Abstract
Objective
To investigate the histopathology of the arachnoid granulations in patients with HIV-associated cryptococcal meningitis and correlate the findings with clinical data, in particular CSF opening pressure.
Design
Case series.
Methods
Post mortems were requested on patients dying during initial hospitalisation with HIV-associated cryptococcal meningitis.
Results
5 post mortems were performed. Large numbers of cryptococcal cells were seen within the arachnoid granulations. The number of fungal cells correlated with CSF pressure. Inflammatory cell infiltrates and disruption of the normal architecture of the granulations were also observed.
Conclusions
The study provides the first direct evidence supporting obstruction to CSF reabsorption at the level of the arachnoid granulations as the main mechanism underlying development of raised CSF pressure in HIV-associated cryptococcal meningitis.
Keywords: Cryptococcal meningitis, Arachnoid granulation, Raised intracranial pressure, Cryptococcus, HIV, Cerebrospinal fluid pressure, Histopathology
Introduction
HIV-associated cryptococcal meningitis is a major cause of death in Sub-Saharan Africa [1]. Raised cerebrospinal fluid (CSF) pressure is an important contributor to the morbidity and mortality associated with cryptococcal meningitis [2,3] and is common, with approximately three-quarters of patients having a CSF opening pressure >25 cm in a large series [2]. Higher cerebrospinal fluid (CSF) opening pressures were associated with higher frequency of headache and neurologic findings. Furthermore, patients with raised intracranial pressure had reduced short-term survival compared to patients with baseline pressures of <25 cm H20 [2]. Given this adverse association, and based on the hypothesis that raised pressure results from mechanical obstruction to CSF outflow by organisms or shed polysaccharide, guidelines recommend aggressive management of raised CSF pressure through repeat lumbar punctures with careful drainage of CSF [4].
Consistent with obstruction to CSF outflow by organism and/or cryptococcal polysaccharide as a mechanism of raised pressure, CSF pressure is positively correlated with CSF cryptococcal antigen (CRAG) titer, the proportion of patients with positive India ink smears, and cryptococcal colony forming units (CFU) in CSF [2, 5]. However, direct evidence for obstruction to CSF outflow has not been presented to date, and alternative theories as to the basis of raised intracranial pressure in cryptococcal meningitis have been proposed, including increased vascular permeability and cerebral oedema resulting from cytokine-induced inflammation, and an osmotic effect of fungal mannitol [6].
Therefore, at Jooste hospital, Cape Town, South Africa, post mortems were requested on patients dying during initial hospitalisation with cryptococcal meningitis. Care was taken to preserve and examine the arachnoid granulations in particular. Samples were taken for routine histology as well as for electron microscopy. The formalin fixed specimens were processed routinely and representative sections were stained with haematoxylin & eosin, mucicarmine, elastic von gieson stain and periodic acid Schiff. The number of cryptococcal cells present in arachnoid granulations was assessed using a Nikon Coolscope to photograph 10 high power fields. Fungal cells in 10 fields were counted and expressed as mean cryptococcal cells per high power field. The findings were correlated with the clinical and CSF opening pressure data.
Histopathological case series
Two patients with high CSF pressure
Case 1
A 26 year old female presented with a four day history of headache and blurred vision. Her mental status was normal. Visual acuity was 6/9 in her right and 6/6 in her left eye. Initial CSF results were: white blood cells (WBCs) 1 cell/mm3, protein 0.24 g/L, glucose 1.8 mmol/L, quantitative culture: 790,000 CFU/ml CSF. The CSF OP was 36cm H2O. The CD4 cell count was 26 ×106/L and HIV viral load 500,000 copies/ml. The patient was treated with amphotericin B and flucytosine. Despite serial lumbar punctures, severe headache persisted and bilateral 6th cranial nerve palsies developed on day 6. OP on day 6 was 80 cm H20. Continued daily lumbar punctures did not control pressure and visual acuity deteriorated to right eye 6/24, left eye 6/9 on day 8, and light perception only on day 12. A lumbar drain was inserted. Despite this, the patient’s condition deteriorated and the patient died on day 14.
Post mortem showed a basal meningitis. The arachnoid granulations contained numerous cryptococcal cells (mean 286 /highpower field, range 96-589) with very little inflammatory reaction (Figure 1). Fungal cells were found in the Virchow-Robin spaces around vessels as well as within the brain, although notably fewer than in the arachnoid granulations.
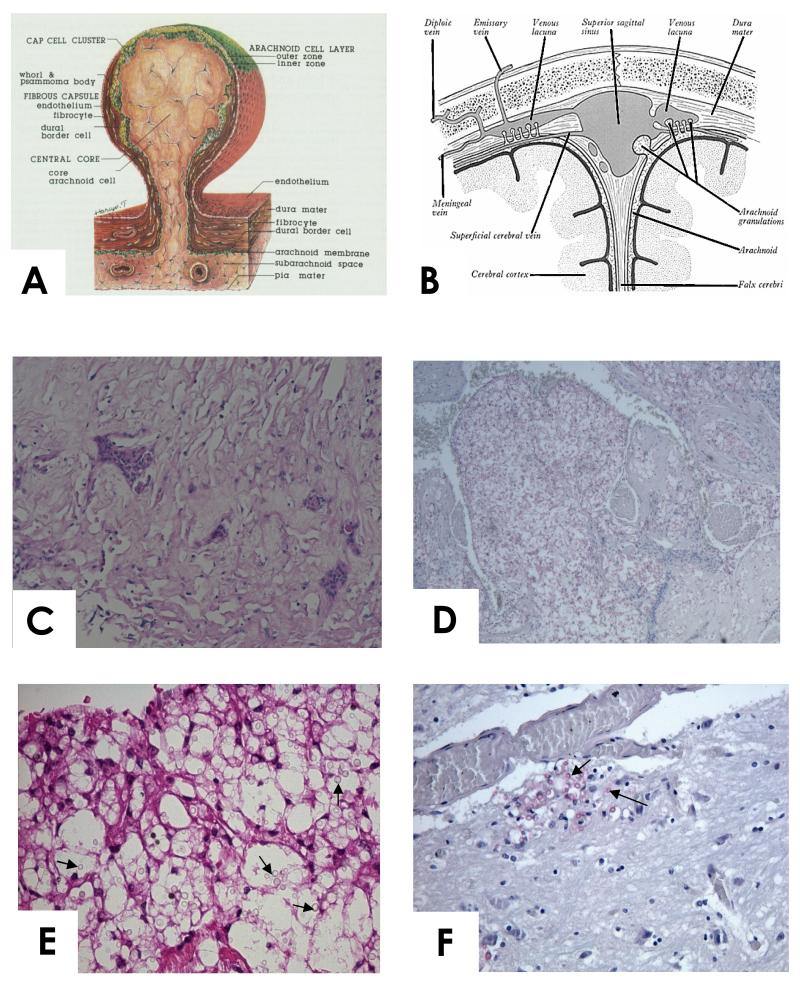
Figure1.
A. Diagram of the structure of human arachnoid granulation (from [10], with permission). B. Diagram illustrating position of the arachnoid granulations between the sub arachnoid space and venous sinus. C. High power view of normal arachnoid granulation from a patient dying of unrelated cause (H&E stain). D and E (high power). Case 1. Arachnoid granulation with large numbers of cryptococcal cells (arrowed) and little inflammation. Mucicarmine stain. F. Case 1. Brain tissue showing cryptococcal cells (arrowed) invading brain adjacent to a blood vessel. Mucicarmine stain. Note the paucity of organisms in the brain tissue compared to the arachnoid granulations.
Case 2
A 31 year old female presented with a 2 day history of headache, seizures, and confusion. Glasgow Coma Scale was 12/15. There was no focal neurology. CSF results were: WBCs 41 cells/mm3, protein 1.15 g/L, glucose 2.7mmol/L, CRAG titre of 1: 2084, quantitative culture: 2,250,000 CFU/ml. OP was 18 cm on admission and 53 cm on day 3. The CD4 cell count was 99 ×106/L and HIV viral load 2,400 copies/ml. On the second day of treatment with amphotericin B and flucytosine, the patient developed respiratory distress, a chest x-ray revealed a right lower lobe infiltrate, and antibiotics for aspiration pneumonia were started. The patient died on day 3.
Post mortem showed cryptococcal cells in the arachnoid granulations (mean 36 /highpower field, range 11-62, which contained a neutrophilic infiltrate, fibrosis and stromal necrosis (Figure 2A,B). The channels were dilated. Venous thrombosis of meningeal vessels at the depths of the sulci was noted.
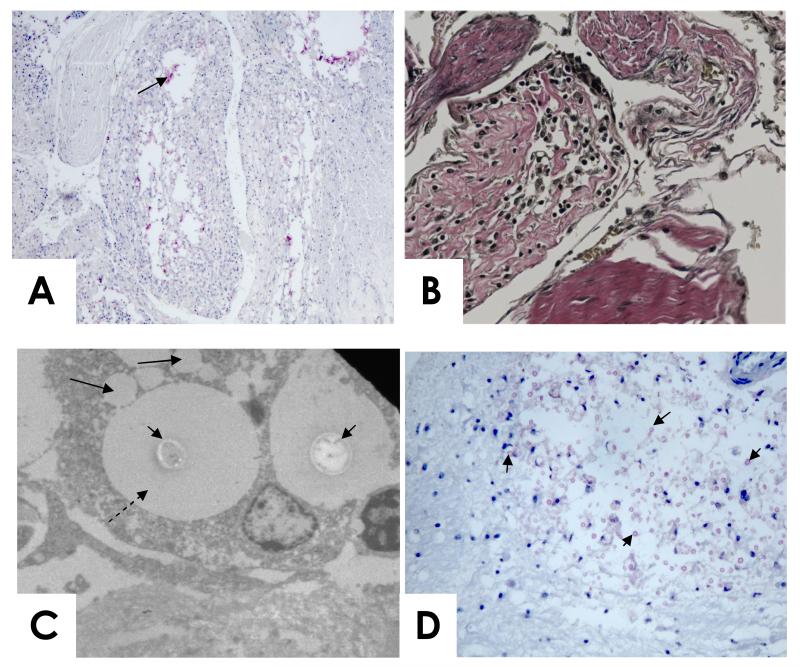
Figure 2.
A. (mucicarmine stain) and B. (high power, elastic Von Gieson stain, which stains collagen pink) Case 2. Arachnoid granulation with cryptococci (arrowed), necrosis, and fibrosis. The channels within the granulation appear compressed.
C. Case 4. Electron microscopy of an arachnoid cell containing two cryptococcal cells (short arrows) with large capsules (dashed arrow). Vacuoles within the cell contain material of the same electron density as the cryptococcal capsule (long solid arrrows).
D. Case 4. Cryptococcal cells (arrowed) in the brainstem. Mucicarmine stain
Two patients with moderately raised CSF pressure
Case 3
A 33 year old male presented with headache. He had a left 6th cranial nerve palsy. CSF showed: white blood cells 0 cells/mm3, protein 0.57g/L, glucose 1.3 mmol/L, quantitative culture 1,130,000 CFU/ml. CSF OP was 28 cm H20. The CD4 cell count and HIV viral load are not known. The patient died the day after admission. Post mortem showed arachnoid granulations with cryptococci (mean 61 /highpower field, range 44-126), chronic inflammation, neutrophils and fibrosis.
Case 4
A 33 year old female presented with a one month history of headache, photophobia, vomiting and seizures. She had a left 6th cranial nerve palsy. Mental status was normal. CSF results were: WBCs 5 cells/mm3, protein 0.37 g/L, glucose 2.2 mmol/L, quantitative culture 695,000 CFU/ml. CSF OP was 25 cm H20. The CD4 cell count was 19 ×106/L and HIV viral load 450,000 copies/ml. The patient died suddenly on the second day of antifungal therapy.
The post-mortem showed disseminated cryptococcosis. Cryptococcomas were found in the lungs, lymph nodes, adrenals, spleen, liver and brain. Cryptococcal meningitis was evident with a giant cell and lymphocytic reaction. The arachnoid granulations contained cryptococci (mean 20 /highpower field, range 3-48) and an inflammatory reaction with giant cells. Electron microscopy showed encapsulated yeasts within arachnoid cells, which also had vacuoles that appeared to contain cryptococcal polysaccharide (Figure 2C). Of note was the invasion of pons and midbrain by cryptococci, without an inflammatory response (Figure 2D).
A patient with normal CSF pressure
Case 5
A 28 year old female was admitted with headache, confusion, and seizures. GCS was 15/15. She had a rash consistent with cutaneous cryptococcosis. A CT brain scan demonstrated brain atrophy with multiple hypodensities consistent with infarcts in the basal ganglia bilaterally and the left cerebellar hemisphere. CSF results were: WBCs 2 cells/mm3, protein 0.22 g/L, glucose 2.7 mmol/L, CRAG titre of 1:2048, quantitative culture: 1,575,000 CFU/ml CSF. The CSF OP was 13cm H2O. The CD4 cell count was 27 ×106/L and HIV viral load 210,000 copies/ml. The patient died on the first day of therapy.
Post-mortem showed cryptococcal meningitis and disseminated cryptococcosis, with multiple intracerebral cryptococcomas. Relatively few organisms were seen in the arachnoid granulations (mean 19 /highpower field, range 2-40) in which the channels were widely dilated. There were sparse lymphocytes, plasma cells and multinucleate giant cells.
Discussion
This study provides the first direct evidence of the extent to which the arachnoid granulations accumulate cryptococcal cells in patients with cryptococcal meningitis. There were more organisms in the granulations than in the rest of the brain parenchyma, consistent with the concentration of viable and perhaps dead organisms in the granulations as a consequence of the circulation of CSF, as has been observed with red blood cells in patients with sub-arachnoid hemorrhage [7]. Although this is a very small series, the CSF opening pressure correlated with the mean number of organisms per high power field in the granulations (opening pressures 80, 53, 28, 25, 13 for cases 1 to 5, with respective mean cryptococcal cells of 286, 36, 61, 20, and 19, Spearmans correlation coefficient 0.9, p = 0.04). The findings add to the evidence supporting obstruction to CSF outflow as the primary mechanism leading to raised CSF opening pressure in patients with cryptococcal meningitis.
The study also serves to illustrate the widespread involvement of the brain, as well as the meninges, in patients with cryptococcal meningo-encephalitis [8]. We postulate that invasion of the brainstem, or other vital structures, by cryptococcal cells, as demonstrated in case 4, may be one mechanism leading to sudden death in patients with cryptococcal meningitis.
In man, the arachnoid granulations probably play a predominant role in CSF re-absorption, although lymphatic drainage around perineural sheaths of cranial and somatic nerve roots, and even transependymal passage of CSF into the brain may contribute [9]. The core of the arachnoid granulation consists of bundles of connective tissue fibres forming a porous meshwork with channels [9-11]. The barrier to CSF flow is provided by the arachnoid cell layer which at the apex of the granulation is in direct contact with the venous blood (Figure 1A). Here the layer is 3-5 cells thick, forming a cap cell cluster with a complex architecture of interdigitating cell processes.
Electron microscopy studies suggest both paracellular and transcellular, via intracellular vacuoles and pinocytosis, passage of CSF and particles. In monkeys, passage across granulations of Saccharomyces cerevisiae yeasts (3-6 μm), and goat (4 μm) and monkey (7 μm) erythrocytes, was demonstrated, while latex spheres of 6-12 μm were excluded [12]. In dogs, 25% of labelled red blood cells injected in the cisterna magna were recovered intact in the peripheral blood while the remainder were enmeshed within the arachnoid [13]. In man, free passage of particles up to 2 μm in diameter [14] has been shown while red blood cells appear within the granulations of patients following sub-arachnoid hemorrhage [15]. Of note some of the encapsulated cryptococcal fungal cells we observed in arachnoid cells by electron microscopy would have exceeded these dimensions. Cryptococci are spheroidal to ovoid in shape and measure from 3-20μm in diameter with the majority in the 6-9μm range. High concentrations of particles have been shown to impede CSF outflow [12].
In subarachnoid hemorrhage, accumulation of red blood cells in the granulation leads to an inflammatory response [7]. In our cases, despite low CD4 cell counts, lymphocytic and granulomatous responses, stromal necrosis, and fibrosis were seen within the arachnoid granulations. There was no immunologic reaction in only one case. It seems likely that the accumulation of encapsulated fungal cells and free lying polysacccharide in the granulations of patients with severe disease and high organism burdens, together with any associated inflammatory response, could obstruct arachnoid granulation function and reduce CSF outflow.
The pathophysiological basis of raised pressure is of great clinical significance since it underlies current recommendations to treat raised pressure with repeat lumbar puncture and careful CSF drainage. Adherence to these guidelines was shown to be associated with improved outcome in a small series [16], and in a large cohort of patients treated with repeat LPs, no association was found between OP and survival [5], in contrast to earlier studies upon which the guidelines were based in which repeat LPs were not routinely implemented [2].
Many questions remain to be answered. Although a high organism load and cyptocooccal antigen titer appear necessary for the development of high pressure, they are not sufficient [5]. Other factors that may be involved include the organism phenotype in particular the capsule phenotype [17]. It is also unclear how long the defect in reabsorption lasts, and whether it may become irreversible. While some patients respond to a number of days of repeat lumbar punctures others do not. Some of these non-responding patients respond to a temporary lumbar drain, but experience with this intervention is still limited, and the usual duration of drainage required is unknown.
Acknowledgements
We thank Gertrude Ntombomzi Williams and Nomqondiso Sidibana for help with obtaining consent for the postmortems at GF Jooste Hospital.
Financial support: Medicines Research Council UK, Wellcome Trust UK
Sources of support: Medical Research Council (UK), Wellcome Trust (UK)
Footnotes
Conflicts of interest: All authors: No conflicts
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Park B, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller T. Estimation of the global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. 2009;23(4):525–530. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Graybill JR, Sobel J, Saag M, van Der Horst C, Powderly W, Cloud G, et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. The NIAID Mycoses Study Group and AIDS Cooperative Treatment Groups. Clin Infect Dis. 2000;30(1):47–54. doi: 10.1086/313603. [DOI] [PubMed] [Google Scholar]
- 3.Pappas PG. A Phase II Randomized Trial of Amphotericin B Alone or Combined with Fluconazole in the Treatment of HIV-Associated Cryptococcal Meningitis. Clin Infect Dis. 2009;48:1775–83. doi: 10.1086/599112. [DOI] [PubMed] [Google Scholar]
- 4.Saag MS, et al. Practice guidelines for the management of cryptococcal disease. Clin Infect Dis. 2000;30:710–8. doi: 10.1086/313757. [DOI] [PubMed] [Google Scholar]
- 5.Bicanic T, Brouwer AE, Meintjes G, Rebe K, Limmathurotsakul D, Chierakul W, et al. Relationship of CSF pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS. 2009;23:701–6. doi: 10.1097/QAD.0b013e32832605fe. [DOI] [PubMed] [Google Scholar]
- 6.Denning DW, Armstrong RW, Stevens DA. Elevated cerebrospinal fluid pressures in patients with cryptococcal meningitis and acquired immunodeficiency syndrome. The American Journal of Medicine. 1991;91:267–272. doi: 10.1016/0002-9343(91)90126-i. [DOI] [PubMed] [Google Scholar]
- 7.Massicote EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurgery. 1999;91:80–84. doi: 10.3171/jns.1999.91.1.0080. [DOI] [PubMed] [Google Scholar]
- 8.Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol. 1996;27:839–47. doi: 10.1016/s0046-8177(96)90459-1. [DOI] [PubMed] [Google Scholar]
- 9.Kapoor KG, Katz SE, Grzybowski DM, Lubow M. Cerebrospinal fluid outflow: An evolving perspective. Brian Res Bull. 2008;77:327–34. doi: 10.1016/j.brainresbull.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Kida S, Yamashima T, Kubota T, Ito H, Yamamoto S. A light and electron microscopic and immunohistochemical study of human arachnoid villi. J Neurosurgery. 1988;69:429–35. doi: 10.3171/jns.1988.69.3.0429. [DOI] [PubMed] [Google Scholar]
- 11.Conegero CI, Chopard RP. Tridimensional architecture of the collagen element in the archnoid granulations in humans. Arq Neuropsiquiatr. 2003;61:561–5. doi: 10.1590/s0004-282x2003000400007. [DOI] [PubMed] [Google Scholar]
- 12.Welch K, Pollay M. Perfusion of particles through arachnoid villi of the monkey. Am J Physiol. 1961;201:651–4. doi: 10.1152/ajplegacy.1961.201.4.651. [DOI] [PubMed] [Google Scholar]
- 13.Adams JE, Prawirohardjo S. Neurology. 1959;9:561. [Google Scholar]
- 14.Glimcher SA, Holman DW, Lubow M, Grzybowski DM. Ex vivo model of cerebrospinal fluid outflow across human archnoid granulations. Invest Opthalmol Vis Sci. 2008;49:4721–8. doi: 10.1167/iovs.08-2238. [DOI] [PubMed] [Google Scholar]
- 15.Yamashima T. Ultrastructural study of the final cerebrospinal fluid pathway in human arachnoid villi. Brain Res. 1986;384:68–76. doi: 10.1016/0006-8993(86)91220-5. [DOI] [PubMed] [Google Scholar]
- 16.Shoham S, Cover C, Donegan N, Fulnecky E, Kumar P. Cryptococcus neoformans meningitis at 2 hospitals in Washington, D.C.: adherence of health care providers to published practice guidelines for the management of cryptococcal disease. Clin Infect Dis. 2005;40:477–479. doi: 10.1086/427213. [DOI] [PubMed] [Google Scholar]
- 17.Fries BC, Lee SC, Keenan R, Zhao W, Casadevall A, Goldman DL. Phenotypic switching of Cryptococcus neoformans can produce variants that elicit increased intracranial pressure in a rat model of cryptococcal meningiencephalitis. Infect Immun. 2005;73:1779–1787. doi: 10.1128/IAI.73.3.1779-1787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]