140:2621–2627. doi: 10.1210/endo.140.6.6802. [DOI] [PubMed] [Google Scholar]
- 40.Goodman RL. A quantitative analysis of the physiological role of estradiol and progesterone in the control of tonic and surge secretion of luteinizing hormone in the rat. Endocrinology. 1978;102:142–150. doi: 10.1210/endo-102-1-142. [DOI] [PubMed] [Google Scholar]
- 41.Smith MS, Neill JD. Inhibition of gonadotropin secretion during lactation in the rat: relative contribution of suckling and ovarian steroids. Biol Reprod. 1977;17:255–261. doi: 10.1095/biolreprod17.2.255. [DOI] [PubMed] [Google Scholar]
- 42.Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A, Franceschini I. Mapping of kisspeptin fibres in the brain of the pro-oestrus rat. J Neuroendocrinol. 2011;23:52–64. doi: 10.1111/j.1365-2826.2010.02053.x. [DOI] [PubMed] [Google Scholar]
- 43.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol. 2009;21:527–537. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- 44.Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98:1359–1364. doi: 10.1210/endo-98-6-1359. [DOI] [PubMed] [Google Scholar]
- 45.Todd BJ, Ladyman SR, Grattan DR. Suppression of pulsatile luteinizing hormone secretion but not luteinizing hormone surge in leptin resistant obese Zucker rats. J Neuroendocrinol. 2003;15:61–68. doi: 10.1046/j.1365-2826.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 46.Ahima RS, Dushay J, Flier SN, Prabakaran D, Flier JS. Leptin accelerates the onset of puberty in normal female mice. J Clin Invest. 1997;99:391–395. doi: 10.1172/JCI119172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D, De Rosa V, Perna F, Fontana S, Mantzoros CS. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA. 2006;103:8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med. 2004;351:987–997. doi: 10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 49.Szymanski LA, Schneider JE, Friedman MI, Ji H, Kurose Y, Blache D, Rao A, Dunshea FR, Clarke IJ. Changes in insulin, glucose and ketone bodies, but not leptin or body fat content precede restoration of luteinising hormone secretion in ewes. J Neuroendocrinol. 2007;19:449–460. doi: 10.1111/j.1365-2826.2007.01551.x. [DOI] [PubMed] [Google Scholar]
- 50.McCowen KC, Chow JC, Smith RJ. Leptin signaling in the hypothalamus of normal rats in vivo. Endocrinology. 1998;139:4442–4447. doi: 10.1210/endo.139.11.6301. [DOI] [PubMed] [Google Scholar]
- 51.Forbes S, Li XF, Kinsey-Jones J, O’Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett. 2009;460:143–147. doi: 10.1016/j.neulet.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 52.Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152:1541–1550. doi: 10.1210/en.2010-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pal R, Sahu A. Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology. 2003;144:3789–3798. doi: 10.1210/en.2002-0148. [DOI] [PubMed] [Google Scholar]
- 54.Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Correia ML, Morgan DA, Sivitz WI, Mark AL, Haynes WG. Leptin acts in the central nervous system to produce dose-dependent changes in arterial pressure. Hypertension. 2001;37:936–942. doi: 10.1161/01.hyp.37.3.936. [DOI] [PubMed] [Google Scholar]
- 56.Pearson PY, O’Connor DM, Schwartz MZ. Novel effect of leptin on small intestine adaptation. J Surg Res. 2001;97:192–195. doi: 10.1006/jsre.2001.6153. [DOI] [PubMed] [Google Scholar]
- 57.Wetzler S, Dumaz V, Goubern M, Tome D, Larue-Achagiotis C. Intraperitoneal leptin modifies macronutrient choice in self-selecting rats. Physiol Behav. 2004;83:65–72. doi: 10.1016/j.physbeh.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 58.Sindelar DK, Havel PJ, Seeley RJ, Wilkinson CW, Woods SC, Schwartz MW. Low plasma leptin levels contribute to diabetic hyperphagia in rats. Diabetes. 1999;48:1275–1280. doi: 10.2337/diabetes.48.6.1275. [DOI] [PubMed] [Google Scholar]
- 59.Nishiyama M, Makino S, Asaba K, Hashimoto K. Leptin effects on the expression of type-2 CRH receptor mRNA in the ventromedial hypothalamus in the rat. J Neuroendocrinol. 1999;11:307–314. doi: 10.1046/j.1365-2826.1999.00331.x. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140:4551–4557. doi: 10.1210/endo.140.10.6966. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 62.Ziotopoulou M, Erani DM, Hileman SM, Bjorbaek C, Mantzoros CS. Unlike leptin, ciliary neurotrophic factor does not reverse the starvation-induced changes of serum corticosterone and hypothalamic neuropeptide levels but induces expression of hypothalamic inhibitors of leptin signaling. Diabetes. 2000;49:1890–1896. doi: 10.2337/diabetes.49.11.1890. [DOI] [PubMed] [Google Scholar]
- 63.Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- 64.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 65.Nagatani S, Tsukamura H, Maeda K. Estrogen feedback needed at the paraventricular nucleus or A2 to suppress pulsatile luteinizing hormone release in fasting female rats. Endocrinology. 1994;135:870–875. doi: 10.1210/endo.135.3.8070380. [DOI] [PubMed] [Google Scholar]
- 66.Chan YM, Broder-Fingert S, Wong KM, Seminara SB. Kisspeptin/Gpr54-independent gonadotrophin-releasing hormone activity in Kiss1 and Gpr54 mutant mice. J Neuroendocrinol. 2009;21:1015–1023. doi: 10.1111/j.1365-2826.2009.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castellano JM, Roa J, Luque RM, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. KiSS-1/kisspeptins and the metabolic control of reproduction: physiologic roles and putative physiopathological implications. Peptides. 2009;30:139–145. doi: 10.1016/j.peptides.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Tena-Sempere M. Ghrelin as a pleotrophic modulator of gonadal function and reproduction. Nat Clin Pract Endocrinol Metab. 2008;4:666–674. doi: 10.1038/ncpendmet1003. [DOI] [PubMed] [Google Scholar]
- 69.Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun. 2001;288:780–785. doi: 10.1006/bbrc.2001.5854. [DOI] [PubMed] [Google Scholar]
- 70.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220–1225. doi: 10.1006/bbrc.2001.4518. [DOI] [PubMed] [Google Scholar]
- 71.Shibata K, Hosoda H, Kojima M, Kangawa K, Makino Y, Makino I, Kawarabayashi T, Futagami K, Gomita Y. Regulation of ghrelin secretion during pregnancy and lactation in the rat: possible involvement of hypothalamus. Peptides. 2004;25:279–287. doi: 10.1016/j.peptides.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 72.Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett. 2004;362:103–107. doi: 10.1016/j.neulet.2004.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.