Abstract
Recent studies indicate that chemotherapeutic agents may increase the anti-tumoral immune response. Based on the pivotal role of dendritic cells (DCs) in host tumour-specific immune responses, we investigated the effect of commonly used chemotherapeutic drugs dexamethasone, doxorubicin, cisplatin and irinotecan and glucocorticoids on monocyte-derived DCs (moDCs). Dexamethasone displayed the strongest inhibitory effect on DC differentiation. The effect of cisplatin and irinotecan was moderate, while only weak effects were noticed for doxorubicin. Surprisingly, when the functional consequence of chemotherapy-treated CD14+ monocytes and their capacity to activate CD4+ T responders cells were investigated, cisplatin-treated monocytes gave rise to increased T cell proliferation. However, dexamethasone, doxorubicin and irinotecan-pretreated monocytes did not stimulate any increased T cell proliferation. Further investigation of this observation revealed that cisplatin treatment during DC differentiation up-regulated significantly the interferon (IFN)-β transcript. By contrast, no effect was evident on the expression of interleukin (IL)-1β, tumour necrosis factor (TNF)-α, IL-6 or IFN-α transcripts. Blocking IFN-β attenuated the cisplatin-enhanced T cell proliferation significantly. In conclusion, cisplatin treatment enhanced the immune stimulatory ability of human monocytes, a mechanism mediated mainly by the increased production of IFN-β.
Keywords: antigen presentation, chemotherapeutic drugs, cisplatin, dendritic cell, immunotherapy
Introduction
Conventional treatment for solid tumours relies on a combination of surgery, therapies including neoadjuvant or adjuvant chemotherapy and/or radiotherapy. Chemotherapy is believed conventionally to act via direct cytotoxic effects and has common side effects to impair host immunity 1,2. However, recent studies indicate that some chemotherapeutic agents increase the anti-tumoral immune response and cause tumour regression 1,3. Furthermore, it has been demonstrated for several tumour types that the presence of tumour-infiltrating lymphocytes (TILs) is a positive prognostic factor. In one study on urinary bladder cancer a cohort of 514 patients were evaluated. In the multivariate analysis, including clinical stage (T category), World Health Organization (WHO) grade, papillary status, six morphometric nuclear factors and volume-corrected mitotic index (M/V index), dense TILs were a highly significant indicator of favourable prognosis 4. Results in a prospective trial on 22 patients with urinary bladder cancer led to the conclusion that a higher number of TILs was associated with better clinical outcome and reduced occurrence of metastases 5. In addition, the clinical outcome in conjunction with responses to chemotherapy is also increased in the presence of TILs 6,7. In a prospective trial including 25 patients with breast cancer, results suggested that development of TILs after neoadjuvant treatment with paclitaxel had a favourable clinical outcome 8.
Cancer immunotherapy is an evolving attractive additional treatment modality, which offers potentially targeted therapy with fewer side effects compared with conventional therapy. Furthermore, immune-based treatment may trigger immunological memory and provide long-standing effects that are responsive to tumour rechallenges 2. Novel immune-based treatment strategies against cancer include monoclonal antibodies and adoptive lymphocyte transfer. In active specific immunotherapies, a vaccination-induced response is created by injecting tumour proteins, irradiated tumour cells and/or tumour cell lysates or by transfusion of dendritic cells (DCs) pulsed with peptides or lysates or transfected with tumour antigen-expressing constructs 2.
Dendritic cells have a pivotal role in protecting the host from tumour development by initiating, programming and regulating tumour-specific immune responses 9,10. Based on their potent antigen-presenting capacity, DCs have been used in many tumour immunotherapy trials and have been shown to induce anti-tumour immunity 11. In these treatment protocols, patient-derived autologous DC precursor cells such as CD34+ bone marrow cells or CD14+ monocytes from peripheral blood are often used as precursors to derive DCs for further use as antigen-presenting cells (APCs). Although cytotoxic anthraquinone derivatives have been reported to increase DC differentiation and function 12, chemotherapeutic agents may mainly induce suppression of DCs. Prolonged exposure to doxorubicin could impair the capacity of c-KIT+CD34+ precursors to differentiate into Langerhans cells 13. Glucocorticoids are often added to the chemotherapeutic cocktail for treatment of several lymphoproliferative diseases and malignancies, where it induces the expression of the pattern-recognition receptors Toll-like receptor (TLR)-2 and TLR-4, but at the same time they severely impair the differentiation and antigen-presenting ability of DCs in vitro and in vivo 14. Interestingly, breast cancer patients carrying a loss-of-function point mutation in the TLR-4 receptor have shorter disease-free survival, demonstrating the importance of the immune system in controlling malignancy 15. Immune adjuvant effects of radiotherapy and chemotherapy on malignant cells include calreticulin exposure and high-mobility group box chromosomal protein 1 (HMGB1) release. These adjuvant regimens address DCs for increased antigen uptake and presentation 16.
In clinical studies the combination of chemotherapy and immunotherapy has shown promising results. Chemotherapy has been reported to alleviate adverse effects and prolong survival for patients with late-stage non-small cell lung cancer receiving chemo-immunotherapy 17. Chemo-immunotherapy programmes in clinical oncology ought, ideally, to be evaluated initially for the effects on immune function. However, most treatment regimens today are based on the lymphocyte count recovery since the last given dose. This will permit the development of drug dosing and time schedules, allowing recovery of immune function, and may lead possibly to synergistic and augmented anti-tumour responses.
The effect of chemotherapy drugs on DC function needs to be investigated further. We set out to investigate how three conventional chemotherapeutic drugs, doxorubicin, cisplatin and irinotecan, affect human monocyte differentiation to DCs and to examine their effect on DC functionality.
Materials and methods
Cell culture media, cytokines and reagents
L-glutamine, penicillin, streptomycin and fetal calf serum (FCS) (Hyclone, Logan, UT, USA), Ficoll-Paque (Pharmacia Biotech, Uppsala, Sweden), Roswell Park Memorial Institute (RPMI)-1640 cell culture medium (Sigma-Aldrich, St Louis, MO, USA), recombinant human granulocyte–macrophage colony-stimulating factor (GM-CSF) and recombinant human IL-4 (Invitrogen Biosource, Camarillo, CA, USA) were purchased for culture of human cells. The study was approved by the ethical committee at Karolinska Institute. Informed written consent in connection with human buffy coat specimens was obtained from all participants (permit no.: 2008/2017-31). Human CD14+ monocytes and CD4+ T cells were treated with dexamethasone 1 μM, doxorubicin 0·2 μM, cisplatin 25 μM or irinotecan 125 μM. Rabbit polyclonal anti-human interferon (IFN)-β-neutralizing antibody (Merck Millipore, Darmstadt, Germany) and rabbit immunoglobulin (Ig)G isotype control (Abcam, Cambridge, UK) were used at a concentration of 3000 units/ml.
Isolation of cells
Human peripheral blood mononuclear cells (PBMCs) were isolated from fresh heparinized buffy coats (Department of Transfusion Medicine, Karolinska University Hospital) by Ficoll-Paque (Pharmacia Biotech) density gradient centrifugation. CD4+ T lymphocytes and CD14+ monocytes were isolated from PBMCs by magnetic sorting using CD4+ T cell isolation kits and CD14+ monocyte isolation kit II, respectively, and an autoMACS separator (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer's standard protocol.
Differentiation of human monocyte-derived DCs (moDCs)
DCs were generated as described previously 18. Briefly, purified human monocytes were cultured (106 cells/ml) in RPMI-1640 medium supplemented with 10% FCS, 50 ng/ml GM-CSF and 20 ng/ml IL-4 only as control, or with different chemotherapeutics as described above for 6 days. The cells were fed with fresh medium (half the original medium volume) containing GM-CSF, IL-4 and the respective chemotherapeutic drug at double the original concentration on days 2 and 4. During differentiation the cells were harvested daily for analysis by flow cytometry, as indicated.
T cell proliferation assay
The original concentrations of the chemotherapy drugs were doxorubicin 2 mg/ml (Meda, Holzkirchen, Germany), irinotecan 20 mg/ml (Pfizer, Sollentuna, Sweden) and cisplatin 1 mg/ml (Hospira, Stockholm, Sweden). The drugs were added to 96-well plates to make a 1:2 dilution series for doxorubicin (20–0·01 μM), cisplatin (400–0·2 μM) and irinotecan (400–0·2 μM). A concentration of 1 × 105 PBMCs was then added to the wells with the addition of staphylococcal enterotoxin B (SEB) 5 μg/ml (Sigma) and incubated in 37°C for 3 days. Proliferation was measured on day 4 by adding 1 μCi of [3H]-thymidine/well (PerkinElmer, Waltham, MA, USA) 18 h prior to harvesting.
Flow cytometry analysis for blast transformation of T cells
Isolated CD4+ T cells and CD14+ monocytes were incubated separately overnight at 37°C at a concentration of 1 × 106 cells/ml with RPMI-supplemented medium alone or in combination with one of the chemotherapeutic drugs. The next day the cells were washed twice with RPMI-supplemented medium. The treated 1·8 × 105 CD14+ and 3·6 × 105 CD4+ cells (at a 1 : 2 ratio) were then aliquoted and mixed into 5 ml polystyrene tubes (Becton Dickinson, Franklin Lakes, NJ, USA) to a final volume of 1 ml. Staphylococcal enterotoxin B 5 μg/ml was then added. The mixture was incubated at 37°C for the indicated time-period until analysed for blast transformation by flow cytometry.
Flow cytometry
For moDC differentiation analysis by flow cytometry, antibodies for human moDCs multiple colour staining were CD14 [fluorescein isothiocyanate (FITC); mouse monoclonal CD14 antibody (M5E2)], human leucocyte antigen D-related (HLA-DR) [peridinin chlorophyll (PerCP); L243], CD 80 [phycoerythrin (PE); L307·4], CD 86 (APC; B70) (BD Pharmingen, Franklin Lakes, NJ, USA) and CD1a (Pacific Blue; HI149) (Bioscience, San Diego, CA, USA). Approximately 1 × 106 cells were harvested and stained with antibodies for 30 min, according to standard protocols. After incubation, the cells were washed with fluorescence-activated cell sorting (FACS) buffer and finally suspended in 500 μl FACS buffer. For multiple marker staining, the cells were incubated with the combination of FITC-conjugated CD14, Pacific Blue-conjugated CD1a, APC-conjugated CD86, PE-conjugated CD80 and PerCP-conjugated HLA-DR. Fifty thousand cells were acquired and analysed using a FACS Aria flow cytometer (BD Biosciences, Heidelberg, Germany). An appropriate gate was set on the basis of scatter properties for excluding dead cells, and only cells within this gate were analysed. Cells exhibiting a higher mean fluorescence intensity (MFI) value than exhibited by cells stained with isotype control were considered positive (Supplementary Fig. S1a).
For the flow cytometric analysis of blast transformation of T cells, the cells were stained with anti-CD3 (PerCP; SK7), anti-CD4 (APC; RPA-T4), anti-CD45RO (PE; UCHL1) and anti-HLA-DR (FITC; L243) (Becton Dickinson). Fifty thousand cells were acquired and analysed using a FACS Aria flow cytometer (BD Biosciences). Small non-granular lymphocytes (SNLs) and large granular lymphoblasts (LGLs) were identified on dot-plots based on forward-scatter (FSC) versus side-scatter (SSC). Large granular lymphoblasts were analysed further on dot-plots displaying CD3 versus CD4. Double-positive cells (CD3+CD4+) were analysed further on a histogram to identify HLA-DR+ cells, and these cells (CD3+CD4+ HLA-DR+) were analysed further to identify CD45RO+ cells on a histogram. CD3+CD4+ HLA-DR+CD45RO+ cells were considered to be lymphoblasts. The ratio of lymphoblasts to T cells was calculated as number of lymphoblasts (CD3+CD4+HLA-DR+CD45RO+)/number of total T cells (SNLs+LGLs).
Quantitative reverse-transcription polymerase chain reaction
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). Total RNA (1 μg) was treated with deoxyribonuclease (Dnase) I (Promega, Madison, WI, USA) and then reverse-transcribed using a complementary deoxyribonucleic acid (cDNA) synthesis kit (Bio-Rad, Hercules, CA, USA). The synthesized cDNA was used as template in a real-time polymerase chain reaction (PCR) mix according to the manufacturer's standard protocol (iQ SYBR Green supermix reagents). The reactions were performed in a total volume of 20 μl with 2 μl of respective cDNA sample (7500 fast real-time PCR system; Applied Biosystems, Carlsbad, CA, USA). As a control for the specificity of the real-time PCR, a sample without template was included. All the measurements were performed in triplicate for each sample; the relative amounts of mRNA were calculated using the comparative threshold (Ct) method and normalized against human RNA polymerase II (RP-II) or mouse glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). All primer sequences are provided in Table 1.
Table 1.
Primers for real-time polymerase chain reaction (PCR) amplification.
| Forward (5′–3′) | Reverse (5′–3′) | |
|---|---|---|
| IL-1β | ACAGATGAAGTGCTCCTTCCA | GTCGGAGATTCGTAGCTGGAT |
| TNF-α | CACGCTCTTCTGCCTGCTG | GATGATCTGACTGCCTGGGC |
| IL-6 | GACAGCCACTCACCTCTTCA | AGTGCCTCTTTGCTGCTTTC |
| IFN-α | AGCCATCTCTGTCCTCCATGA | CATGATTTCTGCTCTGACAACC |
| IFN-β | GATTCCTACAAAGAAGCAGCAA | CAAAGTTCATCCTGTCCTTGAG |
| RP-II | GCACCACGTCCAATGACAT | GTGCGGCTGCTTCCATAA |
IFN: interferon; IL: interleukin; RP-II: RNA polymerase II; TNF: tumour necrosis factor.
Statistical analysis
Statistical comparisons between groups were made using analysis of variance followed by a paired t-test or Student's t-test. Statistical significance is indicated in the Figures (*P < 0·05; **P < 0·01).
Results
Titration of chemotherapeutic drugs
To investigate the effect of chemotherapeutic drugs on DC function in vitro, we optimized the concentrations of each of the tested drugs. The relevant doses of chemotherapeutic agents were obtained by serial dilutions of the respective drugs. CD4+ T cell proliferation was measured by [3H]-thymidine incorporation after stimulation with SEB in the presence of chemotherapeutic drugs for 3 days. Based on the results, the doses resulting in 50% inhibition of proliferation (LD50) were chosen for further experiments (doxorubicin 0·2 μM, cisplatin 25 μM, irinotecan 125 μM) ( Fig. 1).
Fig. 1.
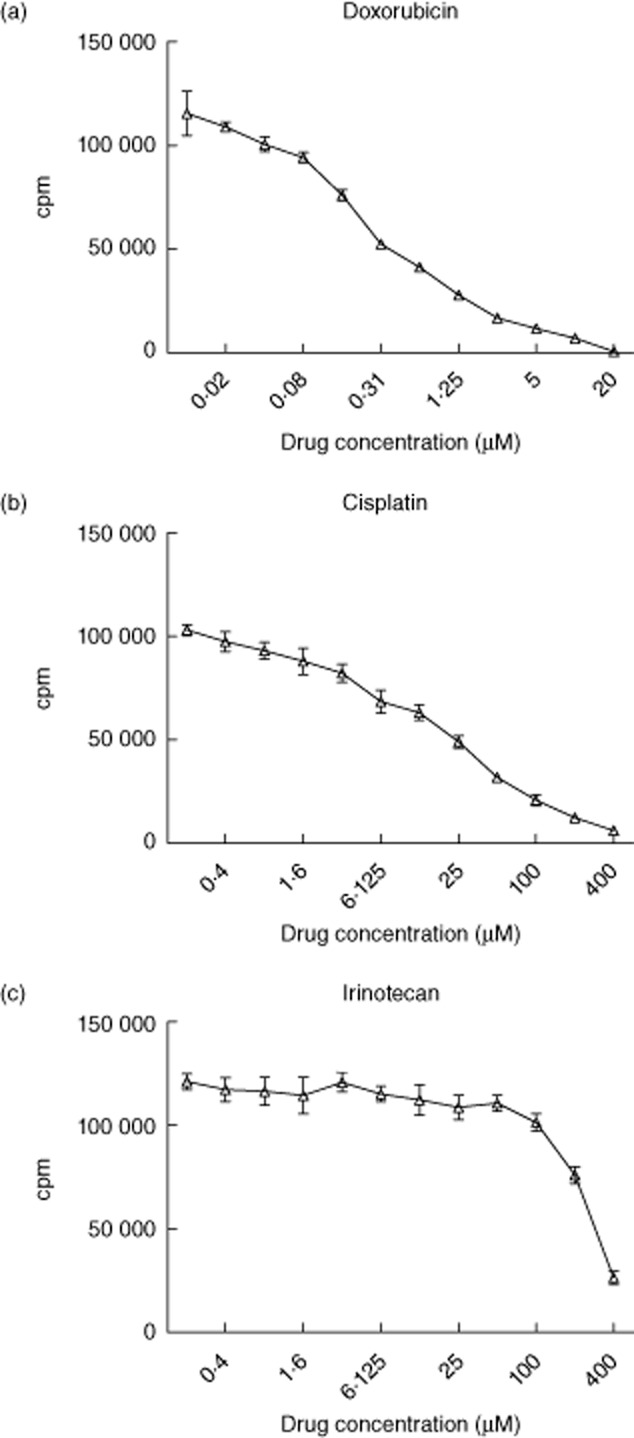
Determining the inhibitory concentration of chemotherapeutic drugs inhibiting T cell proliferation. Dilution series of 1:2 were established for doxorubicin, cisplatin and irinotecan from their original concentrations. A total of 1 × 105 peripheral blood mononuclear cells (PBMCs) were incubated with indicated concentration of chemotherapeutic drugs and staphylococcal enterotoxin B (SEB) 5 μg/ml in 37°C for 3 days, and 1 μCi of [3H]-thymidine/well was added 18 h prior to harvesting on day 4. Radioactivity [counts per minute (cpm)] was measured for doxorubicin (Fig. 1a), cisplatin (Fig. 1b) and irinotecan (Fig. 1c). Data shown are the means of quadruplicate determinations for each condition from three independent experiments.
Chemotherapeutic agents inhibited differentiation of human moDCs
First, we studied the effect of the chemotherapeutic drugs on the differentiation of human moDCs. Relevant human DC markers were analysed by flow cytometry during differentiation in the presence of doxorubicin, cisplatin, irinotecan or dexamethasone. Dexamethasone has been well demonstrated to inhibit moDC differentiation 19, and was therefore included as a positive control in the experiment.
We began to investigate the changes in the relative number of cells expressing DC markers from all the donors (Fig. 2a). Dexamethasone treatment delayed the down-regulation of the human monocyte marker CD14 significantly, as demonstrated by the increased fraction of immature DCs expressing CD14 (Fig. 2a, top left panel). At the same time, dexamethasone caused a significantly smaller fraction of DCs expressing CD1a as a sign of delayed maturation (Fig. 2a, top right panel). Different effects of dexamethasone treatment on the expression of co-stimulatory molecules were observed; there was no dexamethasone effect on the fraction of cells expressing CD80, while the fraction of cells expressing the inducible co-stimulator CD86 and major histocompatibility complex (MHC)-II was increased significantly (Fig. 2a). Doxorubicin treatment (Fig. 2a, middle bars) showed a mild inhibition in the differentiation of moDCs. Although the fraction of cells expressing CD14 was decreased significantly, no obvious change in the expression of CD1a was observed. Interestingly, the fraction of cells expressing CD80 was enhanced after doxorubicin treatment (Fig. 2a). The increased expression of MHC-II was increased to a similar extent as observed with monocytes treated with dexamethasone, but by contrast, doxorubicin did not cause any effects on CD86 expression. Cisplatin (Fig. 2a, middle right) and irinotecan (Fig. 2a, far right) showed a similar, moderate inhibitory effect with regard to the proportion of cells expressing CD14, CD1a and MHC-II, but did not induce increased cell expression of CD86 compared with control values. The fraction of cells expressing the co-stimulatory molecule CD80 was inhibited by irinotecan, while cisplatin had no effect on cells expressing CD80 (Fig. 2a).
Fig. 2.
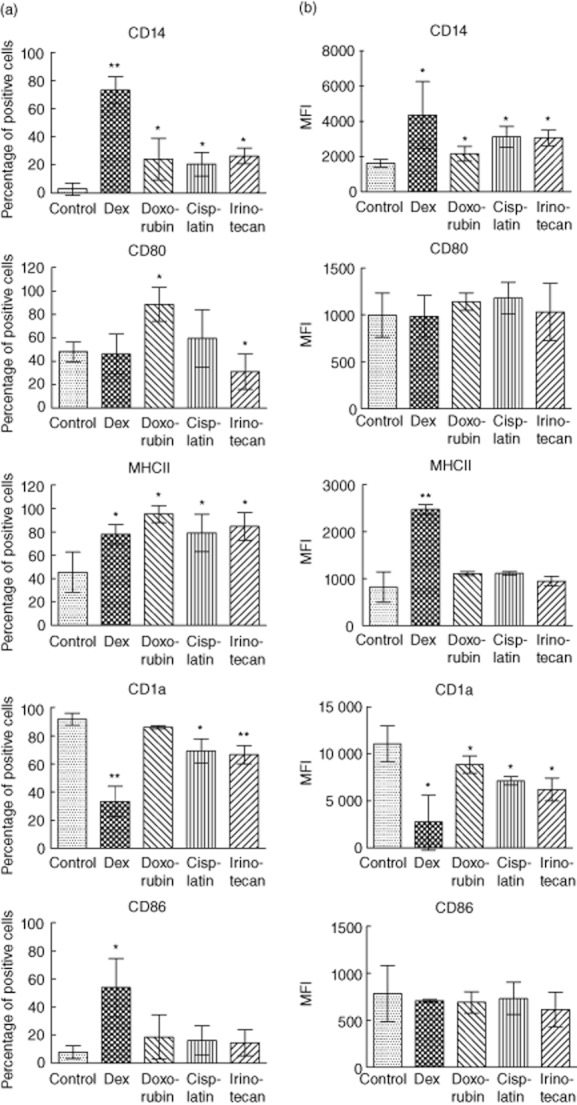
Inhibition of human monocyte-derived dendritic cell (moDC) differentiation by chemotherapeutic agents. Enriched human monocytes were cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 for 6 days. Chemotherapy agents doxorubicin 0·2 μM, cisplatin 25 μM, irinotecan 125 μM and dexamethasone 1 μM, respectively, were added and were present for the whole dendritic cell (DC) differentiation process. The cells were harvested, stained and analysed by flow cytometry on day 6. Percentages of positive cells (Fig. 2a) and mean fluorescent intensity (MFI) (Fig. 2b) for indicated surface markers were measured for the different groups. Data shown are the means from three donors for each condition. Significant results by comparing the chemotherapy-treated group with the control group are indicated (*P < 0·05; **P < 0·01).
In addition, we measured the MFI of maturation markers on monocyte cultured with chemotherapeutic drugs (Fig. 2b). The mean expression levels of CD14 and CD1a (Fig. 2b) mirrored the effect on the fraction of cells expressing the markers (Fig. 2a) for all the tested drugs. However, none of the tested drugs had an obvious effect on the expression levels CD80 and CD86. With regard to expression of MHC-II, dexamethasone induced a significant increase in MHC-II expression, while the remaining drugs did not have any effect on class II expression. Supplementary Fig. S1b shows the representative histograms for each condition. A time–course with investigations of marker expression was performed daily and the proportion of cells and MFI expression levels is presented for all the markers (Supplementary Figs S2 and S3). We conclude that chemotherapy drugs change the differentiation of human moDCs.
Chemotherapeutic drugs enhance moDC function in vitro
The ability of APCs to activate T cells can be evaluated by a flow cytometric assay of specific cell-mediated immune response in activated whole blood (FASCIA) 20. To study the functional consequences of the chemotherapeutic drugs, we used the principle of FASCIA with isolated CD14+ moDCs as APCs and isolated CD4+ lymphocytes as responder cells. Monocytes or lymphocytes treated with chemotherapeutic drugs were co-cultured and activated with the superantigen SEB. Lymphoblast developments among cells treated with chemotherapy were compared with control cells for a 4-day period. Gating strategies for analysis of the lymphoblasts are illustrated in Fig. 3.
Fig. 3.
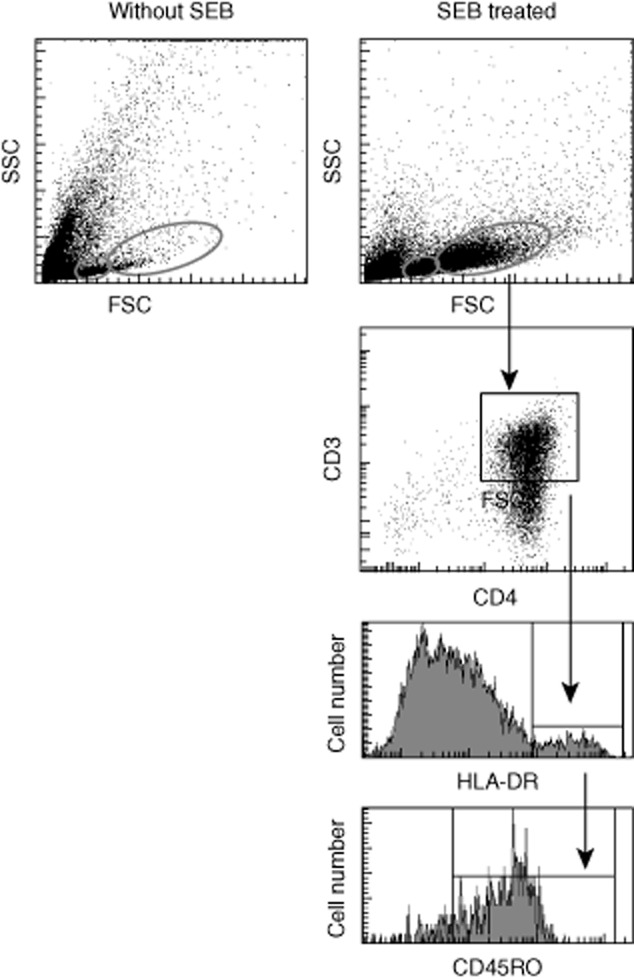
Illustration of gating strategies for analysis of lymphoblasts. Enriched human CD4+ T cells and CD14+ monocytes were incubated separately with medium alone or with one of the chemotherapeutic drugs overnight. The next day, the treated CD14+ and CD4+ cells were mixed with staphylococcal enterotoxin B (SEB) 5 μg/ml and incubated for the time-period indicated for flow cytometric measurement of lymphoblast development. Small non-granular lymphocytes (small red circles) and large granular lymphoblasts (big red circles) were identified on dot-plots, and then large granular lymphoblasts were further analysed based on CD3 versus CD4 dot-plots. Double positive cells were analysed further on a histogram to identify human leucocyte antigen D-related (HLA-DR+) cells, and these cells were analysed further to identify CD45RO-positive cells on a histogram. The cells CD3+, CD4+, Cd45RO+ and HLA-DR+ were considered lymphoblastic.
Monocytes and lymphocytes cultured with SEB had peak activation, with maximum blast transformation, on day 3 (Fig. 4). When monocytes were combined with pretreated CD4+ T cells, we noticed that neither dexamethasone nor doxorubicin affected lymphoblast formation compared with controls (Fig. 4a). We found a slight decrease in lymphoblast formation on day 3 for irinotecan and day 4 for cisplatin. Therefore, pretreatment of CD4+ lymphocytes with chemotherapeutic drugs seems to have a slight effect on T cell activation (Fig. 4a). CD4+ lymphocyte culture in the presence of monocytes pretreated with cisplatin resulted in increased activation of T cells, reaching significance on day 4 (Fig. 4b). However, dexamethasone, doxorubicin and irinotecan-treated monocytes did not cause increased activation of T cells (Fig. 4b). These results demonstrate that cisplatin enhances the ability of moDCs to simulate T cell activation.
Fig. 4.
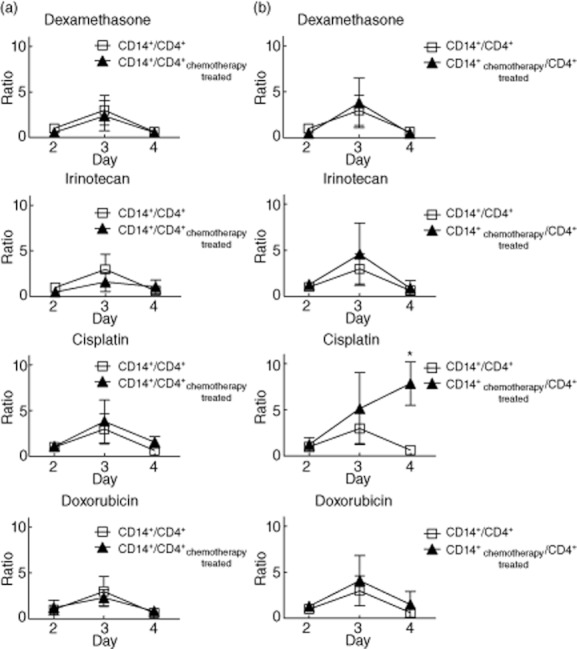
The effect of chemotherapeutic drugs on monocyte-derived dendritic cell (moDC) function. Enriched human CD4+ T cells and CD14+ monocytes were incubated separately with medium alone or with medium in combination with the respective chemotherapy drug overnight. On the next day the treated 1·8 × 105 CD14+ and 3·6 × 105 CD4+ cells were mixed in a final volume of 1 ml after twice washing. Staphylococcal enterotoxin B (SEB) 5 μg/ml was added subsequently and then the mixture was incubated for the indicated time-period for flow cytometric measurement. The mixed samples without drug pretreatment were considered as control. The proliferation ratio of lymphoblasts to pretreated T cells and untreated monocytes (Fig. 4a) and untreated T cells and pretreated monocytes (Fig. 4b) was calculated at the indicated time-periods, and then normalized to the value of the day 2 control sample that was set to 1. Data shown are the mean values from three donors. Error bars illustrate standard deviation. Significance comparing the chemotherapy-treated groups with the control group is indicated (*P < 0·05; **P < 0·01).
Cisplatin increases IFN-β secretion during DC differentiation
To investigate the mechanism of cisplatin-enhanced DC function, we dissected the production of proinflammatory cytokines during DC differentiation. The transcripts of the proinflammatory cytokines IL-1β, TNF-α, IL-6, IFN-α and IFN-β were not affected in the control group during the 4-day differentiation process (Fig. 5). Cisplatin treatment did not regulate IL-1β, TNF-α, IL-6 and IFN-α expression. Surprisingly, the expression of the IFN-β mRNA was significantly up-regulated on day 2 and continued to increase (Fig. 5). Consequently, IFN-β is a likely candidate explaining the enhanced DC function after cisplatin treatment.
Fig. 5.
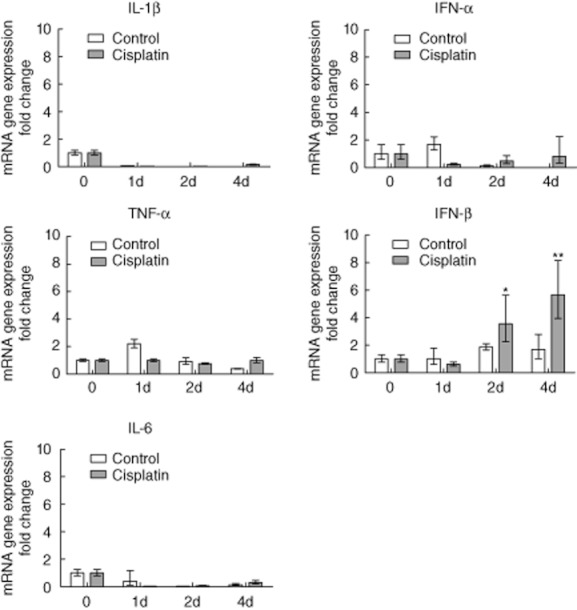
The effect of chemotherapeutic agents on proinflammatory cytokine messenger ribonucleic acid (mRNA) expression during human monocyte-derived dendritic cell (moDC) differentiation. Enriched human monocytes were cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 for 6 days. Chemotherapy agents doxorubicin 0·2 μM, cisplatin 25 μM and irinotecan 125 μM, respectively, were added and were present for the whole dendritic cell (DC) differentiation process. Quantitative real-time polymerase chain reaction (PCR) was used to measure IL-1β, tumour necrosis factor (TNF)-α, IL-6, interferon (IFN)-α and IFN-β mRNA expression during the moDC differentiation process in the indicated time-periods. Data shown are the means of triplicate determinations from three independent experiments. Values for time-point 0 were set to 1. Significance comparing the chemotherapy-treated group with the control group is indicated (*P < 0·05; **P < 0·01).
Blocking IFN-β attenuates the cisplatin-enhanced T cell proliferation
To demonstrate the role of IFN-β in this setting, we blocked IFN-β function using a neutralizing antibody, and then measured its effect on cisplatin-enhanced T cell proliferation. Staphylococcal enterotoxin B-induced T cell proliferation occurred as expected in the control group. Pretreatment with cisplatin enhanced the SEB-induced T cell proliferation significantly (Fig. 6). However, when IFN-β was blocked by the neutralizing antibody, the cisplatin-enhanced T cell proliferation was attenuated significantly (70% reduction) compared with the isotype control group (Fig. 6). This suggests that IFN-β is a major candidate in mediating cisplatin-enhanced T cell proliferation.
Fig. 6.
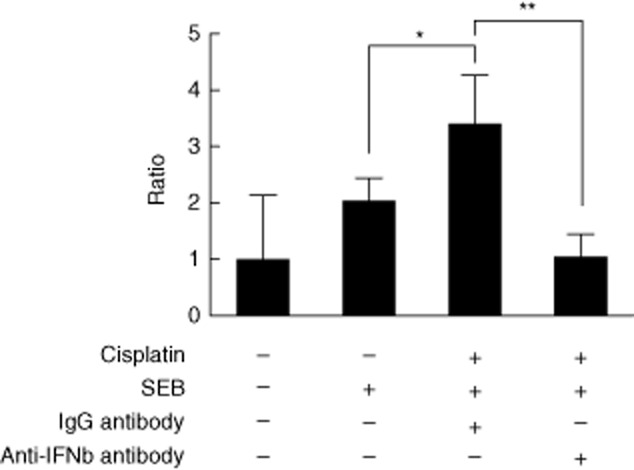
Attenuation of cisplatin-enhanced T cell proliferation by neutralizing interferon (IFN)-β. Enriched human CD4+ T cells and CD14+ monocytes were incubated with medium and cisplatin, respectively, overnight. On the next day the treated 1·8 × 105 CD14+ and 3·6 × 105 CD4+ cells were mixed in a final volume of 1 ml after twice washing. Interferon-β-neutralizing antibody and its immunoglobulin (Ig)G isotype control were added separately to the cell mixture. After 1 h, staphylococcal enterotoxin B (SEB) 5 μg/ml was added and then the mixture was incubated for 5 days for analysis by flow cytometry. The mixed samples without treatment were considered as control. The proliferation ratio of lymphoblasts was calculated for different treatment groups, and then normalized to the value of the control group, which was set to 1. Data shown are the mean values for three donors. Error bars illustrate standard deviation. Significance comparing the groups is indicated (*P < 0·05; **P < 0·01).
Discussion
In this study, we investigated the effect of chemotherapy drugs on the differentiation and functionality of human moDCs in vitro. We found that co-stimulatory molecules showed different expression profiles on DCs when cells had been treated with dexamethasone, cisplatin, irinotecan or doxorubicin. CD80 expression was increased by doxorubicin, but decreased by irinotecan (Fig. 2a), while the expression of CD86 was increased by dexamethasone treatment only. All four tested compounds increased the expression levels of MHC-II on the DCs (Fig. 2a). However, dexamethasone, doxorubicin and irinotecan were unable to stimulate T cells in a functional assay (Fig. 4). Surprisingly, the blast transformation of T cells in a FACS-like analysis revealed that cisplatin pretreatment of CD14+ human monocytes increased their ability to activate CD4+ T helper cells (Fig. 4), despite the finding of no increase in the expression of co-stimulatory molecules (Fig. 2b). To reveal the mechanism behind the cisplatin-induced ability of DCs to stimulate T cell activation, we measured levels of cytokine transcripts. Cisplatin treatment of DCs did not regulate the mRNA expression of the proinflammatory cytokines IL-1β, TNF-α, IL-6 and IFN-α. However, IFN-β mRNA expression was up-regulated significantly (Fig. 5). Furthermore, blocking IFN-β with a neutralizing antibody significantly attenuated the cisplatin-enhanced T cell proliferation (Fig. 6), thereby demonstrating a role for IFN-β in the enhanced dendritic T cell-stimulating capacity after cisplatin treatment. IFN-β has been shown to play a critical role in inducing increased antigen-presenting ability in DCs 21,22. In our study, IFN-β was up-regulated more than fourfold after 2 days of cisplatin treatment, and the expression was consistent over the time-period of increased T cell activation (Fig. 4).
Singh and Sodhi report that, in their study, cisplatin treatment enhanced the antigen-presenting ability of macrophages and increased keyhole limpet haemocyanin (KLH)-primed T cell proliferation in a biphasic manner. In addition to IL-1 and TNF-α, Ca2+, they suggest involvement of calmodulin and calmodulin-dependent kinases in the second phase of antigen presentation 23. In our study, cisplatin showed a similar ability to increase DC function, causing T cell activation (Fig. 4). Cisplatin inhibited DC differentiation but no significant effects were seen on the expression of the co-stimulatory molecules CD80 and CD86. Dendritic cell antigen presentation to T cells requires the combined action of signals 1, 2 and 3. Therefore, the increased MHC expression (signal 1) in combination with the increased expression of the proinflammatory cytokine IFN-β (signal 3) is compatible with the effect we observed for cisplatin regarding T cell activation.
Dexamethasone has been well demonstrated to inhibit human DC differentiation, maturation and antigen-presenting function 14,24. Our results are consistent with earlier findings 24: the DC differentiation process was inhibited, as shown by affected CD14 and CD1a markers and decreased CD80 and CD86 co-stimulatory markers. MHC-II was expressed by a high MFI in our experiment instead of decreased expression. This was caused probably by different doses of drugs used, as reported previously 24.
Doxorubicin pretreatment in non-cytotoxic concentrations (2 or 10 nM) up-regulated the ability of mouse bone marrow-derived DCs to present antigens to antigen-specific CD8+ T cells, and the function was associated with the up-regulation of co-stimulatory molecules, such as CD80, CD86, CD40 and MHC-II 25. However, prolonged doxorubicin exposure (at 90 nM) in a human cell line model interfered with the differentiation capacity of DC precursor into Langerhans cells, a particular DC subset, with decreased DC surface molecule expression for CD1a, CD40, CD86 and MHC-II 13. This revealed the dual effect of doxorubicin on DC differentiation, with enhancement at low concentrations, but inhibition at a higher concentration. In our study a high concentration of doxorubicin (0·2 μM) therefore inhibited the differentiation of human moDCs slightly, but did not affect the antigen presentation ability.
Data about the effect of irinotecan on DCs are sparse. Bourquin et al. demonstrated that in a mouse colon carcinoma model the combination of DC-based immunotherapy with irinotecan did not affect its efficacy and, furthermore, that it strongly decreased the toxicity of chemotherapy 26. We are the first to reveal an effect of irinotecan on DC differentiation and antigen presentation. Although irinotecan could inhibit the differentiation of human moDCs moderately, the treatment did not affect antigen-presenting ability, which may partly explain the obtained in vivo data 26.
Exposure to chemotherapeutic agents may lead to short-term activation of DCs. Put into perspective, a novel understanding of the chemotherapeutic effects on the immune system may lead potentially to new and optimized treatment strategies. Several studies have shown that the combination of chemotherapy and immunotherapy may have synergistic effects. However, setting the time and dose schedule is not trivial, as chemotherapy may have devastating effects on the immune system when administered in a vulnerable phase of leucocyte activation, differentiation and proliferation. In conclusion, we have shown that cisplatin treatment of human DCs leads to increased T cell activation, a beneficial effect of cisplatin mediated by increased expression of the cytokine IFN-β by DCs.
Acknowledgments
This work was supported by the Swedish Cancer Society, the Wallenberg Foundation, The Söderberg Foundation, Center for Immune Modulatory Therapies for Autoimmunity and Cancer (IMTAC) and the Swedish Research Council.
Disclosure
We declare there are no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1a. Illustration of gating strategies of human monocyte-derived dendritic cells (moDCs). Enriched human monocytes were cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 and different chemotherapy agents for 6 days. The cells were harvested, stained and analysed by flow cytometry on day 6. A gate was set on the basis of scatter properties to exclude dead cells on dot plots. The cells within this gate were displayed on histogram for different cell surface markers. Cells exhibiting a higher mean fluorescence intensity (MFI) value compared with cells stained with respective isotype control (right) were considered positive.
Fig. S1b. Characterization of different chemotherapy-treated human monocyte-derived dendritic cells (moDCs). Enriched human monocytes were cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 for 6 days. Chemotherapy agents doxorubicin 0·2 μM, cisplatin 25 μM, irinotecan 125 μM and dexamethasone 1 μM, respectively, were added and were present for the whole dendritic cell (DC) differentiation process. The cells were harvested, stained and analysed by flow cytometry on day 6. Histograms represent data on three donors for each condition.
Figs S2 and S3. Inhibition of human monocyte-derived dendritic cell (moDC) differentiation by chemotherapeutic agents. Enriched human monocytes were cultured in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 for 6 days. Chemotherapy agents doxorubicin 0·2 μM, cisplatin 25 μM, irinotecan 25 μM and dexamethasone 1 μM, respectively, were added and were present for the whole dendritic cell (DC) differentiation process. The cells were harvested, stained and analysed by flow cytometry at indicated time-periods. The histogram shows changes in indicated surface molecules in cells from the different groups. The percentage of positive cells (Supplementary Fig. 2) and mean fluorescent intensity (MFI) (Supplementary Fig. 3) for indicated surface markers were measured for the different groups. Data shown are the means from three donors for each condition. Significant results from comparing the chemotherapy-treated group with the control group are indicated (*P < 0·05; **P < 0·01).
References
- 1.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 2.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 3.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipponen PK, Eskelinen MJ, Jauhiainen K, Harju E, Terho R. Tumour infiltrating lymphocytes as an independent prognostic factor in transitional cell bladder cancer. Eur J Cancer. 1992;29A:69–75. doi: 10.1016/0959-8049(93)90579-5. [DOI] [PubMed] [Google Scholar]
- 5.Tsujihashi H, Matsuda H, Uejima S, Akiyama T, Kurita T. Immunocompetence of tissue infiltrating lymphocytes in bladder tumors. J Urol. 1988;140:890–894. doi: 10.1016/s0022-5347(17)41851-9. [DOI] [PubMed] [Google Scholar]
- 6.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 8.Demaria S, Volm MD, Shapiro RL, et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 9.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 10.Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ. 2008;15:39–50. doi: 10.1038/sj.cdd.4402247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 12.van de Ven R, Reurs AW, Wijnands PG, et al. Exposure of CD34(+) precursors to cytostatic anthraquinone-derivatives induces rapid dendritic cell differentiation: implications for cancer immunotherapy. Cancer Immunol Immunother. 2012;61:181–191. doi: 10.1007/s00262-011-1039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van de Ven R, Verbrugge SE, Reurs AW, et al. High susceptibility of c-KIT(+)CD34 (+) precursors to prolonged doxorubicin exposure interferes with Langerhans cell differentiation in a human cell line model. Cancer Immunol Immunother. 2011;60:943–951. doi: 10.1007/s00262-011-1003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rozkova D, Horvath R, Bartunkova J, Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin Immunol. 2006;120:260–271. doi: 10.1016/j.clim.2006.04.567. [DOI] [PubMed] [Google Scholar]
- 15.Zitvogel L, Tesniere A, Apetoh L, Ghiringhelli F, Kroemer G. [Immunological aspects of anticancer chemotherapy] Bull Acad Natl Med. 2008;192:1469–1487. discussion 87–9. [PubMed] [Google Scholar]
- 16.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 17.Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60:1497–1502. doi: 10.1007/s00262-011-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Winqvist O, Flores-Morales A, Wikstrom AC, Norstedt G. SOCS2 influences LPS induced human monocyte-derived dendritic cell maturation. PLoS ONE. 2009;4:e7178. doi: 10.1371/journal.pone.0007178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Heuvel MM, van Beek NM, Broug-Holub E, et al. Glucocorticoids modulate the development of dendritic cells from blood precursors. Clin Exp Immunol. 1999;115:577–583. doi: 10.1046/j.1365-2249.1999.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svahn A, Linde A, Thorstensson R, Karlen K, Andersson L, Gaines H. Development and evaluation of a flow-cytometric assay of specific cell-mediated immune response in activated whole blood for the detection of cell-mediated immunity against varicella-zoster virus. J Immunol Methods. 2003;277:17–25. doi: 10.1016/s0022-1759(03)00111-x. [DOI] [PubMed] [Google Scholar]
- 21.Zietara N, Lyszkiewicz M, Gekara N, et al. Absence of IFN-beta impairs antigen presentation capacity of splenic dendritic cells via down-regulation of heat shock protein 70. J Immunol. 2009;183:1099–1109. doi: 10.4049/jimmunol.0803214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh RA, Sodhi A. Antigen presentation by cisplatin-activated macrophages: role of soluble factor(s) and second messengers. Immunol Cell Biol. 1998;76:513–519. doi: 10.1046/j.1440-1711.1998.00769.x. [DOI] [PubMed] [Google Scholar]
- 24.Piemonti L, Monti P, Allavena P, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–6481. [PubMed] [Google Scholar]
- 25.Shurin GV, Tourkova IL, Kaneno R, Shurin MR. Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol. 2009;183:137–144. doi: 10.4049/jimmunol.0900734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourquin C, Schreiber S, Beck S, Hartmann G, Endres S. Immunotherapy with dendritic cells and CpG oligonucleotides can be combined with chemotherapy without loss of efficacy in a mouse model of colon cancer. Int J Cancer. 2006;118:2790–2795. doi: 10.1002/ijc.21681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


