Abstract
This study examines adenosine 5′-triphosphate-binding cassette (ABC) transporters as a potential therapeutic target in dendritic cell (DC) modulation under hypoxia and lipopolysaccharide (LPS). Functional capacity of dendritic cells (DCs) (mixed lymphocyte reaction: MLR) and maturation of iDCs were evaluated in the presence or absence of specific ABC-transporter inhibitors. Monocyte-derived DCs were cultured in the presence of interleukin (IL)-4/granulocyte–macrophage colony-stimulating factor (GM-CSF). Their maturation under hypoxia or LPS conditions was evaluated by assessing the expression of maturation phenotypes using flow cytometry. The effect of ABC transporters on DC maturation was determined using specific inhibitors for multi-drug resistance (MDR1) and multi-drug resistance proteins (MRPs). Depending on their maturation status to elicit T cell alloresponses, the functional capacity of DCs was studied by MLR. Mature DCs showed higher P-glycoprotein (Pgp) expression with confocal microscopy. Up-regulation of maturation markers was observed in hypoxia and LPS-DC, defining two different DC subpopulation profiles, plasmacytoid versus conventional-like, respectively, and different cytokine release T helper type 2 (Th2) versus Th1, depending on the stimuli. Furthermore, hypoxia-DCs induced more B lymphocyte proliferation than control-iDC (56% versus 9%), while LPS-DCs induced more CD8-lymphocyte proliferation (67% versus 16%). ABC transporter-inhibitors strongly abrogated DC maturation [half maximal inhibitory concentration (IC50): P-glycoprotein inhibition using valspodar (PSC833) 5 μM, CAS 115104-28-4 (MK571) 50 μM and probenecid 2·5 μM], induced significantly less lymphocyte proliferation and reduced cytokine release compared with stimulated-DCs without inhibitors. We conclude that diverse stimuli, hypoxia or LPS induce different profiles in the maturation and functionality of DC. Pgp appears to play a role in these DC events. Thus, ABC-transporters emerge as potential targets in immunosuppressive therapies interfering with DCs maturation, thereby abrogating innate immune response when it is activated after ischaemia.
Keywords: ABC transporter blockers, dendritic cells maturation, immunosuppression, P-glycoprotein
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells whose differentiation, migration and activities are linked intrinsically to the microenvironment. The capacity of DCs to activate and regulate T cell responses is acquired during a complex differentiation and maturation programme 1,2. DCs originate in bone marrow, and at an immature stage (iDC) they migrate through diseased peripheral tissue before reaching their final destination in the lymph node 1,3,4. In view of their physiological function, DCs play an important role in adverse immune reactions such as autoimmune diseases, chronic inflammatory processes and allograft rejection 2,5. Thus, modulation of DC function is a promising strategy in the treatment and prevention of such diseases 6,7. Furthermore, their ability to change phenotype and function, depending on their stage of maturation, is an interesting target in immune system modulation towards tolerance in solid organ transplantation.
One of the most obvious scenarios in which hypoxia may play a role in immune-mediated renal damage is the transplantation setting. It is clear that ischaemia– reperfusion injury during transplantation contributes to the adaptive and innate immune response. In recent years, DCs have been studied regarding their important role in immune response as a bridge between innate and acquired immune responses 1,4,5. In a previous report we investigated the functional changes shown by immature DCs (iDCs) after hypoxia-induced differentiation 8. In that study we confirmed that hypoxia, similar to allogeneic stimulus, induced maturation of DCs, which was associated with an increase in hypoxia-inducible factor (HIF)-1α protein levels and was attenuated by mammalian target of rapamycin inhibition. We presented hypoxia as a novel maturation signal not only for monocyte-derived DCs, but also for renal resident iDCs exposed to ischaemia 8. This new mechanism for renal DC maturation invites speculation about the role of these cells in the immune-mediated response to renal ischaemia. Thus, we might hypothesize that ischaemia-induced maturation of renal DCs drive their migration to regional lymph nodes, as well as bringing about T cell activation and additional immune-mediated damage to the kidney.
Proteins of the adenosine 5′-triphosphate-binding cassette (ABC) transporter superfamily are involved in the active transport of a broad range of substrates, ranging from xenobiotics, peptides and proteins to sugars, metal ions and lipids 9,10. The primary role of these molecules in various physiological processes is as an efflux pump, conferring resistance by driving out cytotoxic xenobiotics, toxic molecules and various cellular products 11,12. ABC proteins identified for their role in cancer multi-drug resistance (MDR) chemotherapy are the MDR1 gene-encoded P-glycoprotein (Pgp; ABCB1) 13 and multi-drug resistance protein 1 (MRP1; ABCC1) 14–16. In fact, ABC transporters are described fully in nephrotoxicity models in kidney allografts, and play a key role in the pharmacokinetics of many immunosuppressors. Pgp and MRP1 have been found to be expressed in skin DC and monocyte-derived DC (interstitial DC), and functionally, both transporters have been described as being required for efficient DC maturation and T cell migration 12. In this field, Pgp is implicated in interleukin (IL)-12 secretion, resulting in the activation of nuclear factor kappaB (NF-κB) in DCs, which is a key event in the initiation of DC maturation 12.
As DCs are the most potent antigen-presenting cells of the immune system, it is important to know which molecules are essential in their function. ABC transporters, Pgp and MRP1, have already been shown to be required for DC differentiation and maturation after tumour necrosis factor (TNF)-α stimuli 17. During hypoxia, extracellular adenosine 5′-triphosphate (ATP) levels often increase and these extracellular ATP act as a find me signal for many phagocytic cells, including DCs. Thus, it is important to understand the effects of hypoxic environment on local or lymph node DCs and other immune cells. As the putative contribution of ABC transporters as well as other mechanisms defined previously in studies of drug resistance to DC functioning is still relatively unknown, we were tempted to explore this issue under hypoxic conditions.
Notably, immune responsiveness might benefit from such mechanisms. Thus, we aimed to study whether ABC transporters were also essential in maturation of DCs in a hypoxic microenvironment, a well-known stimulus in pathological events such as ischaemia–reperfusion injury. Modulation of DC hypoxia-related maturation through ABC transporters could be an interesting target to reduce immunoinflammatory responses in organ transplantation.
Materials and methods
Antibodies and reagents
The following monoclonal antibodies were obtained from Becton Dickinson Pharmingen (San Diego, CA, USA): anti-human CD3-allophycocyanin (APC), CD20-phycoerythrin (PE), CD14-APC, CD11c-PE-cyanin 5 (Cy5), CD40-fluorescein isothiocyanate (FITC), CD80-APC, CD83-APC, CD86-FITC, CD54-APC and human leucocyte antigen D-related (HLA-DR)-FITC. Mouse anti-human JSB1 (Pgp) (Calbiochem, Darmstadt, Germany), rat anti-human 4124 (MRP) (Chemicon International, Temecula, CA, USA), anti-human DC-lysosomal-associated membrane protein (LAMP) (T-20; Santa Cruz, Madrid, Spain) and secondary antibodies were purchased from Invitrogen (Molecular Probes, Eugene, OR, USA) and 4′,6-diamidino-2-phenylindole (DAPI) mounting medium from Santa Cruz (Madrid). The MDR1 Pgp antagonist PSC833 was provided by Novartis AG (Basel, Switzerland). Purified recombinant human IL-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) were purchased from R&D Systems (Minneapolis, MN, USA). Lipopolysaccharide (LPS) (Escherichia coli serotype 011:B4) and phytohaemagglutinin (PHA) were purchased from Sigma-Aldrich (Madrid, Spain) and MK571 was obtained from Alexis Biochemicals (Grupo Taper SA, Madrid, Spain). Medium and supplements were purchased from PAA (Linz, Austria) and Lonza (Verviers, Belgium). Annexin-V and 7-aminoactinomycin D (7-AAD) were purchased from Sigma-Aldrich (Madrid). Anti-human HIF-1α-fluorescein monoclonal antibody and mouse immunoglobulin (Ig)G1 isotype control-CFS was obtained from R&D Systems. Cytometric bead array (CBA) and carboxyfluorescein diacetate succinimidyl ester (CFSE) were from Molecular Probes (Madrid, Spain).
Ethics statement
Human cells were obtained in accordance with protocols approved by the Ethics Committee of the Hospital Bellvitge of Barcelona (Barcelona, Spain) and in accordance with the principles of the Declaration of Helsinki.
Dendritic cell culture and drug treatment
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll density gradient centrifugation of blood obtained from buffy coats from healthy donors. PBMCs (200 × 106 cells/ml) were incubated for 2 h at 37°C in 5% CO2 in 25 cm2 flask plates. After washing, the adherent monocytes were cultured in the presence of 500 U/ml of IL-4 and 1000 U/ml of GM-CSF in RPMI-1640 medium with 10% human serum at 37°C in a humidified atmosphere of 5% CO2, obtaining 90% DC purity at day 7. ABC inhibitors were added once after 48 h of monocyte isolation: MDR1 inhibitor (PSC833, 5 μM), MRP1 and MRP2 inhibitors (MK571, 50 μM) and probenecid (PBN), 2·5 μM. Cells were kept at 37°C in a humidified atmosphere with 5% CO2. Medium with supplements and inhibitors was changed every second day and prior to experiments. The gating of DC populations was validated in our previous study 8. Lymphocytes were obtained by Ficoll-Percoll gradient and purified by non-adherence.
Dendritic cell differentiation under LPS and hypoxia stimuli
Immature DCs (2 × 106 cells/ml RPMI 10% human serum) were exposed at day 5 to hypoxia conditions for 48 h 8. Hypoxic (0·5% oxygen) conditions were generated at day 5, exposing iDCs to hypoxia (0·5% O2, 5% CO2) in a hypoxia atmosphere-controlled incubator (Binder), keeping cells unmanipulated for 48 h, thereby avoiding O2 pressure changes. To compare with a standard stimulus for DCs maturation, LPS (2 μg/ml) was added for 24 h at day 6 after PBMC isolation.
Cell phenotype analysis by flow cytometry
Flow cytometry (fluorescence-activated cell sorting: FACS) analysis was performed using a FACS Canto and diva software (Becton Dickinson). The study subpopulation was defined using different cell markers: CD3 for lymphocytes, CD14 for monocytes, CD20 for B cells and CD56 to stain natural killer (NK) cells. Thereafter, FACS was performed at day 7 of DCs to assess mean fluorescence and expression of mature cell phenotype. CD14, CD11c and CD123 were used to identify the DC nature and different markers were used to define the mature population of DCs (mDCs) (CD40/CD80/CD83/CD86/CD54/HLA-DR). To assess the DC phenotype, we used the markers according to standard methods in the literature for DCs 18–20. Incubation was carried out at 4°C for 30 min. Apoptosis was measured by annexin-V using flow cytometry.
HIF-1α protein expression on hypoxia DCs ± ABC transporter inhibitors
Intracellular HIF-1α was assessed by flow cytometry (FACS Canto; Becton Dickinson). DCs were identified with two membrane markers as HLA-DR+ and CD11c+. After phenotyping, cells were permeabilized with saponine buffer (Sigma, Madrid) and labelled with HIF-1α or isotype control (R&D Systems). Intracellular HIF-1α was analysed in the double-positive region for HLA-DR+ and CD11c+.
Analysis of Pgp and MRP1 expression (iDC and mDC) by immunofluorescence
To assess Pgp and MRP1 expression in iDCs and mDCs, double-surface immunostaining and dual-colour flow cytometry of freshly isolated PBMCs were carried out following incubation overnight at 37°C in human serum. Cells were washed twice in phosphate-buffered saline (PBS), fixed with cold methanol for 3 min and washed twice with PBS. DCs were blocked with fetal bovine serum (FBS) 20% for 2 h. For indirect staining of Pgp in mDCs, 0·5 × 106 DCs were incubated overnight at 4°C with the primary anti-Pgp monoclonal antibody (mAb) JSB1 (1/50 with FBS 10%), anti-MRP1 mAb (4124) and DC LAMP antibody (1/50 with FBS 10%). Before incubation, cells were permeabilized to anti-Pgp mAb JSB1 incubation. After incubation, cells remained for 30 min at room temperature. The DCs were then incubated with the secondary antibodies Alexa 647 and Alexa 488 (1/100 with FBS 1%) for 45 min and washed. Finally, DCs were mounted in DAPI. Analysis of cell surface marker expression was performed using the dual-colour inmunofluorescence technique (Leica TCS-SL confocal espectral microscope, Mannheim, Germany) equipped with image analysis software (Leica confocal software).
Cell proliferation CFSE assay by flow cytometry
Distinguishing DCs from monocytes was also defined functionally by the ability to stimulate an allogeneic mixed leucocyte reaction (MLR) 20,21. Thus, we tested not only phenotypical changes, but also functionally tested CD3 proliferation. We performed a CFSE study to analyse the effector function of these DCs; the results supported the phenotypical changes and also emphasized the distinction from macrophages. Lymphocytes were stained with CFSE and exposed to mDCs (under hypoxia or LPS stimuli) with or without ABC transporter inhibitors. After 24 h, medium was removed and co-culture was performed with fresh medium. Allogeneic CFSE-labelled PBMCs (2 × 105) were cultured alone (negative control) or in the presence of DCs collected at the end of the 7-day culture after stimuli exposure (DC : T cell ratio 1:10; final volume 200 μl RPMI 10% FBS). As positive control responder, PBMCs were stimulated with 1 μg/ml (PHA). After 5 days of culture (37°C, 5% CO2) the proliferation of responder cells was determined by flow cytometry after labelling with CD20, CD4 and CD8 antibody to exclude DCs and to define different B and T lymphocyte subpopulations. No ABC transporter inhibitors were used in T and DC co-cultures.
In addition, MLR with purified T and B cells was performed with the RosetteSep human T cell enrichment cocktail and the RosetteSep human B cell enrichment cocktail, respectively (Stemcell Technology, Grenoble, France) After cell isolation the MLR technique was carried out as described.
Cell cytokine analysis by flow cytometry
Flow cytometry analysis was performed using FACS Canto and diva software (Becton Dickinson). Interleukin-2, -4, -6, -10, -17a, TNF-α and interferon (IFN)-γ secretion protein levels from cell supernatant were measured quantitatively following cell stimulation by CBA (BD Biosciences). Cytokine quantification was performed on stimulated and non-stimulated, and treated and non-treated (with ABC inhibitors) DCs, and on lymphocytes after MLR.
Statistical analysis
Each experiment was performed at least three times and representative data are shown. Significant differences between samples were determined by analysis of variance (anova) with the spss statistical package. Data in bar graphs are given as the mean ± standard deviation (s.d.). A value of P < 0·05 was considered significant.
Results
Analysis of monocyte, iDC and mDC populations after hypoxia and LPS
Monocytes were isolated and cultured with GM-CSF and IL-4; the resulting iDCs were exposed to hypoxia on day 5 for 48 h or to LPS for 24 h to induce cell maturation. Figure 1a shows the analysis of different cellular subpopulations during the differentiation and maturation of DCs. At day 0 we had a high percentage of monocytes (CD14+) and the presence of several lymphocyte subtypes (CD3+, CD20+ and CD56+). During differentiation, the CD14+ population expressed DCs markers (HLA-DR+ and CD11c+) and the lymphocyte percentage diminished after removing the medium and replacing it with fresh culture medium. At the end of the differentiation (at day 7) the purity of DCs was greater than 90% (Fig. 1b). DC population was gathered in two subpopulations, depending on the degree of maturation according to the forward-/side-scatter profile and specific phenotypic markers established in our previous study 8. We also performed a follow-up of DC differentiation at different time-points. We observed that after hypoxia or LPS stimulus, cells changed their morphology, acquiring a stellate form characteristic of the mDCs shifting to the upper window. LPS stimulus induced a more homogeneous and stronger maturation response, while hypoxia stimulus showed a different magnitude of response (Fig. 1b). To evaluate further the changing phenotype after stimuli of the DC population, FACS analysis was performed at days 1, 5 and 7. CD40 mean fluorescence revealed that mDCs appeared at day 5 of decreasing monocytes and iDCs populations. After LPS and hypoxia stimuli at day 7, DCs were well differentiated from non-stimulated cells.
Fig. 1.
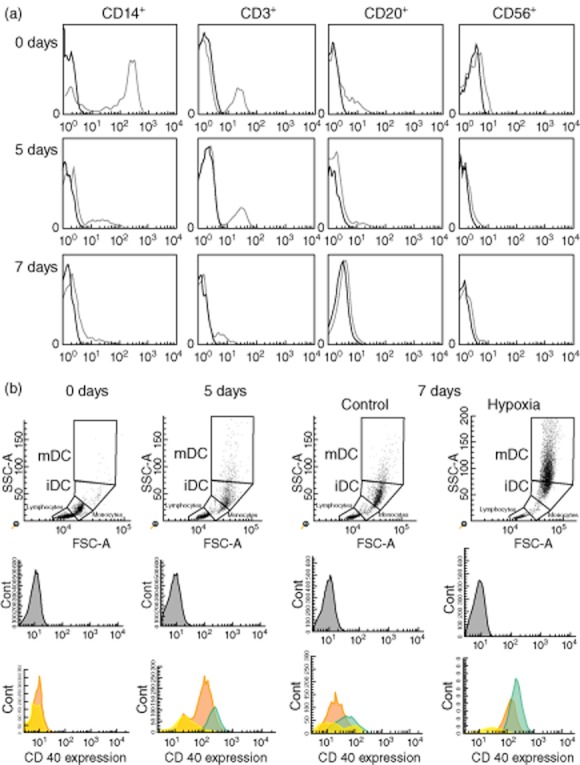
(a) Analysis of different cellular subpopulations during the differentiation and maturation of dendritic cells (DCs). Cells were stained at different times (days 0, 5 and 7) from isolation by antibodies specific for monocytes, T, B and natural killer (NK) cells in control condition (CD14+, CD3+, CD20+ and CD56+, respectively). (b) Follow-up of increasing dendritic cell maturation under hypoxia by flow cytometry. At day 0, monocytes were cultured with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4. At day 5, immature dendritic cells (iDCs) were stimulated for a further 48 h with hypoxia, acquiring mature phenotype with high expression of CD40+ (mDCs) with respect to non-stimulated cells (control). The percentage (%) of monocytes is shown in yellow, iDC in orange and mDC in green.
Analysis of Pgp and MRP1 expression in iDC and LPS/hypoxia-DCs by immunofluorescence
To characterize mDCs we used DC-LAMP, a type I transmembrane glycoprotein restricted to mDCs and expressed in the endosomal/lysosomal compartment. DCs exposed to LPS or hypoxia showed a clear DC LAMP-positive up-regulation, confirming the mature phenotype. Dual staining with the Pgp (JSB1) or MRP1 (4124) antibodies also showed an over-expression of Pgp and MRP1 in those DC-LAMP-positive DCs, differing from non-stimulated cells (P < 0·05) (Fig. 2a,b, respectively). This may indicate that in DC maturation there is an increase in Pgp and MRP1 in the cell membrane. Furthermore, this effect was more evident after LPS stimuli than after hypoxia.
Fig. 2.
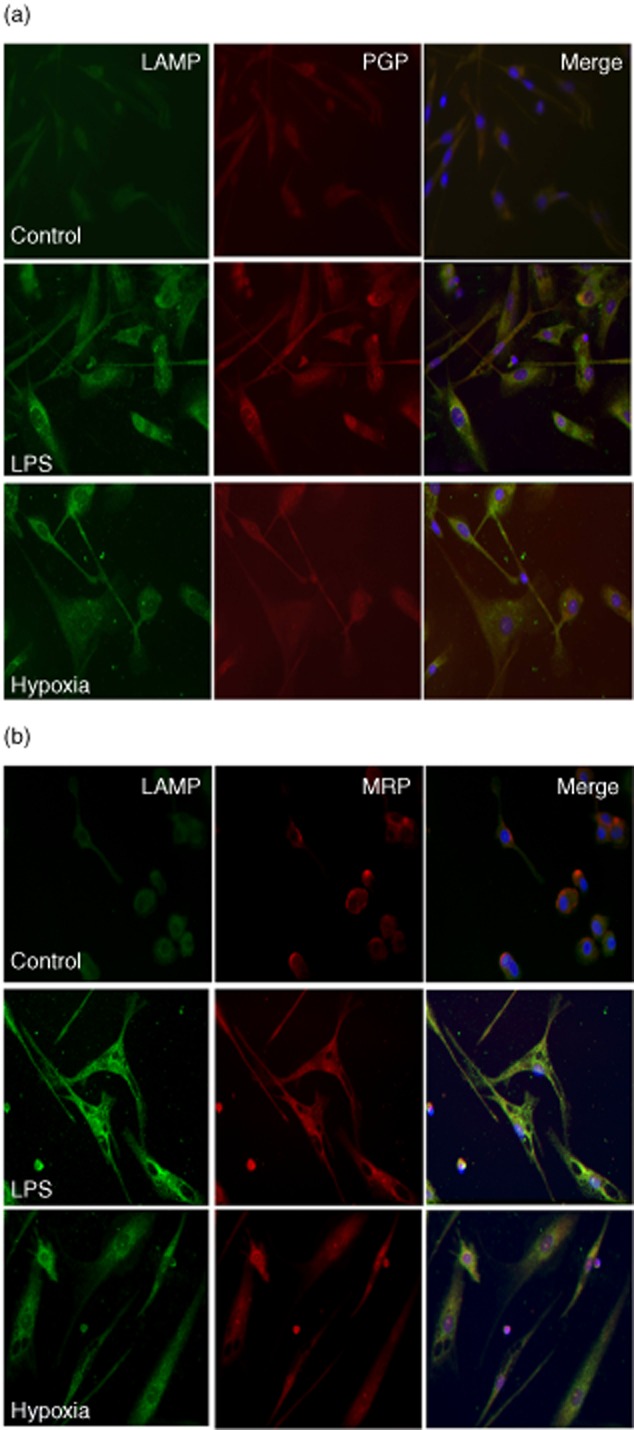
Over-expression of P-glycoprotein (Pgp) and multi-drug resistance protein 1 (MRP1) by immunofluorescence in dendritic cells (DCs) after hypoxia and lipopolysaccharide (LPS). Two-colour immunofluorescence images were obtained from DC lysosomal-associated membrane protein (LAMP) antibody [first panel of each column (1), green] and P glycoprotein antibody (JSB1) Pgp (a) or MRP1 (b) antibody [second panel of each column (2), red]. The two images were merged and double-positive cells are shown in the third panel of each row (3). iDCs appear in the first row, LPS-DC in the second row and hypoxia-DCs in the third row.
Effect of ABC transporter blockade in hypoxia and LPS DC maturation
To evaluate the ABC transporters involvement in DC maturation, PSC833, MK571 or PBN were added to inhibit MDR1, MRP1 and MRP2, respectively.
After hypoxia stimulation the percentage of mature DCs was evaluated by the forward-/side-scatter profile. Hypoxia resulted in an induction of 67·8% of mDCs versus 32·2% of iDCs (Fig. 3), lower compared to LPS, which induced 80·8% of mDCs and 19·2% of iDCs (P < 0·05). The addition of ABC transporter inhibitors shifted the ratio of mature and immature DCs achieved after both stimuli (Fig. 3) (P < 0·05). MDR1 and MRP inhibitors induced a marked decrease in mDCs [half maximal inhibitory concentration (IC50): P-glycoprotein inhibition using valspodar (PSC833 5 μM, CAS 115104-28-4 (MK571) 50 μM and probenecid 2·5 μM] and an increase in iDCs. Thus, after hypoxia, PSC inhibited mDCS to 31·4% (P < 0·05), MK571 to 40% (P < 0·05) and PBN to 45·6% (P < 0·05). The effect of ABC blockers on DC maturation after LPS showed similar results: PSC833 reduced mDCS to 48·8% (P < 0·05), MK571 to 51·6% (P < 0·05) and PBN to 50·6% (P < 0·05). All mean values were analysed for 10 experiments.
Fig. 3.
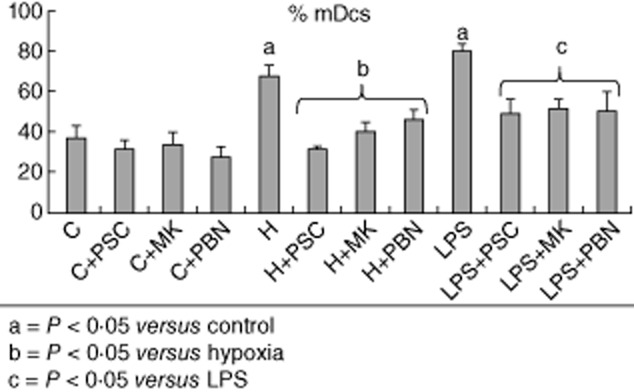
Effect of P-glycoprotein (Pgp) blockade in dendritic cells (DCs) after hypoxia and lipopolysaccharide (LPS) maturation. After DC maturation stimuli (hypoxia or LPS), the percentage of mDCs increased with respect to non-stimulated cells. Additionally, the Pgp inhibitors blockaded the maturation of the DCs. Each solid bar represents the average percentage of 10 different experiments [P < 0·05 versus non-stimulated cells and adenosine 5′-triphosphate-binding cassette (ABC) transporter inhibitors].
To rule out a toxic effect of inhibitors on DC viability, cell apoptosis was analysed by annexin V/7-ADD assay. A comparable percentage of viable cells was observed after hypoxia exposure with or without ABC inhibitors exposure (H: 86·1%, H + PSC: 84·25%, H + MK: 85·29% and H + PBN: 83·7%). We found no statistical changes between hypoxia DC and non-stimulus.
Phenotype switch on DC maturation: effect of ABC transporter blockade
Hypoxic conditions induced a twofold DC maturation compared to control non-stimulated DCs (P < 0·05), analysed as intensity of different maturation markers (CD40, CD83, HLADR and CD54). This confirmed the results validated in a previous study 8. ABC inhibitors showed a clear decrease in both plamacytoid-like and conventional DC phenotype maturation, depending on the stimuli (Table 1).
Table 1.
Representative fluorescence activated cell sorter (FACS) mature phenotype markers for dendritic cells (DCs).
| Control | LPS | Hypoxia | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | LPS | LPS+PSC | LPS+MK | LPS+PBN | H | H + PSC | H + MK | H + PBN | ||||||||||
| % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | % | MFI | |
| Presentation molecules | ||||||||||||||||||
| MHC II | 96·5 | 549·9 | 98·6 | 2260·9a | 96·9 | 1106·6b | 94·2 | 700·7b | 91·8 | 484·2b | 99·2b | 1085·2 | 93·1 | 660·7 | 75·9 | 160·9 | 82 | 367·9 |
| Co-stimulatory/signalling molecules | ||||||||||||||||||
| CD40 | 64·8 | 35·8 | 90·9a | 95·1a | 80·2 | 47·2b | 73·8b | 43·1b | 59·8b | 36·2b | 91·3a | 78·2a | 67·9c | 36·6c | 48·5c | 25·4c | 40·3c | 23·5c |
| CD80 | 5·6 | 10·5 | 69·2a | 48·8a | 24·2 | 19b | 36·3b | 25·4b | 23·7b | 19·4b | 28a·b | 22·1a·b | 3 | 8·7c | 3·2 | 10·1 | 35·3 | 10·5 |
| CD86 | 90·7 | 317·7 | 97·5 | 855·6a | 97 | 592·8 | 90·5 | 326·2b | 86·7 | 404·3b | 96·3 | 389·3b | 88·4 | 391·1 | 71·5 | 143·5 | 81·5 | 321·7 |
| CD54 | 98·8 | 780·2 | 99·3 | 5343a | 98·5 | 2629·5b | 98·1 | 1416·6b | 97·7 | 1197b | 96·8 | 2504·4a·b | 96·2 | 867·4c | 97·7 | 673c | 99·3 | 1197c |
| Maturation antigen | ||||||||||||||||||
| CD83 | 12·4 | 16·4 | 49·7a | 47·3a | 17·4b | 18·8b | 16·6b | 19·1b | 34·9b | 26·5b | 54·1a | 43·8a | 5·2c | 12·1c | 5·4c | 13·3c | 18c | 19·1c |
Versus C P < 0·05,
versus lipopolysaccharide (LPS) P < 0·05,
versus H P < 0·05. Hypoxia and LPS conditions cause phenotypic and morphological changes in immature dendritic cells (iDCs). Stimulated DCs were analysed by flow cytometry for CD40, CD80, CD83, CD86, CD54 and human leucocyte antigen D-related (HLA-DR) surface marker expression. Mean fluorescence intensity (MFI) for hypoxia-DC and LPS-DC was higher than for non-stimulated cells, although conventional markers showed lower MFI intensity under hypoxia compared to LPS. MFI and expression of each marker are presented as mean ± standard deviation of six experiments. MHC: major histocompatibility complex; PSC: P-glycoprotein inhibition using valspodar; PBN: probenecid; MK: CAS 115104-28-4.
When iDCs were stimulated by LPS the mean fluorescence intensity (MFI) of CD80, CD86, HLA-DR and CD54 maturation markers increased MFI threefold with respect to control, and there was a twofold increase of MFI with respect to hypoxia stimulus (Table 1). Interestingly, CD83 and CD40 were similarly up-regulated under both stimuli, and CD86 was down-regulated under hypoxia-achieving control values, suggesting a plasmocytoid-like phenotype pattern with respect to LPS-DC. Despite these differences in the maturation response of DCs after the two stimuli, the up-regulation of maturation markers was abrogated strongly when ABC inhibitors were added at a similar intensity (Table 1). All results are representative of six experiments.
Figure 4 is a representative histogram of the most relevant changes in DC maturation markers after hypoxia or LPS.
Fig. 4.
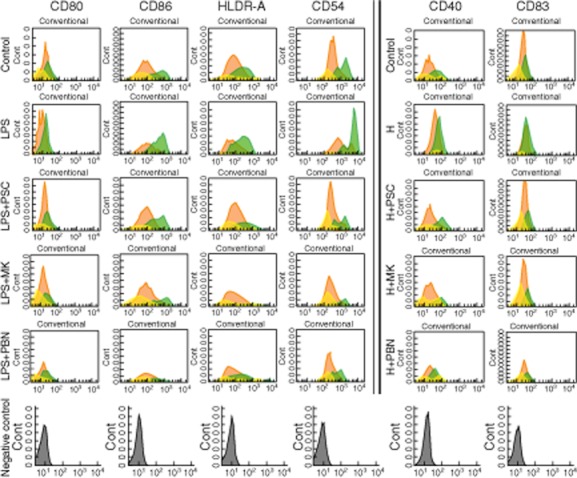
Conventional/plasmacytoid-like phenotype switch on dendritic cell (DC) maturation. Effect of adenosine 5′-triphosphate-binding cassette (ABC) transporter blockade. Human monocyte derived-DCs were used after a 5-day culture with granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4. To inhibit multi-drug resistance (MDR1) protein, DC culture was performed in separate flasks with 5 μM P-glycoprotein inhibition using valspodar (PSC833), and to inhibit multi-drug resistance protein (MRP) with 50 μM CAS 115104-28-4 (MK571) or 2·5 mM probenecid (PBN). At day 5 DCs were stimulated for a further 48 h with hypoxia (hypoxia-mDCs) or for 24 h with lipopolysaccharide (LPS) (1 μg/ml, LPS-mDCs), or left non-stimulated. Cells were then collected and analysed with fluorescence activated cell sorter (FACS) for different mature DC subsets.
HIF-1α protein expression on hypoxia DCs ± ABC transporter inhibitors
HIF-1α expression in control cells was 37·5 ± 5·2%, when DCs stimulated by hypoxia were increased significantly with respect to control (67·6 ± 3·7). Interestingly, when ABC inhibitors were added to hypoxic-DC, HIF-1α results were similar to hypoxia-DCs (H + PSC833 57·5 ± 4·4 and H + MK571 62·3 ± 5·1) (Fig. 5).
Fig. 5.
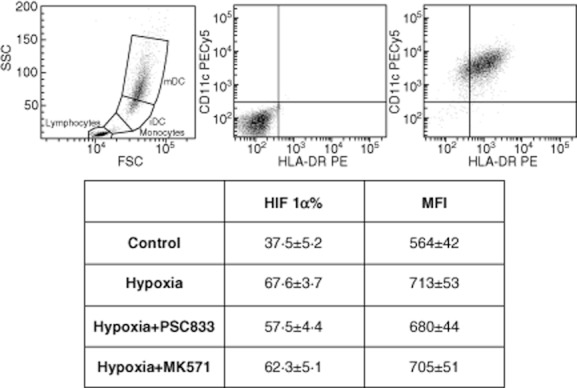
Hypoxia-inducible factor 1 (HIF-1α) over-expression in hypoxia-dendritic cells (DCs). Effect of adenosine 5′-triphosphate-binding cassette (ABC) transporter inhibitors. Representative HIF-1α flow cytometry histogram from a double positive region (human leucocyte antigen D-related+ and CD11c+). Results are presented as mean ± standard error of three experiments. (H, H + PSC and H + MK versus control: P < 0·05).
Impact of ABC transporter inhibition on lymphocyte proliferation
To address the functional impact of ABC transporter inhibition on DCs, we next assessed the effects of these cells on lymphocyte proliferation in the MLR, evaluated by CFSE staining. Hypoxia- and LPS-matured DCs were capable of inducing a significantly (P < 0·05) higher lymphocyte proliferation than non-stimulated iDCs. Functional studies showed a higher T cell proliferation after LPS than after hypoxia stimulus (53·9% with LPS versus 28·5% with hypoxia). ABC transporter inhibitors reduced alloimmune lymphocyte proliferation after exposure to hypoxia and LPS mDCs by approximately 20–30% with respect to non-treated cells (P < 0·05) (data not shown).
To explore further the impact of different DC subtypes on lymphocyte proliferation, lymphocyte subpopulations were assessed. Interestingly, the LPS stimulus induced higher lymphocyte proliferation in the CD8 lymphocyte subtype. Further, plasmocytoid-like hypoxia-DC induced a higher B lymphocyte proliferation than LPS-DC (Fig. 6). MLR performed with purified T and B cells showed similar results to those with unfractionated PBMCs (data not shown).
Fig. 6.
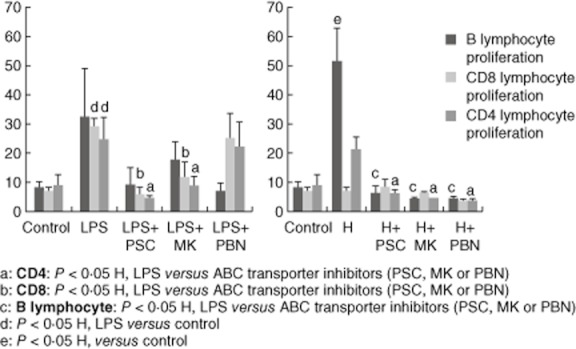
T cell proliferation in the mixed lymphocyte reaction (MLR). Lymphocytes were stained with carboxyfluorescein diacetate succinimidyl ester (CFSE) and exposed to mature dendritic cells (DCs) [under hypoxia or lipopolysaccharide (LPS) stimuli] with or without adenosine 5′-triphosphate-binding cassette (ABC) transporter inhibitors. Cell proliferation was determined by flow cytometry after labelling with CD20, CD4 and CD8 antibodies. The results are representative of six independent experiments and expressed as the mean ± standard error.
Interestingly, when lymphocyte subpopulations were analysed, ABC transporter inhibitors showed a different profile depending on the stimuli for DC maturation; that is, under hypoxia, ABC inhibitors presented a clear inhibition of B and T CD4 lymphocyte proliferation (P < 0·05) (Fig. 6).
Cytokine release from PBMCs after MLR
Cytokine release in the mixed culture with mDCs and lymphocytes showed a different pattern depending on the maturation stimuli. Lymphocytes stimulated by LPS-mDCs presented over-production of IL-2, IL-6, IFN-γ and TNF-α, related mainly to a T helper type 1 (Th1) response, compared with control (P < 0·05). IL-2 and IL-6 were higher in lymphocyte-LPS-mDCs than lymphocyte-hypoxia-mDCs (P < 0·05) (Fig. 7). In contrast, IL-4 was over-expressed in PBMCs exposed to hypoxia-mDCs, suggesting a switch to a Th2 response. IL-17 was up-regulated similarly in PBMCs exposed to the two conditions (Fig. 7).
Fig. 7.
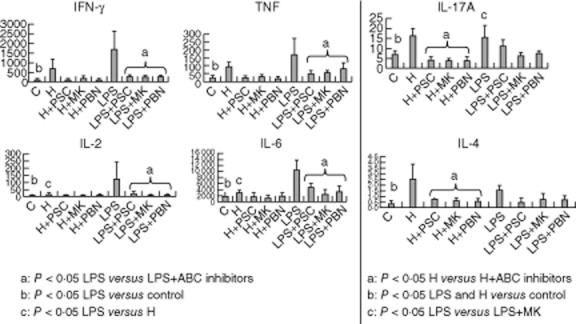
Different pattern on cytokine release observed after hypoxia-dendritic cells (DCs) or lipopolysaccharide (LPS)-DCs exposition. Interleukin-2, -4, -6, -10, -17a, tumour necrosis factor (TNF)-α and interferon (IFN)-γ secretion protein levels from cell supernatant were measured following cell stimulation by fluorescence activated cell sorter (FACS). Cytokine quantification was performed on stimulated and non-stimulated, treated and non-treated [with adenosine 5′-triphosphate-binding cassette (ABC) inhibitors] DCs, and on lymphocytes after mixed lymphocyte reaction (MLR). Cytokine secretion results are expressed as pg/ml (y-axis)
All cytokine release was abrogated by the addition of ABC transporter inhibitors. However, only IL-4 and IL-17 release from PBMCs exposed to hypoxia-mDCs and IL-2, IL-6, IFN-γ, TNF-α and IL-17 release from PBMCs exposed to LPS-mDCs were statistically significantly different compared to samples of DCs not exposed to ABC blockers (P < 0·05) (Fig. 7).
Discussion
Since we first described the impact of hypoxia on DC maturation, there have been further DC studies in the literature confirming a cross-talk between the hypoxic environment and DC maturation 22,23. In the transplant setting, immune-mediated injury is not only caused by alloimmune response, but also points to the ‘injury hypothesis’ as a result of other factors that may play an important role (for example, ischaemia–reperfusion injury). In fact, there is increasing evidence that ischaemia modulates immune and inflammatory responses, but the precise role of hypoxic signalling in renal immune-mediated injury is largely unexplored and unclear 24. Our group proposed hypoxia as a key regulator of DC maturation in the kidney 8, suggesting a novel mechanism by which the lack of oxygen regulates immune responses. This work targets new investigation into the role of molecular oxygen-sensing in dendritic cell maturation and function, which may have implications in acute and chronic renal injuries in both the transplantation and non-transplantation settings.
LPS stimulus has been used classically in several studies to induce DC migration and maturation. Here we show that the LPS stimulus induced a stronger homogeneous maturation effect, while the hypoxia stimulus showed a diverse degree of response. It is well known that in activating innate immunity, LPS induces DC maturation by ligand-driven Toll-like receptor (TLR) activation 25. Our current results show that LPS and hypoxia induced mean fluorescence of mature phenotype DC markers differently from non-stimulated iDCs, but examining these markers individually to compare the two stimuli we found a down-regulation of CD86 for only hypoxia DC. Also, only CD40 and CD83 were expressed to the same degree for both hypoxia and LPS stimulation, whereas for the other surface markers (CD80, CD86, CD54 and HLA-DR) LPS induced a significant up-regulation at least two times greater than did hypoxia. Recently, Jantsch et al. 26 described similar results with an increase in CD80, CD86 and major histocompatibility complex (MHC)-II expression in DCs treated with LPS together with hypoxia, compared to cells treated only with LPS. In contrast, CD80 and CD86 expression decreased slightly under hypoxia alone, whereas MHC-II expression remained unchanged. Sekar et al. 27 generated plasmacytoid-like DC, attenuated IFN-γ production and decreased CD86 as well as MHC-I surface exposure under hypoxia. These findings suggest that LPS probably promotes a more conventional DC profile, while hypoxia appears to create an imbalance in plasmacytoid-like DC phenotypes 28,29.
ABC transporters are described fully in nephrotoxicity models in kidney transplantation, modulating the pharmacokinetics of many immunosuppressors. It is also known that P-glycoprotein is involved in DC maturation. Pendse et al. 12 defined a novel role for Pgp in DC maturation, identifying this transporter as a potential novel therapeutic target in allotransplantation. Schroeijers et al. 30 showed that human monocyte-derived DCs express Pgp at all maturation stages, and that they are up-regulated during DC maturation. Randolph et al. 31 found that Langerhans cells express Pgp and observed that their blockade inhibited migration of these cells. Although there is some consistent literature in this field, the precise role of Pgp and MRP1 in DC migration and maturation is, as yet, not known precisely, especially under hypoxia 32. Concerning our results, the immunofluorescence staining that revealed higher expression of Pgp and MRP1 in DC LAMP-positive mDCs versus iDCs suggested initially that Pgp plays a role in the maturation of iDCs under hypoxia.
To explore further the mechanisms involved in DC maturation under hypoxia, and taking into account the potential role of ABC transporters in this process, we were tempted to analyse the role of the ABC transporters. The addition of three specific inhibitors shifted the ratio of mature and immature DCs achieved after hypoxia or LPS stimuli. Both MDR1 and MRP inhibitors showed a marked decrease in the mean fluorescence of all DC maturation markers except for CD86, which did not reach statistical significance. These results suggest that both MDR1 and MRPs are involved in DC maturation under LPS and hypoxia. In fact, our results under hypoxia point to a possible downstream mechanistic pathway via hypoxia-induced expression of HIF-1α. Interestingly, HIF-1α achieved similar values in hypoxia-DCs under both ABC transporter (MDR1 and MRPs) inhibitors to those under hypoxia alone. These findings are in agreement with recent studies in cancer therapy which argue for the contribution of HIF-1α in drug resistance, as HIF-1α is able to activate MDR1 33. Currently, it is well known that DCs are a bridge between innate and adaptative immunological responses and that LPS and hypoxia are involved in DC stimulation, but the role of ABC transporters in this context has been not explored 34. Also, this link between hypoxia and LPS-DCs and ABC transporters may be inhibited by some of the most potent immunosuppressive drugs such as cyclosporin, tacrolimus and sirolimus, and this suggests an excellent target for preventing ischaemia-derived inflammation mediated by innate immunity.
As described previously, hypoxia is able to increase the release of proinflammatory cytokines and the expression of co-stimulatory molecules by murine and human DCs, thus enhancing their potential to induce allogeneic lymphocyte proliferation 8,26. Hypoxia- and LPS-matured DCs induced significantly higher T cell proliferation than immature untreated DCs, achieving different degrees of T cell proliferation depending on the stimuli. Interestingly, when different subpopulations were assessed, CD8 lymphocyte proliferation was up-regulated remarkably in DCs treated with LPS, while the proliferation of B lymphocytes was higher under hypoxia. Recently it has been reported that plamacytoid DCs are able to induce B lymphocyte proliferation, which lends support to our findings 35. DCs differentiated in the presence of MDR1 and MRP inhibitors reduced alloimmune T cell proliferation twofold. Furthermore, ABC transporter inhibitors showed different profiles of lymphocyte proliferation inhibition depending on DC maturation stimuli. Thus, inhibiting ABC transporters could be an effective approach to reducing the stimulatory capacity of DC, thereby decreasing lymphocyte proliferation.
DCs are usually exposed to diverse pathological and physiological conditions. In fact, LPS and hypoxia are some of the possible in-vitro stimuli that can simulate the different environments that arise in wide-ranging types of cytokines that may trigger assorted inflammatory processes. However, the effects of these stimuli on phenotype differentiation patterns of DC and of the cytokine prompt cascade remain unclear 36,37. In our study, we showed that lymphocytes exposed to LPS-DCs generated higher levels of proinflammatory cytokines (IL-2, IL-6, IL-10, IFN-γ and TNF-α), balanced mainly to the Th1 response. Higher levels of immunoregulatory cytokine IL-4 were found in lymphocytes stimulated by hypoxia-DCs, suggesting a switch to the Th2 response. In fact, plasmacytoid DCs have just been found to secrete substantial amounts of IL-4-producing Th2 cells 27,38. Cytokine secretion was abrogated by the addition of MDR1 and MRP1 inhibitors. The inhibition of DC maturation through ABC transporter blockers probably has a downstream impact on cytokine release.
These findings allow us to suggest that the modulation of different DC phenotype profiles depends upon the initial stimulus and defines subsequent diverse cytokine activators, markers and functions. This is the first time that the role of ABC blockers as inhibitors of DCs maturation after hypoxia and LPS stimuli has been described. The impact of this immune activation, depending on DC maturation stimulus leading to different lymphocyte subtype proliferation, confirms the plasticity of the immunological response in the face of pathological stimuli. In addition, both ABC transporter MDR1 and MRP1 blockers interfere in DC differentiation and maturation, modifying mature DC phenotype and lymphocyte activation. ABC transporters could be a potential target in DC-based immunosuppressive therapies designed to abrogate innate immune response when it is activated after ischaemia or endotoxin stimulus. The cellular and molecular mechanisms underlying the innate adaptive immune response to ischaemia–reperfusion are an active area of research with much more to tell us. These findings add more information about the specific functional role of ABC transporters as a potential therapeutic target in alloimmunity modulation.
Acknowledgments
We are especially grateful to the Servei Cientific-Tècnic team (Esther Castaño, Eva Julià and Benjamín Torrejón) and Nuria Bolaños and Cristian Varela for the technical support in immunological analyses. We thank Novartis in Basel for kindly providing PSC833. This study was supported by Astellas European Foundation Award (13th European Society of Transplantation), Instituto de Salud Carlos III (CP06/00067), Universitat de Barcelona and the Ministerio de Sanidad y Consumo (FIS PI07/0768 and PS09/00897).
Disclosure
None.
References
- 1.Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- 2.Thomson AW, Robbins PD. Tolerogenic dendritic cells for autoimmune disease and transplantation. Ann Rheum Dis. 2008;67(Suppl. 3):iii90–96. doi: 10.1136/ard.2008.099176. [DOI] [PubMed] [Google Scholar]
- 3.Ribatti D, Nico B, Crivellato E, Vacca A. Macrophages and tumor angiogenesis. Leukemia. 2007;21:2085–2089. doi: 10.1038/sj.leu.2404900. [DOI] [PubMed] [Google Scholar]
- 4.Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 5.Stockwin LH, McGonagle D, Martin IG, Blair GE. Dendritic cells: immunological sentinels with a central role in health and disease. Immunol Cell Biol. 2000;78:91–102. doi: 10.1046/j.1440-1711.2000.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsue H, Kusuhara M, Matsue K, Takashima A. Dendritic cell-based immunoregulatory strategies. Int Arch Allergy Immunol. 2002;127:251–258. doi: 10.1159/000057741. [DOI] [PubMed] [Google Scholar]
- 7.Thompson AG, Thomas R. Induction of immune tolerance by dendritic cells: implications for preventative and therapeutic immunotherapy of autoimmune disease. Immunol Cell Biol. 2002;80:509–519. doi: 10.1046/j.1440-1711.2002.01114.x. [DOI] [PubMed] [Google Scholar]
- 8.Rama I, Bruene B, Torras J, et al. Hypoxia stimulus: an adaptive immune response during dendritic cell maturation. Kidney Int. 2008;73:816–825. doi: 10.1038/sj.ki.5002792. [DOI] [PubMed] [Google Scholar]
- 9.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim Biophys Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 10.Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 11.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 12.Pendse SS, Behjati S, Schatton T, Izawa A, Sayegh MH, Frank MH. P-glycoprotein functions as a differentiation switch in antigen presenting cell maturation. Am J Transplant. 2006;6:2884–2893. doi: 10.1111/j.1600-6143.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- 13.Juliano RL, Ling V. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7. [DOI] [PubMed] [Google Scholar]
- 14.Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 16.Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 17.van de Ven R, de Jong MC, Reurs AW, et al. Dendritic cells require multidrug resistance protein 1 (ABCC1) transporter activity for differentiation. J Immunol. 2006;176:5191–5198. doi: 10.4049/jimmunol.176.9.5191. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal S, Gollapudi P, Elahimehr R, Pahl MV, Vaziri ND. Effects of end-stage renal disease and haemodialysis on dendritic cell subsets and basal and LPS-stimulated cytokine production. Nephrol Dial Transplant. 2010;25:737–746. doi: 10.1093/ndt/gfp580. [DOI] [PubMed] [Google Scholar]
- 19.Verkade MA, van Druningen CJ, Vaessen LM, Hesselink DA, Weimar W, Betjes MG. Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol Dial Transplant. 2007;22:128–138. doi: 10.1093/ndt/gfl519. [DOI] [PubMed] [Google Scholar]
- 20.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 22.Naldini A, Morena E, Pucci A, et al. Hypoxia affects dendritic cell survival: role of the hypoxia-inducible factor-1alpha and lipopolysaccharide. J Cell Physiol. 2012;227:587–595. doi: 10.1002/jcp.22761. [DOI] [PubMed] [Google Scholar]
- 23.Bosco MC, Pierobon D, Blengio F, et al. Hypoxia modulates the gene expression profile of immunoregulatory receptors in human mature dendritic cells: identification of TREM-1 as a novel hypoxic marker in vitro and in vivo. Blood. 2011;117:2625–2639. doi: 10.1182/blood-2010-06-292136. [DOI] [PubMed] [Google Scholar]
- 24.Mancino A, Schioppa T, Larghi P, et al. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–3734. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AE, Swan DJ, Sayers BL, et al. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. J Leukoc Biol. 2009;85:243–250. doi: 10.1189/jlb.0608374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jantsch J, Chakravortty D, Turza N, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 27.Sekar D, Brune B, Weigert A. Technical advance: generation of human pDC equivalents from primary monocytes using Flt3-L and their functional validation under hypoxia. J Leukoc Biol. 2010;88:413–424. doi: 10.1189/jlb.0809543. [DOI] [PubMed] [Google Scholar]
- 28.Bratke K, Klein C, Kuepper M, Lommatzsch M, Virchow JC. Differential development of plasmacytoid dendritic cells in Th1- and Th2-like cytokine milieus. Allergy. 2011;66:386–395. doi: 10.1111/j.1398-9995.2010.02497.x. [DOI] [PubMed] [Google Scholar]
- 29.Elia AR, Cappello P, Puppo M, et al. Human dendritic cells differentiated in hypoxia down-modulate antigen uptake and change their chemokine expression profile. J Leukoc Biol. 2008;84:1472–1482. doi: 10.1189/jlb.0208082. [DOI] [PubMed] [Google Scholar]
- 30.Schroeijers AB, Reurs AW, Scheffer GL, et al. Up-regulation of drug resistance-related vaults during dendritic cell development. J Immunol. 2002;168:1572–1578. doi: 10.4049/jimmunol.168.4.1572. [DOI] [PubMed] [Google Scholar]
- 31.Randolph GJ, Beaulieu S, Pope M, et al. A physiologic function for p-glycoprotein (MDR-1) during the migration of dendritic cells from skin via afferent lymphatic vessels. Proc Natl Acad Sci USA. 1998;95:6924–6929. doi: 10.1073/pnas.95.12.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;80:2735–2739. [PubMed] [Google Scholar]
- 33.Rohwer N, Cramer T. Hypoxia-mediated drug resistance: novel insights on the functional interaction of HIFs and cell death pathways. Drug Resist Updat. 2011;14:191–201. doi: 10.1016/j.drup.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Izawa A, Schatton T, Frank NY, et al. A novel in vivo regulatory role of P-glycoprotein in alloimmunity. Biochem Biophys Res Commun. 2010;394:646–652. doi: 10.1016/j.bbrc.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaw J, Wang YH, Ito T, Arima K, Liu YJ. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood. 2010;115:3051–3057. doi: 10.1182/blood-2009-08-239145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosco MC, Puppo M, Blengio F, et al. Monocytes and dendritic cells in a hypoxic environment: spotlights on chemotaxis and migration. Immunobiology. 2008;213:733–749. doi: 10.1016/j.imbio.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 38.Gregori S. Dendritic cells in networks of immunological tolerance. Tissue Antigens. 2011;77:89–99. doi: 10.1111/j.1399-0039.2010.01615.x. [DOI] [PubMed] [Google Scholar]


