Abstract
In this study, we examined the effect of ethyl pyruvate (EP) on pulmonary inflammation in rats with severe pancreatitis-associated acute lung injury (ALI). Severe acute pancreatitis (SAP) was induced in rats by the retrograde injection of 5% sodium taurocholate into the pancreatic duct. Rats were randomly divided into the following experimental groups: control group, SAP group and EP-treated group. The tissue specimens were harvested for morphological studies, Streptavidin–peroxidase immunohistochemistry examination. Pancreatic or lung tissue oedema was evaluated by tissue water content. Serum amylase and lung tissue malondialdehyde (MDA) and myeloperoxidase (MPO) were measured. Meanwhile, the nuclear factor-κB (NF-κB) activation, tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) levels and HMGB1 protein expression levels in the lung were studied. In the present study, we demonstrated that treatment with EP after SAP was associated with a reduction in the severity of SAP and lung injury. Treatment with EP significantly decreased the expression of TNF-α, IL-1β, HMGB1 and ameliorated MDA concentration, MPO activity in the lung in SAP rats. Compared to SAP group, administration of EP prevented pancreatitis-induced increases in nuclear translocation of NF-κB in the lung. Similarly, treatment with EP significantly decreased the accumulation of neutrophils and markedly reduced the enhanced lung permeability. In conclusion, these results demonstrate that EP might play a therapeutic role in pulmonary inflammation in this SAP model.
Keywords: ethyl pyruvate, high mobility group box 1, lung injury, severe acute pancreatitis
Introduction
Acute lung injury (ALI) is the most common extrapancreatic complication associated with the high rates of morbidity and mortality in severe acute pancreatitis (SAP) 1. Despite significant advances in understanding the pathogenesis of ALI in SAP and its management, the mortality rate remains unacceptably high. Studies have shown that pancreatic damage due to SAP leads to the release of systemic inflammatory cytokines, including tumour necrosis factor (TNF)-α and interleukin (IL)-1β. These proinflammatory cytokines may result in distant organ damage and the development of ALI. ALI is characterized by an intense inflammatory process in the lungs, with accumulation of activated neutrophils and the development of interstitial oedema 2–4. Related studies found that the transcriptional induction of genes involved in the release of inflammatory cytokines associated with SAP is controlled by various regulated factors, including the nuclear factor-kappa B (NF-κB) signalling pathway. NF-κB appears as the primary activator of proinflammatory mediators 5.
High mobility group box 1 (HMGB1) has been shown to be a late-acting mediator in lethal systemic inflammation 6,7. Extracellular HMGB1 can trigger acute lung injury 8,9 and a lethal inflammatory process 10 by inducing nuclear translocation of NF-κB and increasing significantly the release of inflammatory cytokines such as TNF-α, IL-1β, etc. 11–14. Therefore, HMGB1 can stimulate the release of cytokines 15 and, conversely, cytokines can control the further release of HMGB1 into the extracellular space 16. As such, HMGB1 enhances the inflammatory response in various pathological conditions. These observations demonstrate why extracellular HMGB1 might be a potential novel therapeutic target. These findings suggest that HMGB1 plays a critical role in ALI. Accordingly, HMGB1 inhibitors might be beneficial in the treatment of various inflammatory diseases.
Recently, our laboratory and others have demonstrated that ethyl pyruvate (EP), a simple aliphatic ester derived from pyruvic acid, is an effective anti-inflammatory agent 17–19. EP has been shown to improve survival and ameliorate organ dysfunction in a wide variety of animal models of severe sepsis, haemorrhagic shock, ischaemia/reperfusion-induced intestinal mucosal injury and ileus induced by bowel manipulation in mice 20–23. EP inhibits lipopolysaccharide-induced NF-κB activation in cultured RAW264·7 murine macrophage-like cells, and reduces HMGB1 release and TNF-α gene expression both in vitro and in vivo 20,22,23. Therefore, we reasoned that EP might also be protective in pancreatitis-associated ALI, depending on inhibition of early and late inflammatory cytokines. The present study was designed to evaluate whether EP could be beneficial in a rat model of pancreatitis-associated ALI 24.
Materials and methods
Animals
Healthy male Wistar rats weighing 250–300 g were purchased from the Experimental Animal Center of China Medical University. The animals were kept under standardized conditions with a 12-h light/dark cycle. Rats were fasted from solid food for 24 h (but allowed water ad libitum). All experimental procedures were approved by the Animal Ethics Committee of China Medical University and carried out in accordance with established International Guiding Principles for Animal Research.
Reagents
The reagents used were as follows: serum amylase, malondialdehyde (MDA) and myeloperoxidase (MPO) kits (Jiancheng Company, Nanjing, China); UltraSensitive™ SP kit (Maxim Company, Fuzhou, China); goat anti-rat polyclonal anti-HMGB1 antibody (Santa Cruz Inc., Santa Cruz, CA, USA); micro-bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL, USA); rabbit anti-HMGB1 polyclonal primary antibody (BD Pharmingen, San Jose, CA, USA); rabbit anti-β-actin monoclonal antibody (Invitrogen, Carlsbad, CA, USA); anti-rabbit horseradish peroxidase-coupled secondary antibody (Bio-Rad, Hercules, CA, USA); enhanced chemiluminescence plus Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA); TNF-α and IL-1β enzyme-linked immunosorbent assay (ELISA) kit (Sengxiong Biotechnology Co. Shanghai, China); nuclear and cytoplasmic extraction reagent kit (NE-PER; Pierce Biotechnology, Rockford, IL, USA); and non-radioactive NF-κB p50/p65 transcription factor assay kit (Chemicon, Temecula, CA, USA). All other chemicals were purchased from Sigma-Aldrich Chemicals (St Louis, MO, USA). EP was prepared in solution with sodium (130 mM), potassium (4 mM), calcium (2·7 mM), chloride (139 mM) and EP (28 mM) (pH 7·0).
Taurocholate-induced pancreatitis
Rats were anaesthetized by intraperitoneal injection of 1% pentobarbital sodium (35 mg/kg body weight) and the operation was performed under aseptic conditions. SAP models were prepared according to the method by Aho et al. 24. After entering the abdomen via median epigastric incision, the bile–pancreatic duct, hepatic hilus and common hepatic duct were identified, and the duodenal papilla inside the duodenum duct wall was identified. A segmental epidural catheter was inserted into the duodenum cavity and then inserted into the bile–pancreatic duct towards the direction of papilla in a retrograde manner; two microvascular clamps were used to nip both ends of the bile–pancreatic duct, then 5% sodium taurocholate (1·5 ml/kg body weight) was injected into the bile–pancreatic duct at a rate of 0·2 ml/min by a microinfusion pump. Five minutes after injection, the microvascular clamp and epidural catheter were removed. After ensuring that there was no active bleeding in the abdominal cavity, the abdomen was closed.
Experimental design
In the first series of experiments, rats were killed at 0, 3, 6, 12, 24 and 48 h after the induction of SAP (n = 12 per group). Zero time refers to the point of first injection of sodium taurocholate. The time–course of the first series of experiments was 48 h. In the first series of experiments, we confirmed the successful induction of the pancreatitis model. Furthermore, we evaluated the trends of these measurements at the designated time-points. Then, we selected the time-points that had shown the most significant differences to determine the effect of EP in the second experiment.
The second experiment was designed to determine the effect of treatment with EP (40 mg/kg) after the induction of SAP. Rats were assigned randomly to one of three groups. The groups were then divided randomly into 3-, 6-, 12- and 24-h subgroups, with 12 rats in each subgroup; in (i) the control group (n = 48), nothing was injected into the bile–pancreatic duct and the remaining procedure was the same as the SAP group; (ii) the SAP group (n = 48) received an infusion of 5% sodium taurocholate into the pancreatic bile duct; and (iii) the EP-treated group (n = 48) was perfused with EP at a dose of 40 mg/kg body weight for 2 min through the tail vein every 6 h after the induction of SAP (0, 6, 12 and 18 after SAP). The control group and SAP group rats were given the same dose of vehicle solution at the same time-point.
Measurement of serum amylase
Blood samples were collected from the abdominal aorta, conserved at room temperature for 10 min and centrifuged at 3000 g for 10 min at 4°C, and the serum were kept at −70°C until measurement. Serum amylase (AMY) activity was determined using AMY kits by automated clinical biochemistry analysis equipment (Hitachi Co., Tokyo, Japan).
Histological analysis
Pancreatic and lung tissue samples were fixed in 10% buffered formalin overnight and subsequently dehydrated through a graded ethanol series. After impregnation in paraffin wax, tissue samples were cut into 4-μm sections. Pancreatic and lung tissues were stained with haematoxylin and eosin (H&E) and examined by light microscopy. Sections were examined for tissue injury by an experienced morphologist who was blinded to the sample identity. For this study, five randomly chosen microscopic fields were examined for each tissue sample and given a histological score for injury according to the previously described method 25,26.
Wet/dry weight (W/D) ratio
Pancreatic or lung tissue oedema was evaluated by tissue water content. A portion of the pancreatic or lung tissue was taken immediately after euthanasia to trim fat and weigh. Tissue water content was determined by calculating the wet weight/dry weight ratio according to the formula: [(wet weight–dry weight)/dry weight] × 100%, where the wet weight was the initial weight of the respective tissue and the weight after incubation at 72°C for 24 h was the dry weight.
Lung tissue MPO and MDA assays
To carry out the assays, 1 g lung tissue samples were thawed, homogenized in 1 M phosphate-buffered saline (PBS) (pH 7·4) and centrifuged at 12 000 g for 10 min at 4°C. The supernatant was assayed for MPO activity and MDA concentration using test kits. All procedures were performed in accordance with the manufacturer's instructions.
Cytokine measurements
Lung tissue was homogenized in ice-cold lysis buffer containing 1 mM protease inhibitor. Homogenates were centrifuged at 14 000 g for 15 min, and supernatants were collected. Levels of TNF-α and IL-1β in lung were determined using commercially available ELISA kits according to the manufacturer's instructions.
Immunohistochemical analysis
Immunohistochemistry was performed to examine the protein expression levels of HMGB1 and to localize HMGB1 expression in the tissue. Lung tissue specimens from control and SAP rats were stained simultaneously. Briefly, tissue samples were isolated and fixed immediately in 10% pH-neutral phosphate-buffered formalin solution. The fixed tissues were then embedded in paraffin and sectioned at 4 μm. Paraffin sections were deparaffinized and hydrated. Antigens were retrieved in a 10 mM sodium citrate buffer (pH 6·0) preheated to 95°C for 20 min. Sections were blocked with peroxidase blocking buffer, followed by serum blocking buffer, avidin blocking buffer and biotin blocking buffer. For the detection of HMGB1, blocked sections were then incubated with goat anti-rat polyclonal anti-HMGB1 primary antibody (1:400 dilution) overnight at 4°C. Subsequently, tissue sections were washed and incubated with biotinylated anti-goat secondary antibody for 20 min at room temperature. After being washed, the sections were incubated with the streptavidin–peroxidase complex for 30 min at room temperature followed by incubation with freshly prepared 0·1% 3,3-diaminobenzidine-tetrahydrochloride containing 0·02% hydrogen peroxidase in PBS. Finally, the sections were counterstained with haematoxylin and subsequently dehydrated, mounted and covered with coverslips. Normal blocking serum without primary antibody was used for the negative control.
Western blotting
Western blotting was used to determine HMGB1 levels in the lungs. Briefly, 100 μg of lung homogenate protein was loaded onto a 10% Tris-HCl-sodium dodecyl sulphate (SDS)-polyacrylamide gel and run for 60 min at 120 V using a Bio-Rad minigel system. Protein was electrotransferred onto a nitrocellulose membrane and then blocked with 5% non-fat dry milk and Tris-buffered saline with 0·1% Tween 20. After being blocked, the membrane was incubated overnight at 4°C with a specific polyclonal rabbit primary antibody to HMGB1 at a dilution of 1:2000 followed by anti-rabbit horseradish peroxidase-coupled secondary antibody at a dilution of 1:5000 for 60 min before detection. After three washings, bands were detected using enhanced chemiluminescence plus Western blotting detection reagents. The membranes were then stripped using stripping buffer (63 mM Tris·HCl, pH 6·8, 2% SDS and 100 mM 2-mercaptoethanol) and reprobed with antibodies specific for β-actin to ensure equal loading of protein on the gel. HMGB1 expression was quantified densitometrically with the use of GelExpert version 3·5 software (Nucleotech, San Mateo, CA, USA).
NF-κB binding assay
Nuclear proteins were extracted from the lung tissue using NE-PER extraction reagents. The DNA binding activity of NF-κB (p50/p65) was determined using an ELISA-based non-radioactive NF-κB p50/p65 transcription factor assay kit. The absorbance at 450 nm was determined using an ELISA reader (Bio-Rad Laboratories).
Statistical analysis
The results are reported as means ± standard error (s.e.). One-way analysis of variance (anova) with Dunnett's multiple comparison tests was performed using the Statistical Package for the Social Sciences (spss) software, version 10·0. P-value < 0·05 was considered statistically significant.
Results
Serum amylase
Evidence of pancreatic injury in SAP induced by administration of taurocholate was confirmed by an increase in serum amylase in the first series of experiments. It showed that the serum amylase in the SAP and the EP-treated groups increased significantly more at each time-point compared to those in the control group (Fig. 1). However, the values in the EP-treated group decreased significantly compared to those in the SAP group at 6 and 12 h after SAP (Fig. 1).
Fig. 1.
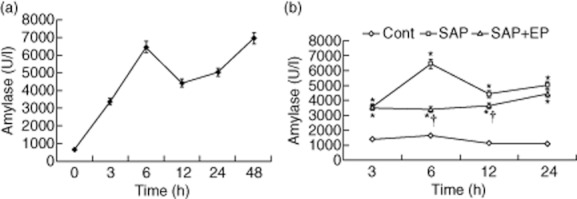
Serum amylase levels are measured in rats of three groups. Male Wistar rats were induced to severe acute pancreatitis (SAP), and serum was collected at different time-points and assayed for amylase levels. Data are shown as the means ± standard error (n = 12 rats per time-point in per subgroup). Lozenges: control group; squares: SAP group; triangles: EP-treated group. * P < 0·05 versus control group and † P < 0·05 versus SAP group, as tested by one-way analysis of variance (anova). The level at zero time means the point of first injection of sodium taurocholate. Cont: control.
Histopathology and morphometry of the pancreas and the lung
In control rats (Fig. 2a), the histological features of the pancreas were typical of a normal architecture. The infiltration of neutrophils and mononuclear cells, interstitial oedema and focal necrotic areas were seen in the pancreatic tissue of untreated SAP rats (Fig. 2b). Treatment of pancreatic rats with EP resulted in a significant amelioration of pancreatic injury. The morphological changes in pancreatic tissue of EP-treated rats included intralobular oedema, inflammatory infiltrate and acinar cell necrosis, but these were greatly reduced and without obvious parenchyma necrosis and haemorrhage (Figs 2c and 3a). Lung tissue from control rats showed a normal structure and no histological changes under a light microscope. In untreated SAP rats, the pathological changes of the lung showed a widespread increase in alveolar wall thickness caused by oedema, severe alveolar congestion, alveolus collapse and obvious inflammatory cell infiltration. The histopathological features were similar to human conditions, and the successful induction of the rat model was confirmed. In the EP-treated rats, the histological changes of lung tissue showed markedly reduced interstitial oedema and inflammatory cell infiltration compared with those in the untreated SAP rats (Fig. 2e,f, 3b).
Fig. 2.
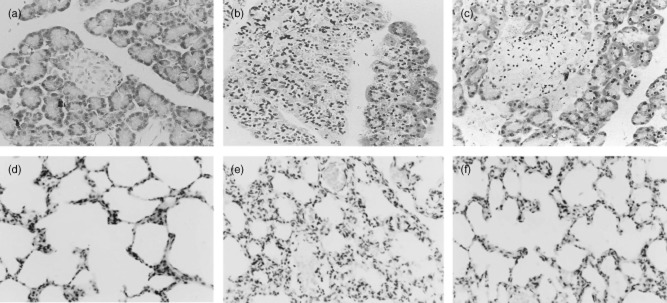
Morphological changes of pancreas and lung stained with haematoxylin and eosin (H&E). (a) No histological alteration of pancreas was observed in the pancreas collected from the control group. (b) Histological examination [at 24 h after severe acute pancreatitis (SAP)] of pancreatic sections from pancreatitic rats revealed oedema and acinar cell necrosis as well as inflammatory cells infiltration and an important alteration of the pancreas was also present. (c) Significantly less histological alteration of the pancreas tissue was observed in pancreatitic rats, which received ethyl pyruvate (EP) treatment. Representative H&E-stained section of lung was examined by light microscopy in control rats (d) and in pancreatitic rats (e), and in pancreatitic rats, which received EP treatment (f). Original magnification: ×400. Figure is representative of at least three experiments performed on different experimental days.
Fig. 3.
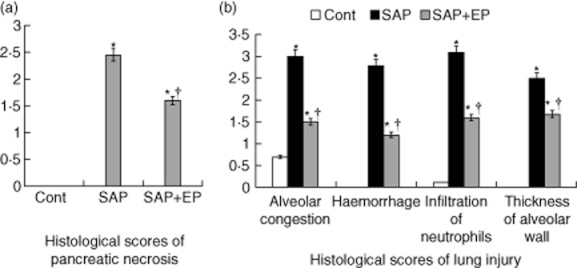
Histological scores of pancreatic necrosis and lung injury. Pancreas and lung were removed 24 h after induction of severe acute pancreatitis (SAP), and haematoxylin and eosin (H&E) staining was performed. (a) The extent of pancreatic acinar cell necrosis was quantitated morphometrically by an observer who was not aware of the sample identity. (b) Changes in lung histology score. The identified histological changes included congestion, oedema, inflammatory cells and haemorrhaging 24 h after induction of SAP. Histological scores were improved significantly in rats treated with EP compared with pancreatitic rats. *P < 0·05 versus control group and †P < 0·05 versus SAP group, as tested by one-way analysis of variance (anova). Cont: control.
W/D ratio of the pancreas and the lung
At 12 h after the induction of SAP, the W/D ratios of the pancreas and the lung in the SAP group were elevated. The W/D ratios of the pancreas and the lung in rats of the SAP group were significantly higher than those of control rats. However, the W/D ratios of the pancreas and the lung in rats of the EP-treated group were significantly lower than those in the SAP group (Fig. 4).
Fig. 4.
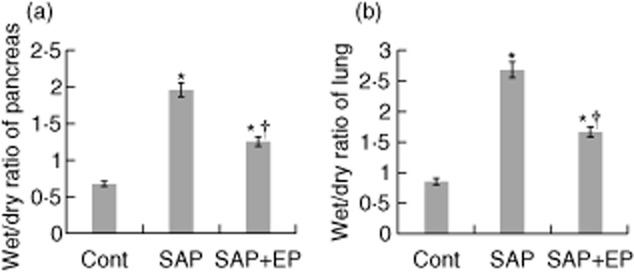
Wet/dry weight ratio of pancreatic and lung tissues in rats with severe acute pancreatitis (SAP). (a) The pancreas wet : dry weight (W/D) ratio was obtained to evaluate pancreas oedema. The W/D ratio in pancreas was determined 12 h after the induction of SAP (n = 12 for each group). (b) Effects of ethyl pyruvate (EP) on the lung W/D ratio of taurocholate-treated rats. The lung W/D ratio 12 h following taurocholate administration was shown for each group (n = 12 for each group). The data are expressed as the means ± standard error. *P < 0·05 versus control group and †P < 0·05 versus SAP group, as tested by one-way analysis of variance (anova). Cont: control.
MDA concentration and MPO activity in the lung
Pulmonary MDA concentrations were increased significantly by taurocholate administration in both SAP- and EP-treated rats (Fig. 5a,c). The increase was significantly smaller in EP-treated rats than in SAP rats at 6 h after the induction of SAP (Fig. 5c). Histological examination of lung sections from SAP rats revealed tissue damage characterized by oedema, neutrophil infiltration and alveolus collapse (Figs 2e, 3b). The infiltration of neutrophils into the lung was also evaluated by pulmonary MPO activity after induction of SAP in the first series of experiments. MPO activity in the lung was increased significantly at 6 h after the induction of SAP. However, rats receiving EP demonstrated a significant reduction of MPO activity in the lung at 6 h after the induction of SAP (Fig. 5d).
Fig. 5.
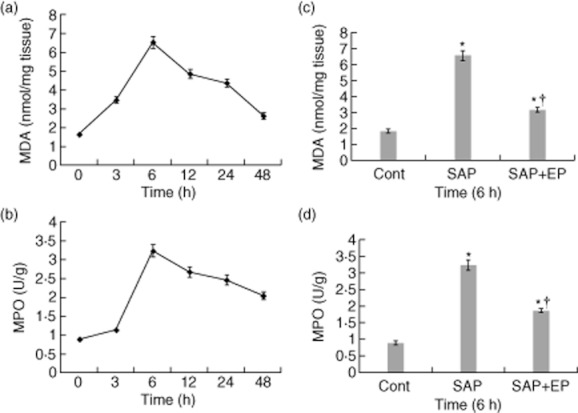
Myeloperoxidase (MPO) activity and malondialdehyde (MDA) levels in the lung of pancreatitic rats. Sample was obtained after induction of severe acute pancreatitis (SAP) at the corresponding time-points. (a,c) Effects of ethyl pyruvate (EP) on MDA concentration in the lung of rats with SAP. Pulmonary MDA concentration was significantly lower in EP-treated rats than in SAP rats at 6 h after the induction of SAP. (b,d) Lung neutrophil infiltration as measured by MPO activity. MPO activity was increased significantly in the lung from taurocholate-treated rats in comparison to control rats. The treatment with EP reduced the taurocholate-induced increase of MPO activity significantly in the lung at 6 h after the induction of SAP. Bars represent the means ± standard error of 12 rats. *P < 0·05 versus control group and †P < 0·05 versus SAP group, as tested by one-way analysis of variance (anova). Cont: control.
Pulmonary TNF-α and IL-1β expression
TNF-α and IL-1β were determined at the designated times in the lung homogenates. As shown in Fig. 6, compared to the control group both TNF-α and IL-1β increased significantly at 3 h in the SAP and EP-treated groups, with an early return to baseline values and no elevation for prolonged periods of time, as did HMGB1. In the SAP group, the changes in the expression of two cytokines in the lung were more significant than in the EP-treated group at 3 h (P < 0·05).
Fig. 6.
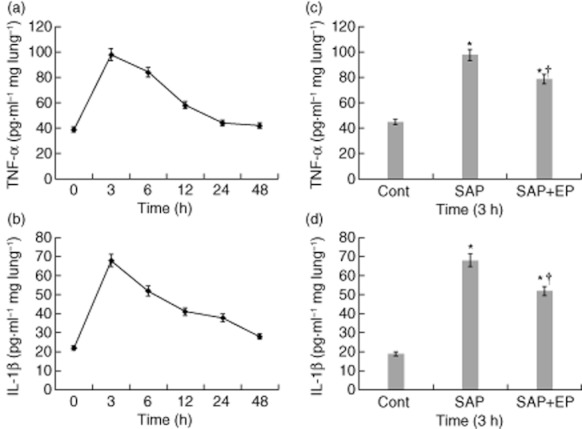
Effects of ethyl pyruvate (EP) treatment on pulmonary tumour necrosis factor (TNF)-α and interleukin (IL)-1β production in pancreatitic rats. (a,b) TNF-α and IL-1β protein of lung in rats was examined by enzyme-linked immunosorbent assay (ELISA) at the designated time-points after SAP. (c, d) At 3 h after the induction of severe acute pancreatitis (SAP), levels of TNF-α and IL-1β were increased compared with control rats. While SAP rats receiving the treatment with EP had a significant reduction in pulmonary TNF-α and IL-1β expression compared with animals receiving vehicle. The number of rats in each group at every time-point was 12. Values are shown means ± standard error. *P < 0·05 versus control group and †P < 0·05 versus untreated SAP group, as tested by one-way analysis of variance (anova). Cont: control.
HMGB1 expression is up-regulated in the lung after SAP
To determine the cellular localization of HMGB1 in the lung, immunohistochemical staining was performed in control lungs and lungs that underwent SAP. At 24 h after SAP, positive staining cells for HMGB1 were rarely observed in lung tissue from the control group (Fig. 7a). Positively staining endothelial cells, macrophages and neutrophils expressing HMGB1 were present in lung tissue from the SAP group. The number of cells expressing HMGB1 was decreased substantially in the EP-treated group compared with the SAP group (Fig. 7b,c). To determine if the HMGB1-mediated injury was associated with changes in protein levels, Western blot analysis was performed on lung lysates from animals that were subjected to SAP. As shown in Fig. 8, in the SAP group HMGB1 concentrations in the lung were increased slightly at 6 h of SAP and increased more markedly at 12 h, with the highest concentration observed at 24 h. Compared with the control group, difference was statistically significant (P < 0·05). HMGB1 concentration started to decrease at 48 h but did not return to basal levels. Moreover, among SAP rats treated with EP, there was a consistent decrease in HMGB1 expression (Fig. 8b).
Fig. 7.
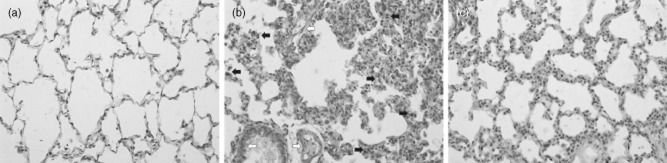
Lung immunohistochemical localization of high mobility group box 1 (HMGB1) is depicted. Immunohistochemical analysis was used to detect HMGB1 protein in lung sections obtained 24 h after severe acute pancreatitis (SAP). Representative specimens from the control group, SAP group and ethyl pyruvate (EP)-treated group are presented. (a) Immunohistochemical stain of HMGB1 from sections of control lung. (b) Staining of lung tissue sections obtained from SAP group with anti-HMGB1 antibody show an intense positive staining in the endothelial cells (white arrows), macrophages and neutrophils (black arrows). (c) The degree of lung staining for HMGB1 was reduced markedly in tissue section obtained from the EP-treated group. The arrows in (b) indicate cells staining positive for HMGB1. All photographs are at ×200 magnification. Images are representative lung sections from 12 rats per group.
Fig. 8.
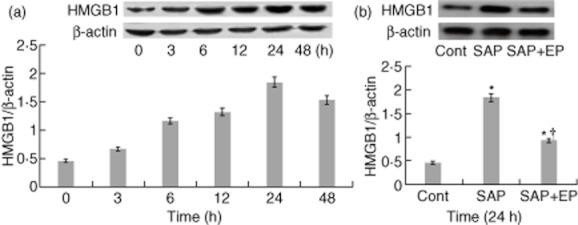
Changes in high mobility group box 1 (HMGB1) protein expression in lung tissue after induction of severe acute pancreatitis (SAP) in rats. (a) The expression of HMGB1 in the lung was detected by Western blot at the designated time-points after SAP. Results show the HMGB1/β-actin ratio from Western blots performed at each time-point. Blot shown is representative of three experiments with similar results. (b) Effect of treatment with EP on pulmonary expression of HMGB1 at 24 h after SAP. Pulmonary expressions of HMGB1 and β-actin determined by Western blot analysis in the control group, SAP group and ethyl pyruvate (EP)-treated group. The data shown are representative of three independent experiments. Values are shown means ± standard error. *P < 0·05 versus control group and †P < 0·05 versus SAP group, as tested by one-way analysis of variance (anova). Cont: control.
EP treatment reduces NF-κB DNA binding activity
Because the NF-κB pathway plays a critical role in the secretion of cytokines, we measured the proinflammatory transcription factor NF-κB in nuclear extracts prepared from lung samples. Whereas there was only a low level of basal DNA binding of NF-κB in pulmonary samples from control animals, NF-κB DNA binding activity increased in the rats with acute pancreatitis (Fig. 9). When the rats from the SAP group were treated with EP the extent of NF-κB DNA binding activity was clearly decreased, although it was still somewhat greater than that observed in the control group (Fig. 9b).
Fig. 9.
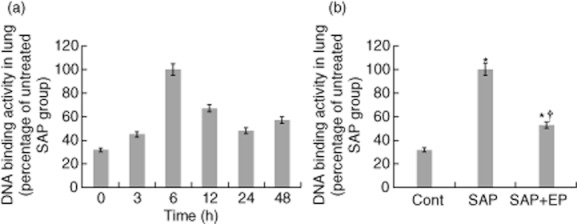
Effect of ethyl pyruvate (EP) on the increase in specific binding of p50 and p65 to DNA in the lung. (a) Changes of p50/p65 DNA binding activity in the lung after induction of severe acute pancreatitis (SAP)at the corresponding time-points. (b) Rats undergoing SAP were treated with EP or vehicle solution. Nuclear fractions were harvested 6 h following taurocholate administration from rats with or without EP treatment (n = 12 for each group). The DNA binding activity assay showed a marked decrease in the p50/p65 binding activity in nuclear fractions from lung tissue in the EP-treated group. Values are shown as means ± standard error. *P < 0·05 compared with control group and †P < 0·05 compared with SAP group. Cont: control.
Discussion
SAP is usually accompanied by obvious inflammatory reactions, and local pathological injuries can lead to systemic inflammatory response syndrome and multiple organ dysfunction syndrome, resulting in high mortality 27. Some degree of pulmonary dysfunction occurs in as many as 40–70% of patients with acute pancreatitis, and respiratory failure occurs in about 45% of patients with pancreatic necrosis 28,29. ALI is an important problem affecting the severity of SAP 30. It is believed that the excess production of inflammatory mediators released by macrophages, neutrophils and other cells of the immune system in a cascade network is the underlying mechanism causing ALI in SAP 31,32. As SAP progresses, neutrophils are also transmigrated into the tissues by inflammatory mediator induction, resulting in microvascular dysfunction and local inflammatory response. These responses include microvascular disorder, alveolar capillary barrier leakage, interstitial and alveolar oedema and eventual cell death 33,34. This study provides evidence that EP attenuates: (i) the rats from taurocholate-induced ALI; (ii) the taurocholate-induced pulmonary expression of TNF-α, IL-1β and HMGB1; (iii) neutrophil infiltration and lipid peroxidation in the lung; and (iv) NF-κB DNA binding activity in the lung. EP has been shown to improve survival and ameliorate organ dysfunction in a wide variety of animal models 20–23. In this study, our results confirm the previous findings and indicate that EP may play a role in reducing the pathology of SAP-associated lung injury, and that the potential mechanism of action is through the inhibition of systemic inflammation.
In the present study, we demonstrated that treatment with EP could prevent the rats from taurocholate-induced ALI. Interestingly, treatment with EP failed to ameliorate the development of hyperamylasaemia at 24 h after SAP. It suggested that, even if treatment of EP could reduce pancreas injury, it could not reverse this trend of pancreas injury. To our knowledge, this is the first study regarding the anti-inflammatory effect of EP on taurocholate-induced lung injury. Moreover, we have demonstrated that treatment with EP improved taurocholate-induced ALI significantly and was associated with a reduction in both early (TNF-α and IL-1β) and late (HMGB1) cytokine levels in rats. Our results also suggested that the inhibition of cytokine secretion was the result of an inhibition of NF-κB activity. Thus, the improvement in taurocholate-induced ALI by EP administration seems to be related to the altered expression of these mediators.
EP reduced the lung permeability index in mice with LPS-induced ALI. Also, there was a dose-dependent reduction in the permeability index 31. In this study, the W/D ratios of lung tissue for each group were measured for assessment of changes in lung vascular permeability. This study found that the EP-treated rats showed a much lower ratio of wet/dry weight in lungs than in the untreated SAP rats. Furthermore, when compared to the untreated SAP rats, the EP-treated rats had lower scores for lung injury, including measurements of alveolar congestion, haemorrhage, infiltration or aggregation of neutrophils in airspace or vessel wall, and thickness of the alveolar wall. According to the MPO assay measurement of neutrophil accumulation, the untreated SAP rats had a higher neutrophil accumulation than the control rats. MDA is one of the final products of lipid peroxidation, and its concentration is directly proportional to the cell damage caused by reactive oxygen metabolites 35. In the present study, the increase in pulmonary MDA production following SAP induction was reduced by EP administration, suggesting that EP is involved in an anti-inflammatory effect. These results lend support to the use of EP as an anti-inflammatory therapy for SAP associated with ALI.
Proinflammatory cytokines, such as TNF-α and IL-1β, are thought to play a crucial role in the pathogenesis of SAP, directly injuring cells and causing necrosis, inflammation and oedema 36,37. In our study, we examined pulmonary levels of TNF-α and IL-1β. These cytokines were normally induced strongly in rats with SAP. However, treatment with EP markedly attenuated this induction, suggesting that EP can reduce the inflammatory response in this rat model of SAP. Cytokines such as TNF-α and IL-1β are secreted during the early phase of the inflammatory response and play an important role in the development of ALI 38. HMGB1 plays an important role in various types of inflammation and is expressed at a relatively late stage following injury 39. As a non-classic proinflammatory cytokine, extracellular HMGB1 plays a pivotal role in the pathogenesis of proinflammatory diseases such as ALI 40. In the present study, pulmonary levels of HMGB1 were increased as soon as 6 h after SAP and remained elevated for 48 h. Thus, delayed release of HMGB1 can participate in the downstream development of lung injury, and HMGB1 may be a distal mediator of acute inflammatory lung injury. Neutrophils appeared to be the primary contributors to the increases in pulmonary HMGB1 levels after SAP. This was supported by the fact that lungs of SAP rats were infiltrated with neutrophils that were strongly positively stained for HMGB1. Moreover, we demonstrated that HMGB1 secretion was inhibited by EP treatment in our SAP model. HMGB1 mediates acute inflammation in animal models of lung injury and plays an important role in the development of sepsis and LPS-induced lung injury 40,41.
Currently, researchers have proved that the activation of NF-κB plays an extremely important role in the development of SAP 42,43. In this study, we observed the increased NF-κB DNA binding activity in lung tissue samples obtained after the induction of SAP. Meanwhile, the increase in NF-κB DNA binding activity was a dynamic course from a lower level to a higher level, and reached the highest level at 6 h after the induction of SAP. The results above indicate that NF-κB could be activated in the early stage of SAP and could be involved in the process of severe pancreatitis-associated lung injury. In the present study, treatment of the SAP rats with EP decreased NF-κB DNA binding activity in lung tissue. Therefore, what is the mechanism of EP inhibition of NF-κB DNA binding activity in lung tissue? On one hand, the molecular mechanism of EP action interferes with signal transduction through the p38 mitogen-activated protein kinase (MAPK) and NF-kB pathways, and to target directly the p65 subunit of the transcription factor 20,44. On the other hand, besides the direct injuries against tissues or cells, reactive oxygen species act as second-messenger molecules and enhance proinflammatory cytokine production through activation of NF-κB 45,46. Furthermore, EP may be an effective scavenger of reactive oxygen species and hydrogen peroxide 47.
In conclusion, the results suggest that administration of EP inhibited the activation of NF-kB, down-regulated downstream inflammatory cytokines and attenuated severe pancreatitis-associated ALI in SAP rats. It might be helpful to use EP in SAP as a therapeutic strategy for future experiments, and we believe this study will be particularly relevant in clinical settings.
Acknowledgments
The authors thank Dr Ying Zhang (Center of Laboratory Technology and Experimental Medicine; China Medical University) for expert technical support.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Renzulli P, Jakob SM, Täuber M, Candinas D, Gloor B. Severe acute pancreatitis: case-oriented discussion of interdisciplinary management. Pancreatology. 2005;5:145–156. doi: 10.1159/000085266. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 3.Solan PD, Piraino G, Hake PW, et al. Liver X receptor α activation with the synthetic ligand T0901317 reduces lung injury and inflammation after hemorrhage and resuscitation via inhibition of the nuclear factor κB pathway. Shock. 2011;35:367–374. doi: 10.1097/SHK.0b013e3181f7d742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jastrow KM, 3rd, Gonzalez EA, McGuire MF, et al. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209:320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Ye X, Steinberg H, Liu SF. Selective blockade of endothelial NF-kappaB pathway differentially affects systemic inflammation and multiple organ dysfunction and injury in septic mice. J Pathol. 2010;220:490–498. doi: 10.1002/path.2666. [DOI] [PubMed] [Google Scholar]
- 6.Ulloa L, Tracey KJ. The ‘cytokine profile’: a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Yu M, Wang H, Ding A, et al. HMGB1 signals through Toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- 8.Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol. 2012;91:590–601. doi: 10.1016/j.ejcb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Park JS, Strassheim D, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958–965. doi: 10.1152/ajplung.00359.2004. [DOI] [PubMed] [Google Scholar]
- 10.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 11.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barsness KA, Arcaroli J, Harken AH, et al. Hemorrhage-induced acute lung injury is TLR-4 dependent. Am J Physiol Regul Integr Comp Physiol. 2004;287:R592–599. doi: 10.1152/ajpregu.00412.2003. [DOI] [PubMed] [Google Scholar]
- 13.Xie K, Yu Y, Huang Y, et al. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock. 2012;37:548–555. doi: 10.1097/SHK.0b013e31824ddc81. [DOI] [PubMed] [Google Scholar]
- 14.Gong Q, Xu JF, Yin H, Liu SF, Duan LH, Bian ZL. Protective effect of antagonist of high-mobility group box 1 on lipopolysaccharide-induced acute lung injury in mice. Scand J Immunol. 2009;69:29–35. doi: 10.1111/j.1365-3083.2008.02194.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Vishnubhakat JM, Bloom O, et al. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery. 1999;126:389–392. [PubMed] [Google Scholar]
- 17.Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Role of high-mobility group box 1 protein in the pathogenesis of intestinal barrier injury in rats with severe acute pancreatitis. Pancreas. 2010;39:216–223. doi: 10.1097/MPA.0b013e3181bab5c5. [DOI] [PubMed] [Google Scholar]
- 18.Luan ZG, Zhang H, Yang PT, Ma XC, Zhang C, Guo RX. HMGB1 activates nuclear factor-κB signaling by RAGE and increases the production of TNF-α in human umbilical vein endothelial cells. Immunobiology. 2010;215:956–962. doi: 10.1016/j.imbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Luan ZG, Zhang H, Ma XC, Zhang C, Guo RX. Therapeutic treatment with ethyl pyruvate attenuates the severity of liver injury in rats with severe acute pancreatitis. Pancreas. 2012;41:729–737. doi: 10.1097/MPA.0b013e31823cd3ef. [DOI] [PubMed] [Google Scholar]
- 20.Ulloa L, Ochani M, Yang H, et al. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351–12356. doi: 10.1073/pnas.192222999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang R, Gallo DJ, Baust JJ, et al. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2002;283:G212–221. doi: 10.1152/ajpgi.00022.2002. [DOI] [PubMed] [Google Scholar]
- 22.Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer's ethyl pyruvate solution ameliorates ischemia/reperfusion-induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513–1518. doi: 10.1097/00003246-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Harada T, Moore BA, Yang R, Cruz RJ, Jr, Delude RL, Fink MP. Ethyl pyruvate ameliorates ileus induced by bowel manipulation in mice. Surgery. 2005;138:530–537. doi: 10.1016/j.surg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- 25.Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284–289. doi: 10.1016/s0039-6060(96)80299-6. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka A, Minoguchi K, Chen X, et al. Activated protein C attenuates leukocyte elastase-induced lung injury in mice. Shock. 2008;30:153–158. doi: 10.1097/SHK.0b013e31815dd570. [DOI] [PubMed] [Google Scholar]
- 27.Nathens AB, Curtis JR, Beale RJ, et al. Management of the critically ill patient with severe acute pancreatitis. Crit Care Med. 2004;32:2524–2536. doi: 10.1097/01.ccm.0000148222.09869.92. [DOI] [PubMed] [Google Scholar]
- 28.Imrie CW, Murphy D, Ferguson JC, Blumgart LH. Proceedings: arterial hypoxia in acute pancreatitis. Ann R Coll Surg Engl. 1976;58:322–323. [PMC free article] [PubMed] [Google Scholar]
- 29.Ranson JH, Turner JW, Roses DF, Rifkind KM, Spencer FC. Respiratory complications in acute pancreatitis. Ann Surg. 1974;179:557–566. doi: 10.1097/00000658-197405000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou MT, Chen CS, Chen BC, Zhang QY, Andersson R. Acute lung injury and ARDS in acute pancreatitis: mechanisms and potential intervention. World J Gastroenterol. 2010;16:2094–2099. doi: 10.3748/wjg.v16.i17.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shang GH, Lin DJ, Xiao W, et al. Ethyl pyruvate reduces mortality in an endotoxin-induced severe acute lung injury mouse model. Respir Res. 2009;10:91. doi: 10.1186/1465-9921-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao X, Shi C, Wang X, Andersson R. Protein kinase C modulates the pulmonary inflammatory response in acute pancreatitis. Respir Physiol Neurobiol. 2006;152:16–26. doi: 10.1016/j.resp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Chooklin S, Pereyaslov A, Bihalskyy I. Pathogenic role of myeloperoxidase in acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:627–631. [PubMed] [Google Scholar]
- 34.Ionescu CV, Cepinskas G, Savickiene J, Sandig M, Kvietys PR. Neutrophils induce sequential focal changes in endothelial adherens junction components: role of elastase. Microcirculation. 2003;10:205–220. doi: 10.1038/sj.mn.7800185. [DOI] [PubMed] [Google Scholar]
- 35.Türüt H, Kurutas EB, Bulbuloglu E, et al. Zinc aspartate alleviates lung injury induced by intestinal ischemia-reperfusion in rats. J Surg Res. 2009;151:62–67. doi: 10.1016/j.jss.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Vonlaufen A, Apte MV, Imhof BA, Frossard JL. The role of inflammatory and parenchymal cells in acute pancreatitis. J Pathol. 2007;213:239–248. doi: 10.1002/path.2231. [DOI] [PubMed] [Google Scholar]
- 37.Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24:45–51. doi: 10.1097/01.shk.0000191413.94461.b0. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 39.Chorny A, Delgado M. Neuropeptides rescue mice from lethal sepsis by down-regulating secretion of the late-acting inflammatory mediator high mobility group box 1. Am J Pathol. 2008;172:1297–1307. doi: 10.2353/ajpath.2008.070969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 42.Rakonczay Z, Jr, Hegyi P, Takács T, McCarroll J, Saluja AK. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. doi: 10.1136/gut.2007.124115. [DOI] [PubMed] [Google Scholar]
- 43.Zhang XP, Zhang L, Xu HM, et al. Application of tissue microarrays to study the influence of dexamethasone on NF-kappaB expression of pancreas in rat with severe acute pancreatitis. Dig Dis Sci. 2008;53:571–580. doi: 10.1007/s10620-007-9867-4. [DOI] [PubMed] [Google Scholar]
- 44.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–1105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- 45.Ohashi S, Nishio A, Nakamura H, et al. Protective roles of redox-active protein thioredoxin-1 for severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G772–781. doi: 10.1152/ajpgi.00425.2005. [DOI] [PubMed] [Google Scholar]
- 46.Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1–11. doi: 10.1016/s0014-2999(99)00421-5. [DOI] [PubMed] [Google Scholar]
- 47.Crestanello JA, Lingle DM, Millili J, Whitman GJ. Pyruvate improves myocardial tolerance to reperfusion injury by acting as an antioxidant: a chemiluminescence study. Surgery. 1998;124:92–99. [PubMed] [Google Scholar]


