Abstract
Interleukin (IL)-37 is a member of the IL-1 cytokine family. We investigated IL-37b expression in the inflamed mucosa of inflammatory bowel disease (IBD) patients. Furthermore, we analysed IL-37b expression in human colonic epithelial cells. The human colonic epithelial cell line T84 and human colonic subepithelial myofibroblasts (SEMFs) were used. IL-37b expression in the IBD mucosa was evaluated by immunohistochemistry. IL-37b mRNA and protein expression were determined by real time-polymerase chain reaction (PCR) and Western blotting, respectively. IL-37b was not detected in the normal colonic mucosa. In the inflamed mucosa of IBD patients, epithelial IL-37b expression was increased markedly. In ulcerative colitis (UC) and Crohn's disease (CD) patients, IL-37b expression was enhanced in the affected mucosa. In the intestinal epithelial cell line T84, the expression of IL-37b mRNA and protein was enhanced by tumour necrosis factor (TNF)-α. This IL-37b induction by TNF-α was mediated by nuclear factor (NF)-κB and activator protein (AP)-1 activation. Furthermore, IL-37b inhibited TNF-α-induced interferon-γ-inducible protein (IP)-10 expression significantly in human colonic SEMFs. Epithelial IL-37b expression was increased in IBD patients, especially UC patients. IL-37b may be involved in the pathophysiology of IBD as an anti-inflammatory cytokine and an inhibitor of both innate and acquired immune responses.
Keywords: anti-inflammatory cytokine, innate immunity, TNF-α
Introduction
Inflammatory bowel diseases (IBD) are comprised of two major phenotypes: Crohn's disease (CD) and ulcerative colitis (UC). Recent studies suggest that the chronic inflammation in IBD is due to aggressive cellular immune responses against a subset of luminal bacteria and dietary factors 1–3. This hypothesis is supported by the recent finding that genes encoding innate immune responses, which are triggered by environmental stimuli, are responsible for determining the susceptibility to IBD 4. Furthermore, IBD is often characterized by an imbalance between the effector and the regulatory activities of intestinal immunity, with a preponderance of proinflammatory cytokines 1,5.
The interleukin (IL)-1 family of cytokines possesses a variety of immunoregulatory properties in response to infection and inflammation 6,7. Of the members of the IL-1 family, seven cytokines (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β and IL-36γ) act as agonists, and two are classified as naturally occurring receptor antagonists (IL-1Ra and IL-36Ra). IL-37 is the last member of the IL-1 family, and was reported initially as an orphan IL-1-like cytokine IL-1F7 that, until recently, had no well-known function 8. The major splice variant IL-1F7b is now being dubbed IL-37b. IL-37 was found to be induced by Toll-like receptor (TLR) agonists in monocytes to bind to the IL-18-receptor (R) α chain and also to IL-18-binding protein (BP) 7,9. However, there is no evidence that IL-37 acts as a receptor antagonist for IL-18 10. Endogenous (cytoplasmic) IL-37 exerts anti-inflammatory effects by suppressing innate immune responses through attenuating the production of inflammatory cytokines induced by TLR agonists, as well as that induced by IL-1 and tumour necrosis factor (TNF) 6,11. However, the function of the IL-37b released remains exclusive.
IL-37 is expressed in a variety of normal tissues and tumours in humans, but a mouse homologue has not been identified 7,10. Immunocytochemical staining of peripheral blood mononuclear cells (PBMC) revealed that the IL-37 protein is present mainly in the cytoplasm of monocytes 7,10, whereas in solid tissues it is often associated with plasma cells 7,10. The IL-37 protein is present constitutively at low levels in human PBMCs, and can be up-regulated by inflammatory stimuli and cytokines 6. Staining for IL-37 shows a granular pattern in close proximity to the Golgi and endoplasmic reticulum (ER) and is associated partly with the plasma membrane, a pattern which suggests translocation via secretory vesicles 3,10. In contrast to an extracellular role for IL-37, ∼25% of induced endogenous IL-37 translocates to the nucleus 12. Like other IL-1 family members, caspase-1 and other enzymes are suspected to be involved in IL-37 maturation 11.
Recently, McNamee et al. reported that human IL-37 transgenic mice were protected from dextran sulphate sodium (DSS) colitis 13. They also reported that IL-37-producing haematopoietic cells are crucial for this protective activity 13. However, the behaviour and pathophysiological role of IL-37 in human IBD remains unclear. In the present study, we investigated IL-37 expression in the inflamed mucosa of IBD patients. Furthermore, to characterize the molecular mechanisms responsible for IL-37 expression in the colonic mucosa, we analysed IL-37 expression in the human colonic epithelial cell line T84.
Materials and methods
Reagents
Recombinant human cytokines were purchased from R&D Systems (Minneapolis, MN, USA). Inhibitors of p42/44 mitogen-activated protein kinases (MAPK) (PD98059 and U0216), an inhibitor for p38 MAPK (SB203580) and an inhibitor for phosphatidylinositol 3-kinase (PI3K) (LY294002) were purchased from Cell Signaling Technology (Beverly, MA, USA). All other reagents were purchased from Sigma Chemical Co. (St Louis, MO, USA). Human interferon (IFN)-γ-inducible protein-10 (IP-10) and IL-8 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R&D Systems. Goat anti-human IL-37b antibodies (R&D Systems) were purchased from commercial suppliers.
Tissue samples
The diagnosis of IBD was based on conventional clinical and endoscopic criteria. Samples were used with informed consent. The ethics committee of the Shiga University of Medical Science approved this project.
The clinical activity of IBD was determined according to the colitis activity index for UC 14 and Crohn's disease activity index 15. Five UC and six CD patients received surgical intervention due to resistance to medication or other complications (e.g. massive bleeding, fistula formation or perforation). Histological examinations were performed in macroscopically affected and non-affected areas of each patient. All patients were treated with salicylates and corticosteroids. Normal colorectal tissues were obtained by the surgical resection of colon cancers at distal tumour sites (n = 7). Immunohistochemical analyses were performed according to the method described in our previous report 16.
Culture of human colonic epithelial cell lines and human colonic subepithelial myofibroblasts (SEMFs)
The human colon cancer cell lines T84 and Caco-2 were obtained from the American Type Culture Collection (Manassas, VA, USA) 17. Primary colonic subepithelial myofibroblast (SEMF) cultures were prepared according to the method reported by Mahida et al. 18. The cellular characteristics and culture conditions have also been described in our previous report 19. The studies were performed on passages 3–6 of pooled myofibroblasts isolated from six normal colonic tissues, which were different from the controls in the IL-37 expression study.
Real-time polymerase chain reaction (PCR)
The expression of mRNA in the samples was assessed by real-time reverse transcription–PCR (RT–PCR) analyses. The human IL-37b oligonucleotide primers are as follows: sense: GCTCAG-GTGGGCTCCTGGAA and anti-sense: GCTGACCTCACTGGGGCTCA) (NCBI reference sequence: AF200496) 20. The human IP-10 and IL-8 oligonucleotide primers used in this study have been described in our previous reports 21,22 Real-time RT–PCR was performed using a LightCycler 480 system (Roche Diagnostics, Basel, Switzerland). The PCR was performed using Premix Ex Taq (Takara Bio, Otsu, Japan). The data were normalized versus β-actin for human IL-37b.
Western blot analyses
The stimulated cells were lysed in a sodium dodecyl sulphate (SDS) sample buffer containing orthovanadate. Western blots were then performed according to a method described previously 21. The detection was performed using the enhanced chemiluminescence Western blotting system (GE Healthcare, Tokyo, Japan).
Adenovirus-mediated gene transfers
We used a recombinant adenovirus expressing a stable mutant form of IκBα (Ad-IκBΔN) 23, a recombinant adenovirus expressing a dominant negative mutant of c-Jun (Ad-DN-c-Jun) 24 and a recombinant adenovirus containing bacterial β-galactosidase cDNA (Ad-LacZ). The stable mutant form of IκBα (IκBΔN) l5 lacks the 54 NH2-terminal amino acids of the wild-type IκBα, and is neither phosphorylated nor proteolyzed in response to signal induction, but inhibits the classical pathway of NF-κB activation. The dominant negative mutant c-Jun (TAM67) lacks the transactivational domain of amino acids 3–122 of the wild-type c-Jun, but retains the DNA-binding domain. In preliminary experiments, Ad-LacZ infections of colonic myofibroblasts with a multiplicity of infection (MOI) of 10 showed a maximal expression (85% positive) of β-galactosidase (data not shown). The recombinant adenovirus was transferred into the cells, and the cells were made quiescent for 48 h before being assessed for the effects of the transferred gene.
Statistical analysis
The statistical significance of the differences was determined by Student's t-test. Differences resulting in P-values less than 0·05 were considered to be statistically significant.
Results
IL-37b expression in IBD mucosa
To evaluate the expression of IL-37b protein in the inflamed mucosa, the samples were immunostained with anti-human IL-37b antibodies (Fig. 1). We checked IL-37 expression in several parts of normal ileum and colon, but IL-37b immunoreactivity was not detected in the normal mucosa (Fig. 1a). In contrast, IL-37b immunoreactivity was detected clearly in both active UC and CD patients, mainly in the epithelial cells and infiltrated immune cells in the lamina propria [Fig. 1b (UC) and d (CD)]. The expression of IL-37b protein tended to be stronger in active UC patients than in active CD patients. IL-37b immunoreactivity was also detected in the inactive mucosa of UC and CD patients [Fig. 1c (UC) and e (CD)], but it was much weaker than in the active mucosa. IL-37 expression changed according to the degree of local mucosal inflammation, but was not affected by the location. These results are summarized in Table 1.
Fig. 1.
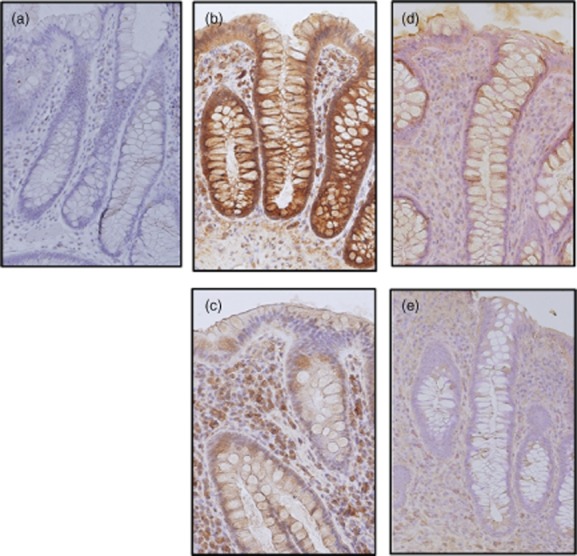
Immunohistochemical analyses of interleukin (IL)-37b expression in the human colon. (a) Normal mucosa, (b) active ulcerative colitis (UC), (c) inactive UC, (d) active Crohn's disease (CD) and (e) inactive CD. Original magnification ×100.
Table 1.
Summary of interleukin (IL)-37b immunoreactivity
| Ulcerative colitis | Crohn's disease | ||||
|---|---|---|---|---|---|
| Normal mucosa | Active lesion | Inactive lesion | Active lesion | Inactive lesion | |
| Epithelial cells | Not expressed | Strong | Moderate | Moderate | Weak |
| Infiltrated cells in the lamina propria | Not expressed | Strong | Moderate | Weak | Weak |
Regulation of IL-37b expression in T84 and Caco-2 colonic epithelial cells
Based on the in-vivo immunoexpression of IL-37b in the inflamed IBD mucosa, we examined IL-37b expression in the human colonic epithelial cell line T84. The cells were stimulated with various cytokines and lipopolysaccharide (LPS) for 12 h, and then the IL-37b mRNA expression was determined by real-time PCR (Fig. 2a, left). Preliminary study showed that there were no significant differences at 6, 12 and 24 h. Significant effects of TNF-α on IL-37 mRNA induction were also confirmed in Caco-2 cells (Fig. 2a, right). Weak IL-37b mRNA expression was detected in unstimulated T84 cells, and TNF-α stimulation enhanced the IL-37b mRNA expression significantly. In contrast, IL-4 and and/or IFN-γ stimulation decreased the IL-37b mRNA expression significantly. Other cytokines and LPS had no effects.
Fig. 2.
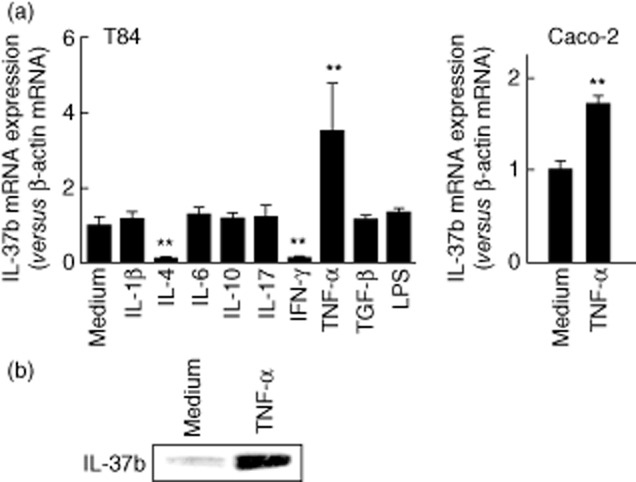
Interleukin (IL)-37b mRNA and protein expression in the human intestinal epithelial cell lines. (a) Intestinal epithelial cell line T84 cells were stimulated with cytokines [IL-1β (10 ng/ml), other cytokines (100 ng/ml) and lipopolysaccharide (LPS) (1·0 μg/ml)] for 12 h, and then the IL-37b mRNA expression was determined by real-time polymerase chain reaction (PCR) (left side). Caco-2 cells were also stimulated with tumour necrosis factor (TNF)-α (100 ng/ml) for 12 h, and the IL-37b mRNA expression was determined by real-time PCR (right side). All values are expressed as means ± standard deviation (n = 3). **P < 0·01; a significant difference from the values for medium alone. (b) T84 cells were stimulated with TNF-α (100 ng/ml) for 24 h, and then the intracellular IL-37b was detected by Western blotting.
These effects were also confirmed at the protein level. T84 cells were stimulated with TNF-α for 24 h, and then the intracellular IL-37b was determined by Western blotting. As shown in Fig. 2, TNF-α induced intracellular IL-37b protein production.
The effects of TNF-α were analysed quantitatively. As shown in Fig. 3a, TNF-α induced IL-37b mRNA expression dose-dependently. Similarly, TNF-α induced IL-37b mRNA expression in a time-dependent manner (Fig. 3b).
Fig. 3.
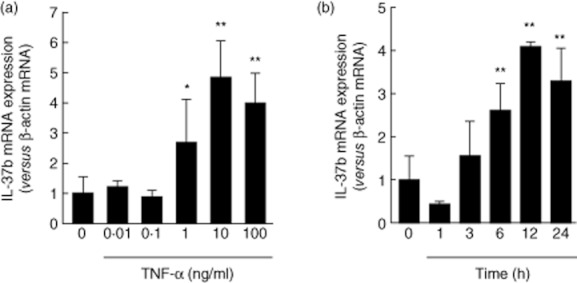
Dose- and time-dependent effects of tumour necrosis factor (TNF)-α on interleukin (IL)-37b mRNA expression in T84 cells. (a) Cells were stimulated for 24 h with increasing concentrations of TNF-α, and then the IL-37b mRNA expression was analysed by real-time polymerase chain reaction (PCR) (b) Cells were stimulated with TNF-α (100 ng/ml) for different time-periods, and then the IL-37b mRNA expression was analysed by real-time PCR. All values are expressed as means ± standard deviation (n = 3).**P < 0·01 and *P < 0·05; a significant difference from the values for medium alone.
Role of MAP- and PI3-kinase activation in IL-37b induction
The MAPK and PI3K/Akt pathways are implicated in cytokine signalling in various cell types. To investigate the molecular mechanisms underlying IL-37b induction in T84 cells, we evaluated the effects of the following inhibitors: p42/44 MAPK inhibitors (PD98059 and U0216) 25,26, a p38 MAPK inhibitor (SB203580) 27 and a PI3K inhibitor (LY294002) 28. Real-time PCR demonstrated that treatment with mitogen-activated, extracellular signal-regulated kinase (MEK) inhibitors (PD98059 and U0216), a p38 MAPK inhibitor (SB203580) and a PI3K inhibitor (LY294002) exerted significant inhibitory effects on TNF-α-induced IL-37b mRNA expression (Fig. 4a). These results suggest that MAPK and PI3K are involved in TNF-α-induced IL-37b mRNA expression in T84 cells.
Fig. 4.
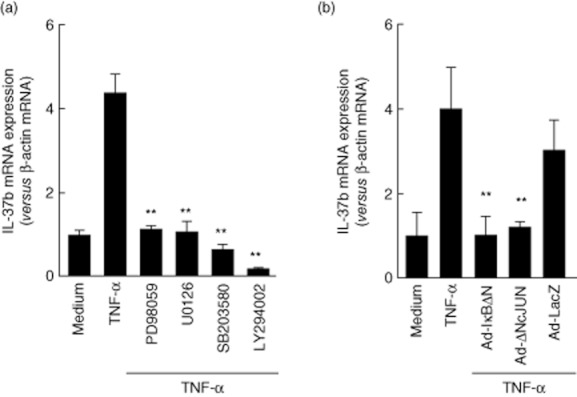
Molecular mechanisms underlying interleukin (IL)-37b mRNA expression. (a) Effects of MAP-and PI3-kinase inhibitors on IL-37b mRNA expression in the intestinal epithelial cell line T84. The cells were pretreated with 10 μM mitogen-activated protein (MAP) kinase inhibitors (SB203580, PD098059 or U02016) and 10 μM PI3-kinase inhibitor (LY294002) for 15 min each, and then stimulated with tumour necrosis factor (TNF)-α (100 ng/ml) for 24 h. The IL-37b mRNA expression was then determined by real-time polymerase chain reaction (PCR). All values are expressed as means ± standard deviation (s.d.) (n = 3). **P < 0·01; a significant difference from the values for TNF-α stimulation. (b) Effects of nuclear factor (NF)-κB and/or activator protein (AP)-1 inhibition on IL-37b mRNA expression. T84 cells were infected with an adenovirus expressing IκBΔN or DN-c-Jun, and at 48 h after infection the cells were stimulated with TNF-α (100 ng/ml) for 24 h. IL-37b mRNA expression was then determined by real-time PCR. All values are expressed as means ± s.d. (n = 3). **P < 0·01; a significant difference from the values for TNF-α stimulation.
Role of NF-κB and activator protein (AP)-1 in IL-37b induction
To assess the role of transcription factors NF-κB and AP-1, we evaluated the effects of a recombinant adenovirus containing a stable mutant form of IκBα (Ad-IκBΔN) and a dominant negative mutant of c-Jun (Ad-DN-c-Jun) on TNF-α-induced IL-37b mRNA expression. As shown in Fig. 4b, T84 cells were infected with a recombinant adenovirus and cultured for 48 h. The cells were stimulated for 12 h with TNF-α (100 ng/ml), and the expression of IL-37b mRNAs was determined by real-time PCR. Both Ad-IκBΔN and Ad-DN-c-Jun inhibited the effects of TNF-α on IL-37b mRNA expression, but the Ad-LacZ gene, which was used as a negative control, did not suppress the effects of TNF-α. These results suggest that NF-κB and AP-1 play a role in TNF-α-induced IL-37b mRNA expression.
Effects of IL-37b on TNF-α-induced IP-10 expression in human colonic SEMFs
To define the biological activities of epithelial cell-derived IL-37b in the inflamed mucosa, we investigated how IL-37b modulates the functions of human colonic SEMFs. We evaluated the effects of IL-37b on TNF-α-induced IP-10 expression in these cells. The cells were preincubated with or without IL-37b (100 ng/ml) for 6 h, and then incubated further with or without TNF-α (100 ng/ml) for 24 h. IL-37b decreased the TNF-α-induced IP-10 expression significantly at both the mRNA and protein levels (Fig. 5).
Fig. 5.
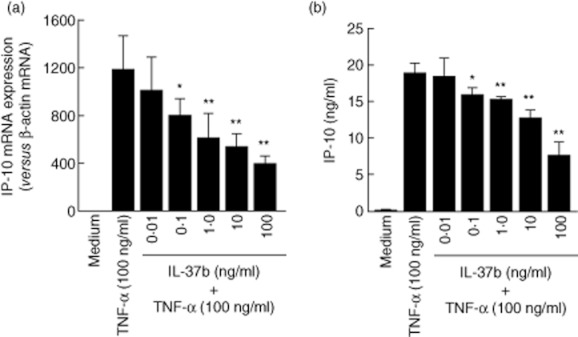
Effects of interleukin (IL)-37b on the tumour necrosis factor (TNF)-α-induced interferon-γ-inducible protein (IP)-10 expression in human colonic subepithelial myofibroblasts. Cells were pretreated with or without IL-37b (100 ng/ml) for 6 h, and then incubated further with or without TNF-α (100 ng/ml) for 24 h. The IP-10 secretion was determined by enzyme-linked immunosorbent assay (ELISA). (a) IP-10 mRNA, and (b) IL-10 protein. All values are expressed as means ± standard deviation (n = 3). **P < 0·01, *P < 0·05; a significant difference from the values for TNF-α stimulation.
Discussion
In the present study, we have demonstrated that the epithelial expression of IL-37b was enhanced in the inflamed mucosa of IBD patients. In-vitro studies using human intestinal epithelial cell (IEC) lines suggest that TNF-α is a major inducer of IL-37b expression. TNF-α has been reported previously as an inducer of IL-37b expression in human PBMCs 6. In PBMCs, IL-37b can also be up-regulated by inflammatory stimuli and cytokines [LPS, IL-1β, IL-18, IFN-γ, transforming growth factor (TGF-β)], whereas other factors [IL-12, IL-32, granulocyte–macrophage colony-stimulating factor (GM-CSF)+IL-4] are inactive or inhibitory 6. In contrast to these responses in PBMCs, LPS, IL-1β and TGF-β had no effects on IL-37b expression in T84 intestinal epithelial cells. In addition, the T helper type 1 (Th1) cytokine IFN-γ and Th2 cytokine IL-4 down-regulated baseline IL-37b expression significantly in T84 cells, although IFN-γ was reported to enhance IL-37b expression in human PBMCs. To our knowledge, this is the first report describing the enhanced expression of IL-37b by intestinal epithelial cells in the inflamed mucosa of IBD patients. Furthermore, we found that the regulation of IL-37b expression in intestinal epithelial cells is different from the regulation reported in PBMCs, suggesting that IL-37b expression is controlled by different mechanisms which are specific for different cell types.
IL-37b has been reported as an inhibitor of innate immunity 6,7. Of particular interest is the finding that PBMC treatment with GM-CSF plus IL-4 [the conditions that induce the differentiation of monocytes to dendritic cells (DCs)] down-regulates IL-37 expression, in agreement with the finding that IL-37 inhibits DC activation 6. In this study, we observed that IL-37b exerted a significant inhibition on TNF-α-induced IP-10 expression in human colonic SEMFs. IP-10 is a ligand for the CXCR3 receptor, the activation of which results in the recruitment of T cells and the perpetuation of mucosal inflammation 29. These observations suggest that epithelial cell-derived IL-37b inhibits T cell activation, as well as DC activation, in the inflamed mucosa of IBD patients. This inhibitory action of IL-37b against DC and T cells is supported by the previous report that IL-37b reduces the surface expression of the co-stimulatory molecule CD86 (B7-2) and major histocompatibility complex (MHC) II (a major activator of CD4+ T cells) on dendritic cells (DCs) together with propagating an anti-inflammatory cytokine 6. It is likely that IL-37b plays an important role in the down-regulation of inflammatory responses that are involved in the pathophysiology of IBD.
The molecular mechanisms underlying IL-37b induction have not been reported previously. In this study, we found that IL-37b mRNA expression induced by TNF-α was mediated by the activation of MAPK and PI3K, transcription factors NF-κB and AP-1. Conversely, these signalling molecules are major mediators of the induction of proinflammatory responses in the inflamed mucosa 30,31. Thus, it became clear that in the inflamed mucosa of IBD patients a negative feedback inhibitor of inflammatory responses (the induction of IL37b) and proinflammatory responses are induced via coupled signalling pathways. It is not unexpected that inflammation induces anti-inflammatory responses to limit the mucosal damage. Therefore, the observation that IL-37b is not expressed constitutively in the intestinal mucosa of healthy subjects, but the expression is high in epithelial cells from patients with IBD, is in accordance with the concept that IL-37 mediates a negative feedback mechanism to curb excessive inflammation.
We have reported recently that expression of IL-33 (IL-1F11), the most recently described cytokine of the IL-1 family, is enhanced in the inflamed mucosa of UC 5. IL-33 stimulates innate immune cells via signalling through the receptor complex ST2/IL–1RAcP and induced Th2 cytokines such as IL-13 and IL-5 32. IL-33 is also considered to have anti-inflammatory actions via the induction of Th2 cytokine secretion in the inflamed mucosa of IBD patients. Although IL-37b binds to the IL-18Rα chain and IL-18BP, at least two members of the IL-1 cytokine family (IL-33 and IL-37b) exert anti-inflammatory actions in the inflamed mucosa of IBD patients, in particular UC. Recent studies have demonstrated that IL-33 is identical to nuclear factor of high endothelial venules (NF-HEV), a nuclear factor that possesses potent transcriptional repressor properties 33. IL-33 is considered to function both as a cytokine and as an intracellular nuclear factor involved in transcriptional regulation. As the translocation of IL-37b into the nucleus has also been reported 12, IL-37b is therefore expected to have transcriptional regulatory functions. Such a function of IL-37b should be defined to understand the exact role of IL-37b in the pathophysiology of IBD.
In conclusion, we have demonstrated that IL-37b expression is enhanced in the inflamed mucosa of IBD patients. Furthermore, we found that IL-37b was induced by TNF-α in human intestinal epithelial cells. IL-37b suppressed the TNF-α-induced IP-10 expression in human colonic myofibroblasts. Thus, our observations suggest that IL-37b mediates a negative feedback mechanism to relieve excessive inflammation in the intestinal mucosa of IBD patients. Previous studies have demonstrated mainly anti-inflammatory actions for IL-37b as an intracellular protein, but the receptor-mediated effects of released IL-37b are poorly understood in all cell types. Further studies on the intracellular and extracellular roles of IL-37b should be performed in the future.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24590940), and a grant for Intractable Diseases from the Ministry of Health, Labor and Welfare of Japan.
Disclosure
The authors disclose no conflicts of interest.
References
- 1.Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9–16. doi: 10.1007/s00535-009-0138-3. [DOI] [PubMed] [Google Scholar]
- 2.Andoh A, Imaeda H, Aomatsu T, et al. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn's disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol. 2011;46:479–486. doi: 10.1007/s00535-010-0368-4. [DOI] [PubMed] [Google Scholar]
- 3.Andoh A, Kuzuoka H, Tsujikawa T, et al. Multicenter analysis of fecal microbiota profiles in Japanese patients with Crohn's disease. J Gastroenterol. 2012;47:1298–1307. doi: 10.1007/s00535-012-0605-0. [DOI] [PubMed] [Google Scholar]
- 4.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobori A, Yagi Y, Imaeda H, et al. Interleukin-33 expression is specifically enhanced in inflamed mucosa of ulcerative colitis. J Gastroenterol. 2010;45:999–1007. doi: 10.1007/s00535-010-0245-1. [DOI] [PubMed] [Google Scholar]
- 6.Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11:1014–1022. doi: 10.1038/ni.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boraschi D, Lucchesi D, Hainzl S, et al. IL-37: a new anti-inflammatory cytokine of the IL-1 family. Eur Cytokine Netw. 2011;22:127–147. doi: 10.1684/ecn.2011.0288. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello C, Arend W, Sims J, et al. IL-1 family nomenclature. Nat Immunol. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bufler P, Azam T, Gamboni-Robertson F, et al. A complex of the IL-1 homologue IL-1F7b and IL-18-binding protein reduces IL-18 activity. Proc Natl Acad Sci USA. 2002;99:13723–13728. doi: 10.1073/pnas.212519099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S, Hanning CR, Brigham-Burke MR, et al. Interleukin-1F7B (IL-1H4/IL-1F7) is processed by caspase-1 and mature IL-1F7B binds to the IL-18 receptor but does not induce IFN-gamma production. Cytokine. 2002;18:61–71. doi: 10.1006/cyto.2002.0873. [DOI] [PubMed] [Google Scholar]
- 11.Sharma S, Kulk N, Nold MF, et al. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J Immunol. 2008;180:5477–5482. doi: 10.4049/jimmunol.180.8.5477. [DOI] [PubMed] [Google Scholar]
- 12.Bufler P, Gamboni-Robertson F, Azam T, Kim SH, Dinarello CA. Interleukin-1 homologues IL-1F7b and IL-18 contain functional mRNA instability elements within the coding region responsive to lipopolysaccharide. Biochem J. 2004;381:503–510. doi: 10.1042/BJ20040217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamee EN, Masterson JC, Jedlicka P, et al. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108:16711–16716. doi: 10.1073/pnas.1111982108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82–86. doi: 10.1136/bmj.298.6666.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn's Disease Activity Index (CDAI) Gastroenterology. 1979;77:843–846. [PubMed] [Google Scholar]
- 16.Fujino S, Andoh A, Bamba S, et al. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dharmsathaphorn K, McRoberts JA, Mandel KG, Tisdale LD, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 18.Mahida YR, Beltinger J, Makh S, et al. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341–1348. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 19.Andoh A, Fujino S, Bamba S, et al. IL-17 selectively down-regulates TNF-alpha-induced RANTES gene expression in human colonic subepithelial myofibroblasts. J Immunol. 2002;169:1683–1687. doi: 10.4049/jimmunol.169.4.1683. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, McDonnell PC, Lehr R, et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem. 2000;275:10308–10314. doi: 10.1074/jbc.275.14.10308. [DOI] [PubMed] [Google Scholar]
- 21.Shimada M, Andoh A, Hata K, et al. IL-6 secretion by human pancreatic periacinar myofibroblasts in response to inflammatory mediators. J Immunol. 2002;168:861–868. doi: 10.4049/jimmunol.168.2.861. [DOI] [PubMed] [Google Scholar]
- 22.Inatomi O, Andoh A, Kitamura K, Yasui H, Zhang Z, Fujiyama Y. Butyrate blocks interferon-gamma-inducible protein-10 release in human intestinal subepithelial myofibroblasts. J Gastroenterol. 2005;40:483–489. doi: 10.1007/s00535-005-1573-4. [DOI] [PubMed] [Google Scholar]
- 23.Obara H, Takayanagi A, Hirahashi J, et al. Overexpression of truncated IkappaBalpha induces TNF-alpha-dependent apoptosis in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:2198–2204. doi: 10.1161/01.atv.20.10.2198. [DOI] [PubMed] [Google Scholar]
- 24.Yasumoto H, Kim S, Zhan Y, et al. Dominant negative c-jun gene transfer inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in rats. Gene Ther. 2001;8:1682–1689. doi: 10.1038/sj.gt.3301590. [DOI] [PubMed] [Google Scholar]
- 25.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 26.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 27.Cuenda A, Rouse J, Doza YN, et al. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 28.Fang J, Ding M, Yang L, Liu LZ, Jiang BH. PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal. 2007;19:2487–2497. doi: 10.1016/j.cellsig.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu M, Guo S, Hibbert JM, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22:121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hata K, Andoh A, Shimada M, et al. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–1044. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 31.Shioya M, Nishida A, Yagi Y, et al. Epithelial overexpression of interleukin-32alpha in inflammatory bowel disease. Clin Exp Immunol. 2007;149:480–486. doi: 10.1111/j.1365-2249.2007.03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011;132:475–481. doi: 10.1111/j.1365-2567.2011.03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]


