Abstract
Opsoclonus–myoclonus syndrome (OMS) is a neuroinflammatory disorder associated with remote cancer. To understand more clearly the role of inflammatory mediators, the concentration of CXCR3 ligands CXCL10, CXCL9 and CXCL11 was measured in 245 children with OMS and 81 paediatric controls using enzyme-linked immunosorbent assay (ELISA), and CXCR3 expression on CD4+ T cells was measured by flow cytometry. Mean cerebrospinal fluid (CSF) CXCL10 was 2·7-fold higher in untreated OMS than controls. Intrathecal production was demonstrated by significantly different CXCL10 CSF : serum ratios. The dichotomized ‘high’ CSF CXCL10 group had higher CSF leucocyte count (P = 0·0007) and B cell activating factor (BAFF) and CXCL13 concentrations (P < 0·0001). CSF CXCL10 did not correlate with clinical severity or relapse using grouped data, although it did in some patients. Among seven types of immunotherapy, including rituximab or chemotherapy, only adrenocorticotrophic hormone (ACTH) monotherapy showed reduced CSF CXCL10, but prospective longitudinal studies of ACTH combination therapies indicated no reduction in CXCL10 despite clinical improvement (P < 0·0001). CXCL10 concentrations were 11-fold higher in CSF and twofold higher in serum by multiplexed fluorescent bead-based immunoassay than enzyme-linked immunosorbent assay, but the two correlated (r = 0·7 and 0·83). In serum, no group differences for CXCL9 or CXCL11 were found. CXCR3 expression on CD4+ T cells was fivefold higher in those from CSF than blood, but was not increased in OMS or altered by conventional immunotherapy. These data suggest alternative roles for CXCL10 in OMS. Over-expression of CXCL10 was not reduced by clinical immunotherapies as a whole, indicating the need for better therapeutic approaches.
Keywords: neuroblastoma, opsoclonus–myoclonus syndrome, paraneoplastic syndrome, paediatric neuroinflammation
Introduction
Interferon (IFN)-γ-inducible protein 10 (CXCL10), a non-glutamic acid–leucine–arginine (ELR) or lymphocyte-specific C-X-C chemokine 1, plays a pivotal role in T cell-mediated immunity in the central nervous system (CNS) 2. CXCL10 is a ligand for CXCR3, its cognate receptor, which is expressed highly on T helper cell type 1 (Th1) cells, and also on CD8+ T cells and natural killer (NK) cells 3. While CXCL10 is expressed in astrocytes, especially reactive astrocytes, and cerebellar Purkinje cells 4, it is also expressed by vascular endothelial cells and fibroblasts 5. As well as its chemoattractant properties, CXCL10 promotes antigen-driven T cell responses 5. Although chemokines and their receptors are potential targets for therapeutic intervention 6, little is known about the effect of immunotherapy on CSF CXCL10 or leucocyte CXCR3 receptors. Gathering more information is essential to assessing adequacy of therapy on immunopathology and being able to manipulate the CXCL10/CXCR3 axis therapeutically.
Opsoclonus–myoclonus syndrome (OMS), a paraneoplastic disorder of children and adults, is characterized by cerebellar gait ataxia, tremulous-appearing action myoclonus and darting opsoclonic eye movements 7. The type of tumour, which is not located in the brain and varies with patient age group, is most commonly neuroblastoma in children 8. Research on OMS is limited by lack of an animal model (antigen is unknown), post-mortem brain tissue (tumour survival is excellent) or a neuroradiological surrogate marker (scans are usually normal). Hence, studies of cerebrospinal fluid (CSF), which is in direct contiguity with the brain, are essential for insights on CNS events. Thus far, B cell and humoral involvement have been implicated by CSF expansion of B cells 9 and intrathecal production of B cell attractant CXCL13 10, B cell activating factor (BAFF) 11 and oligoclonal bands 12. With regard to T cells, skewing of the CD4/CD8 subset and an increase in γδ T cells also occur 9. In preliminary experiments, we also found an elevation in CSF CXCL10 13.
In the present study, we measured the ex-vivo expression of CXCR3 receptors on Th cells (CD4+) by flow cytometry, and the concentrations of three CXCR3 ligands: CXCL10, CXCL9 (monokine induced by IFN-γ: Mig) and CXCL11 (IFN-inducible T cell alpha-chemoattractant: ITAC), which have collaborative, redundant and opposing functions 14. We evaluated the effects of several treatment modalities, such as corticotrophin (ACTH), corticosteroids, intravenous immunoglobulins (IVIG), anti-CD20 monoclonal antibodies (rituximab) and various chemotherapies and steroid-sparers 7.
Materials and methods
Study design
This case–control study had three major aims: to evaluate the role of the CXCL10/CXCR3 axis in OMS, to ascertain the effects of immunotherapy and to determine whether CXCL10 is a useful biomarker of disease activity in OMS. The first goal required chemokine assays of CSF and serum and clinical assessments in a cross-sectional analysis; the second, re-evaluation after open-label treatment with various forms of immunotherapy in a longitudinal study; and the third, correlation of CXCL10 concentrations and CXCR3 with important clinical variables and the lymphocyte immunophenotype. This study is part of the multiplex chemokine profiling project in paediatric OMS to identify biomarkers, which is registered with http://ClinicalTrials.gov NCT 00806182.
Study population
Two-hundred and twenty-five domestic and 20 international patients who met inclusion and exclusion criteria were enrolled through the National Pediatric Myoclonus Center, and parents signed informed consent for this institutional review board (IRB)-approved study. The mean age [standard deviation (s.d.)] was 4·2 ± 3·1 years; there were 110 boys and 135 girls. Each patient received a full neurological assessment by the investigators and a formalized videotaping session. Neuroblastomas detected as part of a diagnostic evaluation were excised prior to inclusion in the study and otherwise handled in accordance with standard practice.
Controls were age- and gender-matched healthy children and others with non-inflammatory neurological disorders, such as headache, ataxia and developmental delay. Records were reviewed to exclude individuals with fever, CSF leucocytosis, bloody CSF, abnormal CSF protein or glucose, evidence of infection or autoimmune disease or treatment with immunotherapy. Serum controls additionally included six unaffected siblings of children with OMS and 13 healthy unrelated children drawn nationally. Samples from children with other inflammatory neurological disorders (OIND), such as multiple sclerosis (MS), neurolupus, encephalitis, acute disseminated encephalomyelitis and different paraneoplastic disorders, were also evaluated. Control samples were acquired throughout the study in order to be equivalent in storage time with the OMS samples.
Evaluation of clinical severity
Children were evaluated and videotaped at every visit. An experienced evaluator, blinded to treatment status, scored all videotapes, using a 12-item motor scale 9. Each scale item was rated 0–3, with a score of 36 indicating maximum abnormality. Total score designated one of three severity categories: 0–12 = mild, 13–24 = moderate and 25–36 = severe.
Cross-sectional immunotherapy groups
Seven groups of children with OMS were defined based on the immunotherapies they were on at initial evaluation, using standard open-label protocols for autoimmune disease 10. Clinical data, videotapes and CSF and serum samples were obtained at intervals after treatment. Children in the ACTH group were taking Acthar gel 80 IU/ml, which had been initiated at 75 IU/m2, given by intramuscular injection twice daily ×1 week, once daily ×1 week, on alternate days ×2 weeks, and then tapered. The IVIG group was receiving 1 g/kg monthly as maintenance after an initial 2 g/kg induction. Two adjunctive therapy groups designated as ‘ACTH + other’ or ‘steroid + other’ included the following agents: rituximab 375 mg/m2 intravenously (i.v.) once weekly ×4 weeks; cyclophosphamide 1 g/m2 i.v. monthly ×6 months; 6-mercaptopurine 2 mg/kg/day per os; methotrexate 15–20 mg/m2 once weekly per os; or mycophenolate mofetil 300 mg/m2 twice daily per os. An eighth group represented prior but not current immunotherapy for comparison.
Enzyme-linked immunosorbent assay (ELISA)
CSF and paired sera were stored at −80°C, and batched assays were run in duplicate by ELISA as per the manufacturer's instructions. The human ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA) and had a detection limit of 0·41–4·46 pg/ml for CXCL10, 1·37–11·31 pg/ml for CXCL9 and 3·4–39·7 pg/ml for CXCL11. The inter- and intra-assay coefficient of variation (CV) for CSF CXCL10 was 10·6% (n = 19) and 2·4% (n = 10), respectively, and for serum CXCL10, 5·8% (n = 11) and 4·8% (n = 8). For CXCL9, the intra-assay CV was 11·3% (n = 19), and the interassay CV was 8·1% (n = 11). For CXCL11, the CV values were 7·0% (n = 11) and 6·2% (n = 5), respectively.
Multiplexed fluorescent bead-based immunoassay
The Beadlyte Human Multi-Cytokine Beadmaster kit was used for CXCL10 assays on the LUMINEX 100 Lab MAP System, according to the manufacturer's directions (Upstate Biotechnology, Lake Placid, NY, USA). CSF samples were processed without dilution, and assays were performed in triplicate. The sensitivity was 6·9 pg/ml. The samples were analysed using the Luminex 100 platform, and data analysis was completed with Beadlyte Beadview Software (Upstate Biotechnology). This method was used originally for this study, but after Millipore (St Charles, MO, USA) acquired Upstate in 2006 it changed antibodies in the kit, and the results with the new kit in our experience were too different to be included in the same data set.
Several quality control measures were taken. Controls were placed onto the same plate as OMS. Samples from a given patient in the longitudinal treatment study were run on a single plate to eliminate interassay variability. For each assay, calibration and control standards were included. Inter- and intra-assay variability tests were performed periodically. The standard curves were inspected visually for proper curve-fitting and to verify that standards were in the expected range. The Beadview software program has built-in checks on the data, if adequate number of beads are counted (50 beads per analyte per well) to choose median fluorescence to use against standard curve. A service technician calibrated the laser annually.
Flow cytometry
CXCR3 receptor expression on CSF and blood lymphocytes was analysed ex vivo in a subgroup of patients by flow cytometry using the method of Kivisäkk and colleagues 15. For CSF staining, 10 ml CSF samples were collected on ice, and centrifuged immediately on arrival in the laboratory within 15–20 min of sampling. The commercial sources for monoclonal antibodies (mAbs) were Becton-Dickinson (San Jose, CA, USA) for CXCR3, CD4, and CD3 and Beckman Coulter (Miami, FL, USA) for CD45. Samples were acquired and analysed on a dual laser fluorescence activated cell sorter (FACS)Caliber flow cytometer (Becton-Dickinson) with CellQuest software (Becton-Dickinson). Lymphocytes were identified on the forward scatter/side scatter (FS-SS) plot and gated on the population of interest. Gates were defined using blood and transferred to CSF sample analysis. All events in CSF were counted: mean 2045 ± 1389 s.d. (n = 25). Peripheral blood staining was performed as specified 15, and the peripheral blood gate was set to count 15 000 events.
Statistical analysis
The dependent variables were continuous, and included CSF and serum CXCL10 concentration, total score and the CSF immunophenotype. Cross-sectional data were analysed by analysis of variance (anova), using the Tukey test as a post-hoc test of means. Pre- and post-treatment data were analysed by paired t-tests. Medians were analysed with the Kruskal–Wallis (K-W) test, using Dunn's test for post-hoc comparisons. For categorical outcomes, the χ2 or Fisher's exact tests were used. Pearson's correlations were used for correlation analysis. In a secondary analysis, Bonferroni corrections were used to identify clinical and immunological differences based on ‘high’ or ‘normal’ CXCL10 concentration as defined by 1 s.d. above the control mean.
Results
Fluorescent bead-based immunoassay CXCL10 determinations
The CXCL10 concentration was higher in CSF than in serum across groups (Fig. 1a). Comparing controls, untreated OMS and treated OMS, the within-group differences between CSF and serum CXCL10 were significant (P = 0·002 anova, P = 0·0003 K-W test). In controls, the delta between the mean CSF and serum CXCL10 concentration was 3·1-fold; in untreated OMS, 8·8-fold; and in treated OMS, 5·7-fold. Untreated OMS and treated OMS differed from controls but not each other in this regard. Inclusion of a small neuroblastoma group without OMS revealed that the elevated CSF CXCL10 is a factor of OMS, not neuroblastoma. CXCL10 concentrations measured by ELISA were correlated with those measured by fluorescent bead-based immunoassay. The Luminex method yielded approximately 11-fold higher results than ELISA for CSF CXCL10 (Fig. 1b) and twofold higher for serum CXCL10 (Fig. 1c), so intrathecal synthesis of CXCL10 was more apparent using it (Fig. 1b).
Fig. 1.
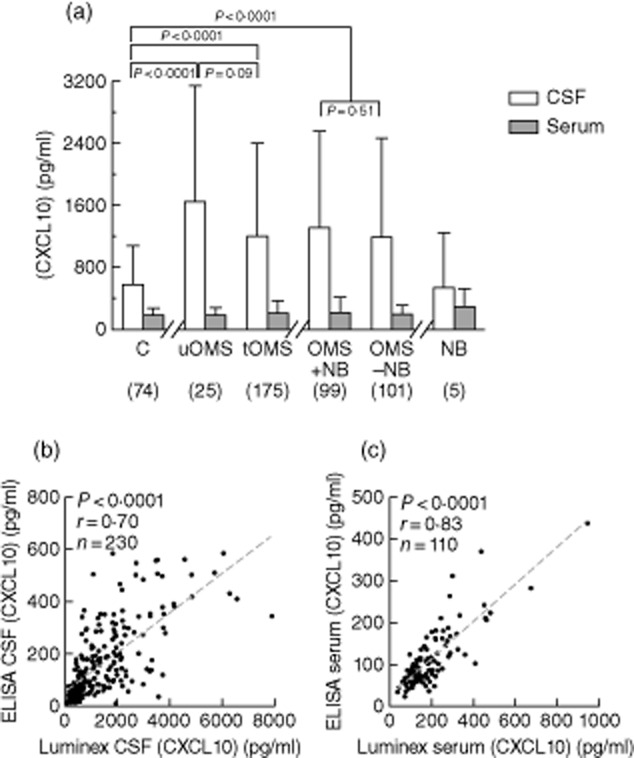
(a) Mean CXCL10 concentration measured by fluorescent bead-based immunoassay was increased in untreated opsoclonus–myoclonus syndrome (OMS) (uOMS) cerebrospinal fluid (CSF) but not in paired serum. Separate statistical comparisons were made between controls, untreated OMS (uOMS) and treated OMS (tOMS); and between controls, OMS without neuroblastoma (OMS − NB), and OMS with neuroblastoma (OMS + NB). Statistical brackets with P-values above them indicate analysis of variance (anova) results; those with P-values underneath indicate t-test results. The neuroblastoma without OMS group had only five patients, so it is presented only for visual comparison. (b) CSF CXCL10 concentration as measured by Luminex versus enzyme-linked immunosorbent assay (ELISA) in entire data set. (c) Serum CXCL10 measured by Luminex versus ELISA in entire data set.
CSF CXCL10 in untreated OMS by ELISA
The remaining chemokine measurements in this report were made by ELISA. There were significant differences between groups in mean (P < 0·0001) and median (P < 0·0001) CSF CXCL10 concentration (Fig. 2a). The mean CSF concentration of CXCL10 was 2·7-fold higher in untreated OMS relative to controls (P < 0·0001). Median CXCL10 was also 2·5-fold higher (P = 0·002). Controls were tight as a group, but in OMS there was considerable interindividual variation. However, even removal of the OMS ‘outlier’ had little effect on the statistical significance. About 32% of untreated OMS had levels above the highest control, some high (range 11–1103 pg/ml). CXCL10 was detectable in 100% of the CSF samples, hence there were no differences in detectability among cross-sectional groups.
Fig. 2.
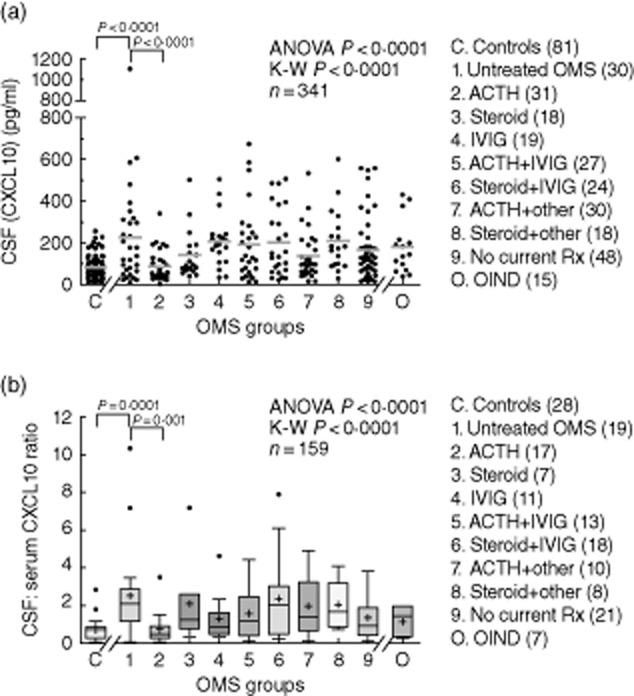
(a) Cross-sectional analysis of mean CXCL10 concentrations measured by enzyme-linked immunosorbent assay (ELISA) from controls, untreated opsoclonus–myoclonus syndrome (OMS) (group 1), various OMS treatment groups (group 2–8), previously but not currently treated OMS (group 9), and other inflammatory neurological disorders (OIND). Statistical brackets signify post-hoc tests. Among the seven OMS active treatment groups, adrenocorticotrophic hormone (ACTH) monotherapy was associated with the lowest cerebrospinal fluid (CSF) CXCL10 level. (b) The CSF: serum CXCL10 ratio showed similar results.
Serum CXCL10 and CSF : serum ratio
Despite the CSF elevations, there was no significant difference in serum CXCL10 concentration (Fig. 1a), indicating that CXCL10 was over-produced in OMS CSF. The mean ratio of CSF to serum concentrations (Fig. 2b) was increased 3·5-fold in untreated OMS (3·4-fold for median).
Comparison of immunotherapy effects in OMS
In the cross-sectional study, despite treatment, most OMS still exhibited high CSF CXCL10 levels (Fig. 2). Approximately 17% of all currently treated OMS had a CSF CXCL10 concentration above the highest control. Only the ACTH-monotherapy group (group 2) had a significantly lower CXCL10 concentration than untreated OMS. Mean ACTH dose (IU/m2/day) did not differ significantly (P = 0·79, anova; P = 0·50, K-W test) between ACTH groups 2 (24 ± 27), 5 (26 ± 20), and 7 (22 ± 16); nor did the median dose.
Relation of CSF CXCL10 to other immunological data
In a secondary analysis (Table 1), the entire OMS group was divided into two subgroups as described. CSF leucocyte count was significantly higher in the ‘high’ CSF CXCL10 group, as were CSF BAFF and CXCL13 concentrations. There were no statistically significant differences between groups in OMS severity category, duration category, percentage of subjects relapsing, number of relapses, OMS aetiology or the frequency of αβ- or γδ-T cells or NK T cells (data not shown).
Table 1.
Comparison of ‘high’ and ‘normal’ cerebrospinal fluid (CSF) CXCL10 concentration groups from all opsoclonus–myoclonus syndrome (OMS) cross-sectional data.
| OMS | ||||
|---|---|---|---|---|
| Controls | ‘High’ | ‘Normal’ | ||
| n | 81 | 48 | 197 | P-value |
| Clinical variables | ||||
| Age (year) | 4·7 ± 3·1 | 3·8 ± 2·2 | 4·4 ± 3·6 | 0·26 |
| Gender ratio (male : female) | 39:42 | 21:27 | 89:108 | 0·87 |
| OMS onset (year) | – | 2·3 ± 1·5 | 1·8 ± 1·0 | 0·01* |
| OMS duration (year) | – | 1·5 ± 2·1 | 2·5 ± 3·7 | 0·06 |
| OMS severity (TS) | – | 14·5 ± 7·7 | 13·0 ± 8·1 | 0·29 |
| Laboratory variables | ||||
| CSF leucocytes/cu mm | 1·2 ± 1·2 | 5·9 ± 10·2 | 1·9 ± 3·0 | < 0·0001*† |
| CSF lymphocyte subsets (%) | ||||
| NK | 7·0 ± 4·2 | 3·7 ± 1·9 | 4·8 ± 3·3 | < 0·0001*† |
| CD19+CD3– | 1·9 ± 2·1 | 4·0 ± 3·7 | 2·9 ± 3·1 | < 0·0001*† |
| CSF cytokines (pg/ml) | ||||
| BAFF (CSF) | 144 ± 88 | 223 ± 184 | 124 ± 73 | < 0·0001*† |
| CXCL13 (CSF) | 1·7 ± 5·2 | 21 ± 42 | 4·1 ± 5·8 | < 0·0001*† |
| CXCL9 (serum) | 79 ± 83 | 103 ± 240 | 59 ± 78 | 0·09 |
| CXCL10 (serum) | 109 ± 51 | 259 ± 399 | 113 ± 66 | 0·001*† |
| CXCL11 (serum) | 37 ± 59 | 105 ± 177 | 31·0 ± 45·3 | 0·005* |
| CSF OCB-positive (%) | 0 | 50 | 31 | 0·21 |
| CSF OCB no. | 0 | 2·9 ± 3·2 | 1·7 ± 2·9 | 0·08 |
| CSF CD4+CXCR3+ (%) | 61 ± 22 | 73 ± 26 | 77 ± 12 | 0·42 |
| Blood CD4+CXCR3+ (%) | 13·7 ± 4·7 | 16 ± 7 | 19 ± 9 | 0·26 |
Significant, but uncorrected, P values.
The Bonferroni correction was calculated separately for clinical variables (P < 0·01) and for laboratory variables (P < 0·004). Except for categorical variables, data are means ± standard deviation; n for oligoclonal bands (OCB) in controls was 17. Three-group comparisons were made by analysis of variance (anova) and two-group comparisons by t-tests, except for ratios, which were analysed by χ2. For all significant anovas, post-hoc comparisons of means were significant at the *** level between controls versus ‘high’, as well as ‘high’ versus ‘normal’, except for serum CXCL10 and CXCL11, which were at the ** level of significance. Also the % natural killer (NK) cells was higher in controls than OMS, but not between OMS groups. BAFF: B cell activating factor BAFF; CSF: cerebrospinal fluid.
When only the untreated OMS group was used in the analysis, the effects were similar but weakened by the small sample size. The percentage of CSF CD19+ B cells was higher in the ‘high CXCL10’ subgroup (6·8 ± 4·2%) compared to the ‘normal CXCL10’ (3·6 ± 1·6%) (P = 0·009) and to neurological controls (P < 0·0001). CSF CXCL13 was higher in the high CXCL10 group (P = 0·004).
Relation of CSF CXCL10 to clinical data
In controls, CXCL10 concentration in CSF (P = 0·99) or serum (P = 0·09) did not correlate with patient age. In OMS, no correlation was found for CSF (P = 0·50) or serum (P = 0·65). Using the entire OMS data set, the CSF concentration of CXCL10 did not correlate with OMS total score or OMS duration.
In the ‘high’ CSF CXCL10 group, OMS duration was slightly shorter than in the ‘normal’ CXCL10 group. A similar trend was seen among OMS duration categories. If there was a weak trend for OMS severity, it was not supported by analysis of OMS severity category. No group differences were found in patient age, gender, tumour incidence or relapse.
Longitudinal treatment
In the longitudinal study (Table 2), no net effect of ACTH- or steroid-combination immunotherapies on CXCL10 concentration in CSF was demonstrated. In contrast, clinical severity (total score) had decreased by 66% across ACTH-based treatments (P = 0·0001). ACTH dose in the combined ACTH groups was tapered to 15·0 ± 7·6 IU/m2 on alternate days.
Table 2.
Effect of prospective immunotherapy on cerebrospinal fluid (CSF) CXCL10 concentration and total score.
| (CXCL10) pg/ml | Reduction in total score | |||||
|---|---|---|---|---|---|---|
| Agents | n | Pretreatment | Treatment | P-value | P-value | |
| ACTH + IVIG | 10 | 224 ± 180 (178) | 173 ± 77 (207) | 0·31 | 55% | < 0·0001* |
| ACTH + IVIG + rituximab | 20 | 181 ± 145 (128) | 190 ± 129 (138) | 0·66 | 72% | < 0·0001* |
Statistical comparisons of means were made by paired t-tests. Patients were on immunotherapy 10·0 ± 3·3 months after the first evaluation in the first group and 7·5 ± 2·6 months in the second group. The data are means ± standard deviation; medians are in parentheses. The mean adrenocorticotrophic hormone (ACTH) dose (IU/m2/day) was 17 ± 11 for the first group and 15 ± 7 for the second (not significantly different). In controls, the CSF CXCL10 concentration was 84 ± 54 pg/ml. IVIG: intravenous immunoglobulin.
Analysis of individual patients
Serial CSF CXCL10 data were available from 93 patients who had had three or more lumbar punctures. The data were graphed and inspected visually. The number of patients with relapse was 21 (22%). Of those, a spike in CSF CXCL10 concentration was seen in 13 (62%). In contrast, a drop in CXCL10 linked to treatment was found in only 13 (15%) of the 73 non-relapsing patients. The clinical/immunological pattern for six patients, whose data was replicated with all the samples on one assay plate to eliminate interassay variability, is shown in Fig. 3.
Fig. 3.
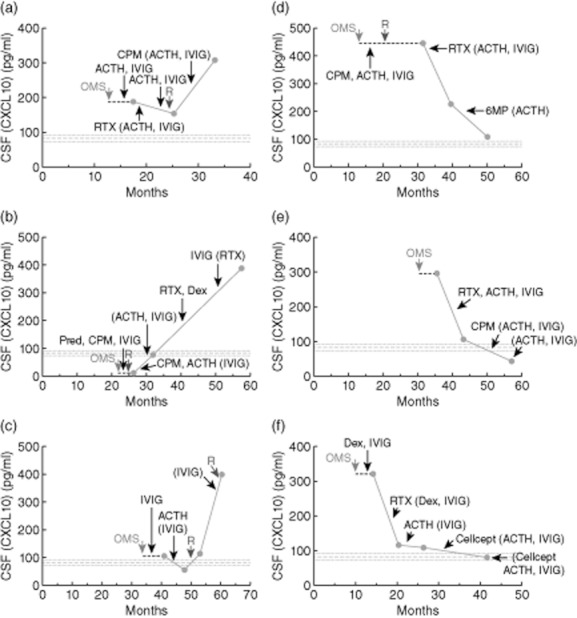
Temporal relation of cerebrospinal fluid (CSF) CXCL10 concentration to relapse (a–d) and immunotherapy (e,f) in six children with opsoclonus–myoclonus syndrome (OMS). (a) A boy status/post (S/P) neuroblastoma with OMS onset at 13 months and our initial evaluation at 17 months. (b) A girl S/P neuroblastoma with OMS onset at 21 months and our first evaluation at 26 months. (c) A girl S/P neuroblastoma with OMS onset at 36 months and our first evaluation at 41 months. (d) A boy S/P neuroblastoma; OMS onset, 14 months; initial evaluation 31 months. (e) A boy without tumour; OMS onset, 31 months; initial evaluation, 32 months. (f) A girl S/P neuroblastoma; OMS onset, 11 months; initial evaluation, 14 months. Cellcept: mycophenolate mofetil; CPM: cyclophosphamide; Dex: pulse dose dexamethasone; 6MP: 6-mercaptopurine; Pred: prednisone; R: relapse of OMS; RTX: rituximab. Parentheses indicate continuing treatments.
Serum CXCL9 and CXCL11
There were no significant group differences in mean serum CXCL9 concentration (P = 0·06, anova): controls 48·1 ± 39 pg/ml; untreated OMS, 75·0 ± 75 pg/ml (Fig. 4b). Serum CXCL11 concentration also did not differ between controls (46·2 ± 69·4 pg/ml) and OMS (114 ± 231 pg/ml) (Fig. 4c). The OIND group had a higher median concentration of CXCL11 (P = 0·048) and CXCL10 (P = 0·017), not CXCL9, than controls, but was not significantly different from untreated OMS. The serum concentrations of CXCL9 and CXCL10 were correlated (r = 0·57, P < 0·0001). Serum CXCL11 and CXCL10 concentrations were not correlated, whether numerous CXCL11 zero values were included (P = 0·10, n = 56) or excluded (P = 0·12, n = 39).
Fig. 4.
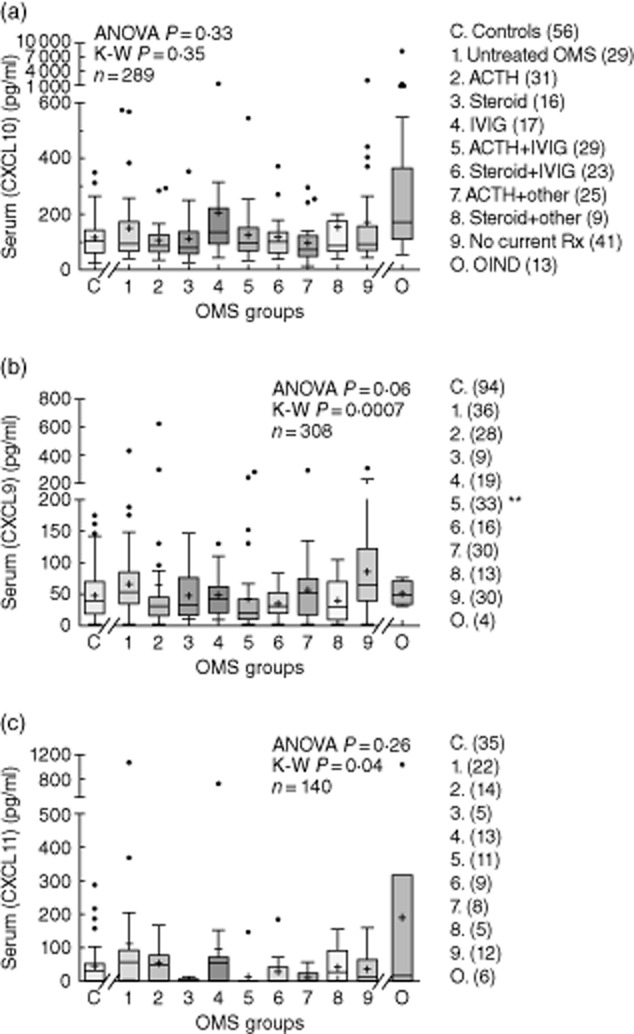
Serum concentrations of CXCL10 (a), CXCL9 (b), and CXCL11 (c). The other inflammatory neurological disorders (OIND)group, not used for statistical analysis, is shown for comparison. The asterisk denotes statistical significance on the Dunn's post-hoc test.
CXCR3 receptors
The frequency of CSF and blood CXCR3+CD4+ T cells was correlated (r = 0·59, P < 0·0001). CXCR3+CD4+ T cells were enriched significantly in CSF compared to blood, as reflected in representative dot-plots (Fig. 5a), in both controls (61% versus 14%) and OMS (79% versus 20%) (Table 3). However, the percentage of CXCR3+ T cells did not differ significantly between controls and untreated OMS in either CSF or blood. Conventional immunotherapy, such as ACTH, steroids or IVIG, also had no significant impact on the percentage of CXCR3+ T cells in CSF or blood. The frequency of CXCR3-expressing CD4+ T cells was not significantly higher in the tumour group. In CSF, CD4+CXCR3+ T cell frequency and CXCL10 concentration were not correlated (P = 0·64). Also, blood CD4+CXCR3+ T cell frequency did not correlate with serum CXCL10 concentration (P = 0·39).
Fig. 5.
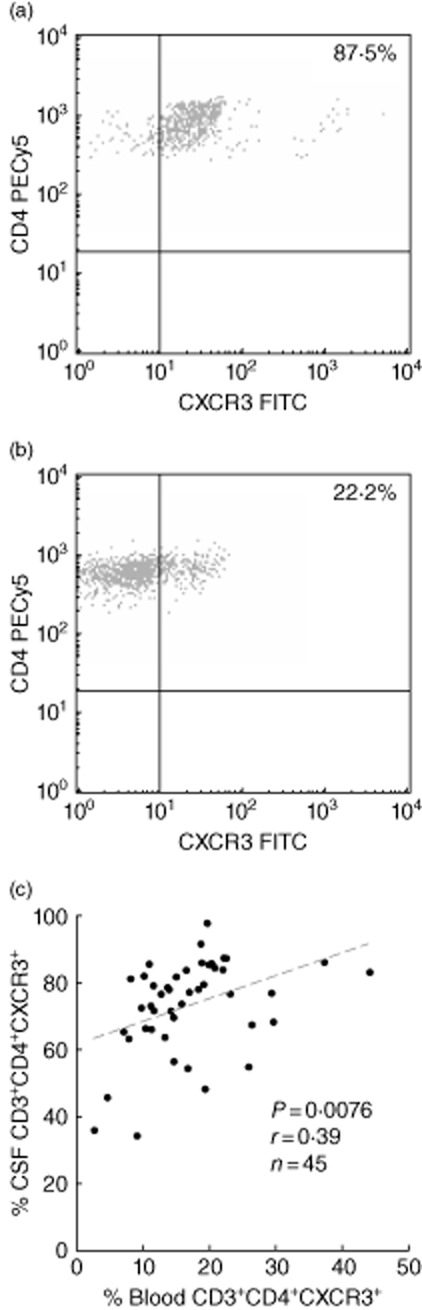
Representative two-colour immunofluorescence analysis of CXCR3+CD4+ T cells in (a) cerebrospinal fluid (CSF) and (b) blood from a patient with untreated opsoclonus–myoclonus syndrome (OMS). (c) Correlation of CSF and blood CXCR3+CD4+ T cells.
Table 3.
Frequency of CXCR3-expressing CD4+ T cells.
| Controls | Untreated OMS | Treated OMS | P-value | Tumour | No tumour | P-value | |
|---|---|---|---|---|---|---|---|
| CSF (%) | 61·0 ± 22 | 78·9 ± 10 | 72·3 ± 15 | 0·2 | 81·5 ± 4·2 | 77·7 ± 8 | 0·08 |
| Blood (%) | 13·7 ± 4·7† | 19·6 ± 10† | 16·6 ± 8·2† | 0·5 | 15·2 ± 7·0† | 20 ± 10† | 0·1 |
| CSF : blood | 4·1 ± 2·7 | 4·9 ± 2·5 | 5·3 ± 2·5 | 0·2 | 5·4 ± 2·8 | 4·8 ± 2·3 | 0·5 |
| n | 4 | 5 | 35 | 19 | 21 | ||
| Age (year) | 2·8 ± 2·0 | 2·7 ± 0·23 | 4·6 ± 3·8 | 0·4 | 4·9 ± 4·2 | 3·7 ± 2·8 | 0·3 |
| Gender (male/female) | 1/3 | 4/1 | 19/16 | 0·2 | 8/9 | 15/8 | 0·2 |
| Mean ± s.d. |
Significant difference compared to cerebrospinal fluid (CSF) by t-test. Statistical tests: analysis of variance (anova) to compare controls, untreated opsoclonus–myoclonus syndrome (OMS), treated OMS; unpaired t-test to compare tumour and no tumour groups; Fisher's exact test for gender comparisons between groups; s.d.: standard deviation.
Discussion
Not demonstrated previously in a paraneoplastic disorder, the concentration of CSF CXCL10 was found to be elevated in paediatric OMS using two different methodologies. The CSF/serum CXCL10 ratio in untreated OMS exceeded that in controls, indicating intrathecal or central overproduction. The association with CSF leucocytosis and up-regulation of BAFF and CXCL13 in the ‘high’ CXCL10 subgroup underscores the presence of CSF inflammation in OMS. Elevated CSF CXCL10 is also noteworthy, because the source of CXCL10 in multiple sclerosis and the experimental autoimmune encephalomyelitis (EAE) animal model has been shown to be the reactive astrocyte 16, which plays a role in the recruitment of inflammatory cells. Whether the same holds true for OMS is unknown. In CSF, our focus was on CXCL10 because it is the only one of the CXCR3 ligands to be present in robust concentration 17, and disease-associated changes in CXCL10 may not be accompanied by changes in CXCL9 or CXCL11 18.
The overall lack of capacity exhibited by various common immunotherapies to modulate CSF CXCL10 concentration was disappointing. Although, in the cross-sectional study, the ACTH monotherapy group had the lowest CSF CXCL10 concentration, patients treated with ACTH combination therapies showed no effect in cross-sectional or longitudinal studies. There are few published data on whether immunotherapy alters CXCL10 concentration in other contexts. In 14 adults with active MS, i.v. methylprednisolone (500 mg × 5 days) reduced median CSF CXCL10 from 279 to 31 pg/ml in 30 days with no effect on serum CXCL10 concentration 19. In another study of 38 acute and 25 stable MS patients, methylprednisolone therapy did not alter the concentration of circulating CXCL10 20. We found no studies about the effects of ACTH or steroids on CXCL10 in children. In vitro, both ACTH and hydrocortisone inhibited only cytokine-induced CXCL10 secretion in human zona fasciculata cells 21.
The increase in CSF CXCL10 we found is not specific for OMS, but comparable in magnitude to that reported in several other diseases, such as multiple sclerosis (3–28-fold) 19,22,23, subacute sclerosing panencephalitis (21-fold) 24, systemic lupus erythematosis (6·3-fold) 25, optic neuritis (1·7-fold) 26, neuroborreliosis 27 and mild Alzheimer disease (2·1-fold) 28. Our paediatric control CSF data fall within the range of adult controls (10–688 pg/ml) 19,24,26. In a study of healthy children, the median CXCL10 serum concentration in those aged < 10 years was slightly lower than in those aged >10 years 29, although we found no significant age effect in our neurological controls. However, those medians (85–98 pg/ml) were similar to our control median of 70 pg/ml in 125 paediatric neurological controls.
CSF CXCL10 studies to date have utilized ELISA, but the multiplexed fluorescent bead-based immunoassay is often used for screening various cytokines. We found no other study using Luminex technology. Our study shows a strong correlation between the two methods, but an approximately 11-fold difference in the results for CSF and twofold for serum. Both methods appear to be valid, but not interchangeable, as has been observed for some cytokines 30.
The CXCR3 receptor, discovered in 1996, is expressed within the CNS in a variety of neuroinflammatory disorders with prominent T cell responses 31. CXCR3-A is the classic form, although other variants exist 32,33. Localization to reactive astrocytes and cerebellar Purkinje cells has been demonstrated 4. Our finding of a higher frequency of CXCR3-bearing CD4+ T cells in control CSF than blood in our study is similar to reports on adults: 64% versus 27%, respectively 26. In CSF, the percentage of CXCR3-expressing CD4+ T cells was not increased in optic neuritis compared to controls 26. In blood of patients with MS, increases, decreases or no changes in the percentage of CXCR3-expressing CD4+ T cells have been found depending on clinical variables 22,23,34–36. Little information is available on the effect of immunotherapy on CXCR3 expression in neuroinflammatory disorders. In asthmatics, a 2-week treatment with low-dose oral prednisolone (20 mg/day) did not change the percentage of CXCR3+ T cells in blood, although it was sufficient to alter the frequency of CCR4+ T cells 37.
In our study, the CSF concentration of CXCL10 did not change greatly over time or with treatment, as also reported in MS 38. As a result, CSF CXCL10 did not correlate with clinical severity of OMS. Our analysis of serial data in individual patients identified some in whom there appeared to be a clinical–immunological relationship. Whether this information could be used to personalize their immunotherapy remains interesting, but untested.
Attenuation of inflammation by blocking CXCR3 remains an attractive therapeutic goal. Discovery of CXCR3 antagonists has been an ongoing process, with efficacy in preclinical disease models for some 39. Our study, in identifying CNS over-production of CXCL10 that responds little to immunotherapy, suggests that a pilot trial of CXCR3 blocker therapy might be considered in paediatric OMS.
Given the disconnect between Th1 cell parameters and OMS parameters, CXCL10 may play alternative roles in OMS. CXCR3 is also expressed on a substantial proportion of B cells in humans 40. Because rituximab effectively depletes CSF and circulating B cell subsets with clinical benefit, it is possible that CXCL10 action in OMS may be more pertinent to B cells. Also, CXCR3 is expressed highly on effector CD8+ T cells 14. Measurement of both CXCR3+ cell types in OMS would be of interest.
Acknowledgments
This study was supported by investigator-sponsored research grants to M.R.P. from the Thrasher Research Fund (grant 02826-2), the Spastic Paralysis Research Foundation (Illinois-Eastern Iowa District of Kiwanis International), the Chicago Institute of Neurosurgery and Neuroresearch (Catherine Gilmore-Lawless and Dr Leonard J. Cerullo), Ronald McDonald House Charities of Central Illinois, and Questcor Pharmaceuticals (Union City, CA). The authors thank the George L. Shields Foundation, Inc. (Robert Brenengan), the Bloomfield Family Trust (Joanne Bloomfield Hunter and Peggy Bloomfield), Cure for OMS (Jennifer R. Harris), and Rhoades Car International for generous research donations. The authors also thank all the families who participated in this research, Ronald McDonald House (Springfield, IL), Miracle Flights for Kids (Green Valley, NV), Kristal J. Adams (Researcher I), Paul W. Phillips (former Researcher I), Tyler J. Allison (former Researcher II), Tammy A. Boyd (Office Support Specialist I) and Carolyn L. Higgason (microcomputer specialist).
Disclosures
M.R.P. was a paid consultant to a summit on Infantile Spasms sponsored by Questcor Pharmaceuticals and held in New York City on 14 June 2010. No funding organization participated in data analysis, manuscript writing or the decision to publish the manuscript. R.M.R. is a member of the Scientific Advisory Board of Chemocentryx and holds stock in the company. He is a member of the Scientific Advisory Board of Vertex.
References
- 1.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Pharmacol Toxicol. 2000;18:217–242. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 2.Lee EY, Lee ZH, Song YW. CXCL10 and autoimmune diseases. Autoimmun Rev. 2009;8:379–383. doi: 10.1016/j.autrev.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Müller M, Carter S, Hofer MJ, et al. Review: the chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity – a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 2010;36:368–387. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg SH, van der Meer P, Hesselgesser J, et al. CXCR3 expression in human central nervous system diseases. Neuropathol Appl Neurobiol. 2001;27:127–138. doi: 10.1046/j.1365-2990.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 5.Klein RS. Regulation of neuroinflammation: the role of CXCL10 in lymphocyte infiltration during autoimmune encephalomyelitis. J Cell Biochem. 2004;92:213–222. doi: 10.1002/jcb.20052. [DOI] [PubMed] [Google Scholar]
- 6.Le Y, Zhou Y, Iribarren P, et al. Chemokines and chemokine receptors: their manifold roles in homeostasis and disease. Cell Mol Immunol. 2004;1:95–104. [PubMed] [Google Scholar]
- 7.Pranzatelli MR, Tate ED. Opsoclonus–myoclonus syndrome. In: Russell CD, Vincent A, editors. Clinics in developmental medicine, no. 184–185, inflammatory and autoimmune disorders of the nervous system in children. London, UK: Mac Keith Press (Cambridge University Press); 2010. pp. 152–173. Chapter 10. [Google Scholar]
- 8.Tate ED, Allison TJ, Pranzatelli MR, et al. Neuroepidemiologic trends in 105 U.S. cases of pediatric opsoclonus–myoclonus. J Pediatr Oncol Nurs. 2005;22:8–19. doi: 10.1177/1043454204272560. [DOI] [PubMed] [Google Scholar]
- 9.Pranzatelli MR, Travelstead AL, Tate ED, et al. B- and T-cell markers in opsoclonus–myoclonus syndrome: immunophenotyping of CSF lymphocytes. Neurology. 2004;62:1526–1532. doi: 10.1212/wnl.62.9.1526. [DOI] [PubMed] [Google Scholar]
- 10.Pranzatelli MR, Tate ED, McGee NR, et al. Key role of CXCL13/CXCR5 axis for cerebrospinal fluid B cell recruitment in pediatric OMS. J Neuroimmunol. 2012;243:81–88. doi: 10.1016/j.jneuroim.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Pranzatelli MR, Tate ED, Hoefgen ER, et al. Therapeutic down-regulation of central and peripheral B-cell activating factor (BAFF) production in opsoclonus–myoclonus. Cytokine. 2008;44:26–32. doi: 10.1016/j.cyto.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Pranzatelli MR, Slev PR, Tate ED, et al. Cerebrospinal fluid oligoclonal bands in childhood opsoclonus–myoclonus. Pediatr Neurol. 2011;45:27–33. doi: 10.1016/j.pediatrneurol.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Pranzatelli MR, Tate ED, Hoefgen ER, et al. Interferon-inducible protein 10 (CXCL10) is increased in the cerebrospinal fluid of children with opsoclonus–myoclonus syndrome. Ann Neurol. 2007;62(Suppl. 11):S112–113. [Google Scholar]
- 14.Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kivisäkk P, Liu Z, Trebst C. Chemokine receptor expression on cerebrospinal fluid leukocytes. Methods. 2003;29:319–325. doi: 10.1016/s1046-2023(02)00355-9. [DOI] [PubMed] [Google Scholar]
- 16.Simpson JE, Newcombe J, Cuzner ML, et al. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2000;26:133–142. doi: 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- 17.Franciotta D, Zardini E, Ravaglia S, et al. Cytokines and chemokines in cerebrospinal fluid and serum of adult patients with acute disseminated encephalomyelitis. J Neurol Sci. 2006;247:202–207. doi: 10.1016/j.jns.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 18.Mahad DJ, Howell SJL, Woodroofe MN. Expression of chemokines in the CSF and correlation with clinical disease activity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;72:498–502. doi: 10.1136/jnnp.72.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreira MA, Tilbery CP, Monteiro LP, et al. Effect of the treatment with methylprednisolone on the cerebrospinal fluid and serum levels of CCL2 and CXCL10 chemokines in patients with active multiple sclerosis. Acta Neurol Scand. 2006;114:109–113. doi: 10.1111/j.1600-0404.2006.00629.x. [DOI] [PubMed] [Google Scholar]
- 20.Franciotta D, Martino G, Zardini E, et al. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J Neuroimmunol. 2001;115:192–198. doi: 10.1016/s0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 21.Rotondi M, Falorni A, De Bellis A, et al. Elevated serum interferon-gamma-inducible chemokine-10/CXC chemokine ligand-10 in autoimmune primary adrenal insufficiency and in vitro expression in human adrenal cells primary cultures after stimulation with proinflammatory cytokines. J Clin Endocrinol Metab. 2005;90:2357–2363. doi: 10.1210/jc.2004-1062. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima H, Fukuda K, Doi Y, et al. Expression of TH1/TH2-related chemokine receptors on peripheral T cells and correlation with clinical disease activity in patients with multiple sclerosis. Eur Neurol. 2004;52:162–168. doi: 10.1159/000081856. [DOI] [PubMed] [Google Scholar]
- 23.Sørensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–815. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saruhan-Direskeneli G, Gürses C, Demirbilek V, et al. Elevated interleukin-12 and CXCL10 in subacute sclerosing panencephalitis. Cytokine. 2005;32:104–110. doi: 10.1016/j.cyto.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto H, Katsumata Y, Nishimura K, et al. Interferon-inducible protein 10/CXCL10 is increased in the cerebrospinal fluid of patients with central nervous system lupus. Arthritis Rheum. 2004;50:3731–3732. doi: 10.1002/art.20598. [DOI] [PubMed] [Google Scholar]
- 26.Sørensen TL, Roed H, Sellebjerg F. Optic neuritis: chemokine receptor CXCR3 and its ligands. Br J Ophthalmol. 2004;88:1146–1148. doi: 10.1136/bjo.2003.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lepej SZ, Rode OD, Jeren T, et al. Increased expression of CXCR3 and CCR5 on memory CD4+ T-cells migrating into the cerebrospinal fluid of patients with neuroborreliosis: the role of CXCL10 and CXCL11. J Neuroimmunol. 2005;163:128–134. doi: 10.1016/j.jneuroim.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Galimberti D, Schoonenboom N, Scheltens P, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006;63:538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 29.Narbutt J, Lesiak A, Sysa-Jedrzeiowska A, et al. The imbalance in serum concentration of Th-1- and Th-2-derived chemokines as one of the factors involved in pathogenesis of atopic dermatitis. Mediators Inflamm. 2009;2009 doi: 10.1155/2009/269541. Article ID 269541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SS, Smith MS, Reda D, et al. Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61:35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 31.Cartier L, Hartley O, Dubois-Dauphin M, et al. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Lazzeri E, Romagnani P. CXCR3-binding chemokines: novel multifunctional therapeutic targets. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:109–118. doi: 10.2174/1568008053174723. [DOI] [PubMed] [Google Scholar]
- 33.Korniejewska A, McKnight AJ, Johnson Z, et al. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes. Immunology. 2011;132:503–515. doi: 10.1111/j.1365-2567.2010.03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balashov KE, Rottman JB, Weiner HL, et al. CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–6878. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Putheti P, Morris M, Stawiarz L, et al. Multiple sclerosis: a study of chemokine receptors and regulatory T cells in relation to MRI variables. Eur J Neurol. 2003;10:529–535. doi: 10.1046/j.1468-1331.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- 36.Sindern E, Patzold T, Ossege LM, et al. Expression of chemokine receptor CXCR3 on cerebrospinal fluid T-cells is related to active MRI lesion appearance in patients with relapsing–remitting multiple sclerosis. J Neuroimmunol. 2002;131:186–190. doi: 10.1016/s0165-5728(02)00263-1. [DOI] [PubMed] [Google Scholar]
- 37.Kurashima K, Fujimura M, Myou S, et al. Effects of oral steroids on blood CXCR3+ and CCR4+ T cells in patients with bronchial asthma. Am J Respir Crit Care Med. 2001;164:754–758. doi: 10.1164/ajrccm.164.5.2008132. [DOI] [PubMed] [Google Scholar]
- 38.Sørensen TL, Sellebjerg F, Jensen CV, et al. Chemokines CXCL10 and CCL2: differential involvement in intrathecal inflammation in multiple sclerosis. Eur J Neurol. 2001;8:665–672. doi: 10.1046/j.1468-1331.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 39.Jenh CH, Cox MA, Cui L, et al. A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol. 2012;13:2. doi: 10.1186/10.1186/1471-2172-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanki T, Takada K, Komano Y, et al. Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2009;11:R149. doi: 10.1186/ar2823. [DOI] [PMC free article] [PubMed] [Google Scholar]


