Abstract
The dose-responsiveness of plasma oxylipins to incremental dietary intake of arachidonic acid (20:4n-6; ARA) and docosahexaenoic acid (22:6n-3; DHA) was determined in piglets. Piglets randomly received one of six formulas (n = 8 per group) from days 3 to 27 postnatally. Diets contained incremental ARA or incremental DHA levels as follows (% fatty acid, ARA/DHA): (A1) 0.1/1.0; (A2) 0.53/1.0; (A3–D3) 0.69/1.0; (A4) 1.1/1.0; (D1) 0.66/0.33; and (D2) 0.67/0.62, resulting in incremental intake (g/kg BW/day) of ARA: 0.07 ± 0.01, 0.43 ± 0.03, 0.55 ± 0.03, and 0.82 ± 0.05 at constant DHA intake (0.82 ± 0.05), or incremental intake of DHA: 0.27 ± 0.02, 0.49 ± 0.03, and 0.81 ± 0.05 at constant ARA intake (0.54 ± 0.04). Plasma oxylipin concentrations and free plasma PUFA levels were determined at day 28 using LC-MS/MS. Incremental dietary ARA intake dose-dependently increased plasma ARA levels. In parallel, ARA intake dose-dependently increased ARA-derived diols 5,6- and 14,15-dihydroxyeicosatrienoic acid (DiHETrE) and linoleic acid-derived 12,13-dihydroxyoctadecenoic acid (DiHOME), downstream metabolites of cytochrome P450 expoxygenase (CYP). The ARA epoxide products from CYP are important in vascular homeostatic maintenance. Incremental DHA intake increased plasma DHA and most markedly raised the eicosapentaenoic acid (EPA) metabolite 17,18-dihydroxyeicosatetraenoic acid (DiHETE) and the DHA metabolite 19,20-dihydroxydocosapentaenoic acid (DiHDPE). In conclusion, increasing ARA and DHA intake dose-dependently influenced endogenous n-6 and n-3 oxylipin plasma concentrations in growing piglets, although the biological relevance of these findings remains to be determined.
Keywords: lipidomics, metabolomics, eicosanoid, prostaglandin, omega-3, omega-6 polyunsaturated fatty acid
Human milk contains the essential fatty acids linoleic acid (18:2n-6) and α-linolenic acid (18:3n-3) and their respective n-3 and n-6 long-chain polyunsaturated fatty acid (LCPUFA) metabolites. Arachidonic acid (20:4n-6; ARA) and docosahexaenoic acid (22:6n-3; DHA) are the most abundant LCPUFA in human milk (1). DHA and ARA accumulate in brain and retina at a high rate during the last trimester of pregnancy and the first months of life (2). DHA and ARA may be synthesized in the body from the precursor essential fatty acids, α-linolenic acid (18:3n-3; ALA) and linoleic acid (18:2n-6; LA), respectively. However, the endogenous conversion efficiency of these precursor fatty acids to ARA and DHA is estimated to be low in infants and not as efficient as preformed ARA and DHA in sustaining ARA and DHA plasma levels (3–6). Many studies indicate that DHA and ARA-enriched formula from birth to one year of age may confer benefits on visual acuity maturation and aspects of cognitive development compared with unenriched formula, particularly in premature infants (7–12).
Fatty acids of the n-3 and n-6 families are dose-dependently incorporated into cell membranes (13, 14). Data from animal studies have demonstrated that during early growth and development, the brain is particularly sensitive to DHA intake (15), while thymus (16), small intestine (17), liver, and heart (18) are particularly sensitive to ARA intake. Nonesterified PUFA can be enzymatically oxidized to generate a wide range of lipophilic signaling molecules known collectively as oxylipins (19). The polyunsaturated fatty acids linoleic acid, dihomo-γ-linolenic acid (20:3n-6; DGLA), ARA, α-linolenic acid, eicosapentaenoic acid (20:5n-3; EPA), and DHA are the main precursors for various oxylipins (19). The three primary enzymes catalyzing the oxygenation of these fatty acids into oxylipins are the cytochrome P450 expoxygenases (CYP), cyclooxygenases (COX), and lipoxygenases (LOX) (19). Other enzymes, such as soluble epoxide hydrolases (sEH), can further metabolize some of the oxylipin species.
Oxylipins are currently the focus of considerable interest as they can act as intercellular signaling molecules and are involved in the regulation of many cell and tissue responses (20). Oxylipins can exert a wide range of effects in biological tissues, including the regulation of cellular proliferation (21, 22), inflammation or inflammation resolution (23–25), and vascular function (23, 26). Novel analytical methodologies to quantify the large spectrum of plasma lipids have emerged and reveal a remarkable diversity of oxylipins in human plasma (19, 27). The biological roles of many of these oxylipins have not yet been fully elucidated. Over the past decade there has been growing interest in pharmacological modulation of oxylipin enzymatic pathways to mediate beneficial vascular and inflammatory effects (28–32). In previous studies with mice (30) and healthy volunteers (19, 31), it was shown that dietary intake of n-3 LCPUFA was able to alter plasma oxylipin levels. Despite intense interest in the biological effects of dietary LCPUFA in infant nutrition, the influence of dietary DHA and ARA on plasma oxylipin levels has not previously been explored in growing mammals, including humans. We report here a lipidomics-based study of plasma oxylipin profile using a dose-response design in growing piglets.
MATERIALS AND METHODS
Animals
The study was conducted in conformity with the Public Health Service Policy on Humane Care and approved by the Institutional Animal Care and Use Committee at Cornell University. Details of the experimental methods have been described elsewhere (18). Briefly, piglets aged three days postnatal, were randomly assigned to receive one of six formula diets or to continue maternal rearing (n = 8 per group; 4 males and 4 females). Piglet weights at day three varied between 1.70 and 2.45 kg. Piglets from the different litters (2–3 piglets per litter) and of different weights were uniformly distributed among the groups. Formula diets were fed from age 3 to 27 days. Total 24 h formula intake was measured daily in the morning. Formula diets consisted of 60% experimental diet (Research Diets, Inc., New Brunswick, NJ) and 40% Birthright baby pig milk replacer (Ralco Nutrition, Inc., Marshall, MN). The composition of the formula diets has been previously reported (18). Formula diets had a caloric density of 0.7 kcal/ml. Diets contained constant DHA concentrations (1.02 ± 0.02 wt% of total fatty acids) in conjunction with increasing ARA concentrations [0.09 (A1), 0.53 (A2), 0.69 (A3–D3), and 1.06 (A4) wt% of total fatty acids, respectively] or constant ARA concentrations (0.67 ± 0.02 wt% of total fatty acids) with incremental DHA concentrations [0.33 (D1), 0.62 (D2), 1.04 (A3–D3) wt% of total fatty acids, respectively]. The dietary ARA levels (0.67 wt%) are reflective of current human infant formulas. The DHA levels (1.02 wt%) are higher than in infant formula (0.33 wt%), reflecting high-end breast milk concentrations of fish-eating populations (1).
Venous blood was collected at day 28, the morning after last formula feeding at day 27. Details have been described elsewhere (18). Plasma levels of free fatty acids were analyzed by LC-MS/MS. In short, protein in the samples was precipitated by adding 50 µl of internal standard solution and 1050 µl of methanol to 50 µl of plasma. After centrifugation, the supernatant was transferred into an injection vial. For (relative) quantification, a mixture of internal standards [32 exogenous calibrants and 12 internal standards] was used. The free fatty acids were analyzed by LC-MS/MS using a Waters AQCUITY UltraPerformance LC (UPLC) System with an ACQUITY UPLC HSS T3 1.8 µm, 2.1 × 100 mm column (Waters, Milford, MA) and an Agilent Q-TOF 6530 High Resolution Mass Spectrometer (Agilent, Santa Clara, CA) using reference mass correction. Data preprocessing was performed using Agilent MassHunter Quantitative Analysis software; all peaks were checked manually. Samples were analyzed in batches with QC samples added after every 10 samples. The QC samples were used to assess data quality and to correct for instrument response variations. Free fatty acid levels were reported as relative quantitative values of target compound to internal standard.
Oxylipin analysis
Oxylipin profiling was done according to the method as described by Strassburg et al. (27). Thus, 250 µl aliquots of piglet plasma were taken and subjected to solid phase extraction with hydrophilic-lipophilic-balanced material (Oasis HLB, Waters, Etten-Leur, The Netherlands) using ethyl acetate to elute analytes. To concentrate, the eluate was gently dried under a nitrogen stream and reconstituted in 50 µl of injection solvent (acetonitrile and methanol, 1:1 v/v). A 5 µl aliquot was injected into the LC-MS for analysis. Separation was done by HPLC (Agilent 1260, San Jose, CA) using an Ascentis Express column (2.1 × 150 mm, 2.7 µm particles; Supelco, Bellefonte, PA) with 0.35 ml/min flow rate during a 28 min gradient. The HPLC was coupled to electrospray ionization on a triple-quadrupole mass spectrometer (Agilent 6460, San Jose, CA). Oxylipins were detected in negative ion mode using dynamic multiple reaction monitoring (MRM). The raw data were preprocessed using Agilent MassHunter Quantitative Analysis software (Version B.04.00), and quantitation of oxylipin response was calculated as the peak area ratios of the target analyte to the respective internal standard. To obtain actual concentrations, calibration samples with spiked oxylipin levels were included in the measurement series. Oxylipins were classified according to the LIPID MAPS Structure Database (33).
Statistics
All statistical analyses were performed using R version 2.13.0. Relative response ratios of oxylipins were converted into actual concentrations (nM) using the R chemCal package (free software, R-project). Second-order (quadratic) regression models were applied to examine dose-response relationships between the 24 day mean dietary DHA or ARA intake and log plasma oxylipin or log PUFA levels. As predictor, variable dietary levels were corrected for effective intake per body weight (BW) throughout growth by expressing as g/kg BW/day. Linear mixed modeling was performed for varying dietary DHA intake (at fixed ARA intake), for gender, and their interactions as independent (fixed) variable, and litter as a random variable. To remove nonsignificant terms, stepwise regression was used. Some dose-response curves followed a curvilinear (quadratic) relationship. Therefore, we also included a quadratic term in our stepwise regression approach. Modeling for varying ARA intake was performed in the same way but with DHA intake as the fixed factor. The maternally reared group was excluded from the analysis as the sow's diet differed from the formula diets in more aspects than only DHA and ARA concentrations. The LC-MS/MS data were log-transformed before modeling. False discovery rate (FDR) control was used to correct for multiple comparisons. Data are presented as the mean ± SD.
RESULTS
Effect of ARA on plasma PUFAs
Dietary intervention groups receiving incremental dietary ARA levels, consumed on average 0.07 ± 0.01, 0.43 ± 0.03, 0.55 ± 0.03, and 0.82 ± 0.05 g of ARA/kg BW/day, respectively, at constant DHA (0.82 ± 0.05g/kg BW/day) over the course of 24 days of formula feeding (Table 1). Mixed model regression analysis showed that incremental ARA intake by the piglets significantly increased the plasma levels of ARA (Table 1). The significant increase in plasma DHA, linoleic acid, and α-linolenic acid levels that occurred with increasing ARA intake disappeared after correcting for multiple testing (Table 1). Supplementary Table I shows the coefficients, P values, and FDRs obtained from linear mixed modeling for the relationships between ARA intake and plasma fatty acid levels.
TABLE 1.
Mean plasma levels (arbitrary values) of polyunsaturated fatty acids after varying intakes of ARA (A1–A4) and DHA (D1–D3)
| Group |
Effecta |
|||||||||||||||||||||||
| A1 |
A2 |
A3–D3 |
A4 |
D1 |
D2 |
DHA | Gender | DHA × Gender | ARA | Gender | ARA × Gender | |||||||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |||||||
| ARA (g/kg BW/day) | 8 | 0.07 | 0.01 | 7 | 0.43 | 0.03 | 7 | 0.55 | 0.03 | 7 | 0.82 | 0.05 | 8 | 0.53 | 0.03 | 8 | 0.54 | 0.05 | ||||||
| DHA (g/kg BW/day) | 8 | 0.80 | 0.07 | 7 | 0.82 | 0.06 | 7 | 0.81 | 0.05 | 7 | 0.83 | 0.05 | 8 | 0.49 | 0.03 | 8 | 0.27 | 0.02 | ||||||
| Plasma levels | ||||||||||||||||||||||||
| 18:2n-6 (LA) | 8 | 2.56 | 1.61 | 7 | 3.03 | 1.51 | 7 | 3.67 | 1.35 | 7 | 3.78 | 1.84 | 8 | 2.97 | 0.79 | 8 | 2.70 | 0.68 | ++ | |||||
| 18:3n-3 (ALA) | 8 | 0.14 | 0.09 | 7 | 0.16 | 0.08 | 7 | 0.19 | 0.07 | 7 | 0.22 | 0.11 | 8 | 0.17 | 0.05 | 8 | 0.14 | 0.03 | ++ | |||||
| 20:4n-6 (ARA) | 8 | 1.06 | 0.26 | 6 | 1.21 | 0.58 | 7 | 1.53 | 0.47 | 7 | 2.01 | 0.27 | 8 | 1.78 | 0.52 | 8 | 1.58 | 0.49 | ++* | |||||
| 20:5n-3 (EPA) | 8 | 0.03 | 0.01 | 7 | 0.02 | 0.01 | 7 | 0.02 | 0.01 | 7 | 0.03 | 0.01 | 8 | 0.02 | 0.01 | 8 | 0.02 | 0.01 | ||||||
| 22:6n-3 (DHA) | 8 | 1.11 | 0.28 | 6 | 0.98 | 0.47 | 7 | 1.25 | 0.33 | 7 | 1.42 | 0.20 | 8 | 0.65 | 0.16 | 8 | 0.90 | 0.28 | ++* | ++ | + | |||
Significances by linear mixed modeling methodology for dose-response effect of dietary ARA or DHA and gender (interaction) effects on fatty acids. +, positive term in the regression model; ++, significant at 95% confidence level; +, significant at 90% confidence level; *, significant after FDR correction.
Effect of DHA on plasma PUFAs
The three dietary DHA levels resulted in a mean DHA intake of 0.27 ± 0.02, 0.49 ± 0.03, and 0.81 ± 0.05 g/kg BW/day, respectively, at constant ARA intake (0.54 ± 0.04 g/kg BW/day) over the 24 days of feeding. The plasma levels of DHA dose-dependently increased with higher amounts of DHA ingested (Table 1). Other plasma fatty acids were not significantly affected. Dietary DHA in our study did not significantly affect plasma EPA levels, but heart and liver tissue phospholipid EPA levels increased with DHA (data not shown). The coefficients, P values, and FDRs for the effects of dietary DHA intake on plasma fatty acids are shown in supplementary Table I.
Detected plasma oxylipins
The metabolites, identified in pig plasma after 24 days of dietary intake, are shown in Tables 2 and 3. Supplementary Fig. I shows MS/MS spectra, MRM transition information, and structures of diols detected in piglet plasma. Despite sufficient sensitivity of the method, only oxylipins known to be enzymatically produced were detected (i.e., no nonenzymatically produced metabolites were detected). Linoleic acid-derived metabolites included 9- and 13-hydroxyoctadecadienoic acid (HODE), 13-ketooctadecadienoic acid (KODE), 9(10)- and 12(13)-epoxyoctadecenoic acid (EpOME), and 9,10- and 12,13-dihydroxyoctadecenoic acid (DiHOME). The prostaglandin F1α (PGF1α) metabolite detected in plasma is a dihomo-γ-linolenic acid-derived oxylipin. Detected ARA metabolites included prostaglandin-E2 (PGE2), prostaglandin F2α (PGF2α), 13,14-dihydro-15-keto-PGF2α, thromboxane (TXB2), 12S-hydroxy-heptadecatrienoic acid (HHTrE), 5-, 8-, 11-, 12-, and 15-hydroxyeicosatetraenoic acid (HETE), and 5,6-, 11,12-, and 14,15-dihydroxyeicosatrienoic acid (DiHETrE). 9-Hydroxyoctatrienoic acid (HOTrE) is derived from α-linolenic acid. Derivatives of EPA comprised prostaglandin-F3α (PGF3α), 5S-hydroxyeicosapentaenoic acid (HEPE), and 17,18-dihydroxyeicosatetraenoic acid (DiHETE). Oxylipins from DHA included 19(20)-epoxydocosapentaenoic acid (EpDPE) and 19,20-dihydroxydocosapentaenoic acid (DiHDPE). The DHA-derivatives 16,17-EpDPE and 5(6)- and 14(15)-epoxyeicosatrienoic acid (EpETrE) were below the detection limits. Plasma oxylipin concentrations were in a similar range as measured previously in humans (19) except for a few metabolites [e.g., 19(20)-EpDPE and 5-HETE], which were exceptionally high in one or two piglets, causing high mean values and standard deviations. Because we had no independent indication that the corresponding measurements were outliers, they were not removed but attributed to biological variation.
TABLE 2.
Mean plasma concentrations (nM ± SD) of metabolites after increasing intake of ARA at constant DHA intake
| Mean ARA Intake (g/kg BW/day) |
Effectb |
|||||||||||||||||||
| Metabolite | Lipid Map IDa | Biosynthetic Pathway | PUFA | 0.07 |
0.43 |
0.55 |
0.82 |
ARA | Gender | ARA Quadratic | ARA × Gender | ARA Quadratic × Gender | ||||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | |||||||||
| 9(10)-EpOME | LMFA02000037 | CYP | 18:2n-6 (LA) | 8 | 2.0 | 0.9 | 8 | 2.0 | 1.3 | 8 | 1.9 | 0.5 | 8 | 2.6 | 1.8 | |||||
| 9,10-DiHOME | LMFA01050350 | CYP | 18:2n-6 (LA) | 8 | 7.0 | 2.0 | 8 | 7.1 | 1.8 | 8 | 7.6 | 2.2 | 8 | 9.7 | 4.3 | ++ | ||||
| 12(13)-EpOME | LMFA02000038 | CYP | 18:2n-6 (LA) | 8 | 2.5 | 1.3 | 8 | 2.8 | 1.4 | 8 | 2.5 | 1.4 | 8 | 3.3 | 2.1 | |||||
| 12,13-DiHOME | LMFA01050351 | CYP | 18:2n-6 (LA) | 8 | 6.9 | 2.0 | 8 | 8.0 | 2.0 | 8 | 8.4 | 2.3 | 8 | 10.3 | 3.6 | − | ++* | |||
| 9-HODE | LMFA01050278 | LOX | 18:2n-6 (LA) | 8 | 27.1 | 7.6 | 8 | 32.7 | 13.0 | 8 | 30.3 | 7.3 | 8 | 38.5 | 23.1 | |||||
| 13-HODE | LMFA01050349 | LOX | 18:2n-6 (LA) | 8 | 51.0 | 15.4 | 8 | 66.8 | 26.5 | 8 | 60.4 | 20.0 | 8 | 72.1 | 38.0 | + | ||||
| 13-KODE | LMFA02000016 | LOX | 18:2n-6 (LA) | 8 | 3.7 | 2.0 | 8 | 3.4 | 1.1 | 8 | 4.3 | 1.7 | 8 | 5.2 | 2.9 | |||||
| 9-HOTrE | LMFA02000024 | LOX | 18:3n-3 (ALA) | 8 | 1.2 | 0.4 | 8 | 1.2 | 0.6 | 8 | 1.3 | 0.4 | 8 | 1.5 | 0.9 | |||||
| PGF1α | LMFA03010137 | COX | 20:3n-6 (DGLA) | 8 | 2.2 | 0.5 | 8 | 2.0 | 0.7 | 8 | 1.9 | 0.6 | 8 | 1.7 | 0.6 | − | ||||
| PGF2α | LMFA03010002 | COX | 20:4n-6 (ARA) | 8 | 3.5 | 1.5 | 8 | 17.3 | 23.5 | 8 | 2.8 | 0.6 | 8 | 5.6 | 6.7 | |||||
| PGE2 | LMFA03010003 | COX | 20:4n-6 (ARA) | 8 | 0.8 | 0.2 | 8 | 1.7 | 1.7 | 8 | 0.9 | 0.3 | 8 | 0.9 | 0.4 | ++ | ||||
| 13,14-dihydro-15-keto-PGFα | LMFA03010027 | COX | 20:4n-6 (ARA) | 6 | 1.4 | 0.3 | 7 | 1.3 | 0.5 | 7 | 1.1 | 0.3 | 8 | 1.8 | 1.5 | |||||
| TXB2 | LMFA03030002 | COX | 20:4n-6 (ARA) | 8 | 4.7 | 2.6 | 8 | 23.7 | 32.2 | 8 | 2.5 | 0.8 | 8 | 8.3 | 9.6 | |||||
| 11-HETE | LMFA03060028 | COX | 20:4n-6 (ARA) | 8 | 2.5 | 3.5 | 8 | 3.4 | 3.3 | 8 | 1.5 | 0.4 | 8 | 2.2 | 0.9 | + | ||||
| 5,6-DiHETrE | LMFA03050004 | CYP | 20:4n-6 (ARA) | 8 | 1.2 | 0.5 | 8 | 1.5 | 0.4 | 8 | 1.8 | 1.0 | 8 | 2.3 | 0.7 | ++* | ||||
| 11,12-DiHETrE | LMFA03050008 | CYP | 20:4n-6 (ARA) | 8 | 1.2 | 0.4 | 8 | 1.2 | 0.4 | 8 | 1.4 | 0.5 | 8 | 1.6 | 0.8 | |||||
| 14,15-DiHETrE | LMFA03050010 | CYP | 20:4n-6 (ARA) | 8 | 2.4 | 0.6 | 8 | 3.8 | 0.9 | 8 | 3.5 | 0.8 | 8 | 4.3 | 1.0 | ++* | ++* | − | ||
| 5-HETE | LMFA03060002 | LOX | 20:4n-6 (ARA) | 8 | 38.3 | 74.6 | 8 | 32.0 | 57.5 | 8 | 17.4 | 8.9 | 8 | 16.3 | 7.1 | |||||
| 8-HETE | LMFA03060006 | LOX | 20:4n-6 (ARA) | 8 | 3.7 | 3.2 | 8 | 3.4 | 2.2 | 8 | 2.7 | 0.8 | 8 | 3.5 | 1.1 | (+) | ++ | − | ||
| 12-HETE | LMFA03060088 | LOX | 20:4n-6 (ARA) | 8 | 3.6 | 4.5 | 8 | 4.2 | 4.2 | 8 | 2.5 | 0.7 | 8 | 3.1 | 0.9 | |||||
| 15-HETE | LMFA03060001 | LOX | 20:4n-6 (ARA) | 8 | 2.3 | 2.3 | 8 | 4.1 | 3.4 | 8 | 1.8 | 0.5 | 8 | 2.1 | 0.7 | |||||
| PGF3α | LMFA03010138 | COX | 20:5n-3 (EPA) | 8 | 6.4 | 3.4 | 8 | 8.9 | 7.8 | 7 | 7.8 | 5.0 | 8 | 6.0 | 3.0 | |||||
| 17,18-DiHETE | LMFA03060078 | CYP | 20:5n-3 (EPA) | 8 | 43.1 | 11.9 | 8 | 45.1 | 12.0 | 8 | 47.9 | 21.8 | 8 | 42.0 | 8.9 | |||||
| 5(S)-HEPE | LMFA03070010 | LOX | 20:5n-3 (EPA) | 8 | 2.3 | 3.4 | 8 | 2.0 | 2.4 | 8 | 1.0 | 0.4 | 8 | 0.8 | 0.4 | − | ||||
| 19(20)-EpDPE | LMFA04000038 | CYP | 22:5n-3 (DHA) | 8 | 372.0 | 781.4 | 8 | 266.7 | 546.4 | 8 | 90.9 | 47.5 | 8 | 81.4 | 31.9 | |||||
| 19,20-DiHDPA | LMFA04000043 | CYP | 22:5n-3 (DHA) | 8 | 17.1 | 4.3 | 8 | 17.6 | 3.0 | 8 | 19.2 | 7.2 | 8 | 18.6 | 4.3 | |||||
Lipid classification according to LIPID MAPS Structure Database (33).
Significances by linear mixed modeling methodology for dose-response effect of dietary ARA or DHA and gender (interaction) effects on fatty acids. +/−, positive/negative term in the regression model; ++/−−, significant at 95% confidence level; +/−, significant at 90% confidence level; (+), other terms; *, significant after FDR correction.
TABLE 3.
Mean plasma concentrations (nM ± SD) of metabolites after increasing intake of DHA at constant ARA
| Mean DHA intake (g/kg BW/day) |
Effectb |
||||||||||||||||
| Metabolite | Lipid Map IDa | Biosynthetic Pathway | PUFA | 0.27 |
0.49 |
0.83 |
DHA | Gender | DHA Quadratic | DHA × Gender | DHA Quadratic × Gender | ||||||
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |||||||||
| 9(10)-EpOME | LMFA02000037 | CYP | 18:2n-6 (LA) | 8 | 1.6 | 0.6 | 8 | 1.9 | 0.9 | 8 | 1.9 | 0.5 | (−) | − | (+) | + | − |
| 9,10-DiHOME | LMFA01050350 | CYP | 18:2n-6 (LA) | 8 | 7.6 | 1.6 | 8 | 6.7 | 1.6 | 8 | 7.6 | 2.2 | −− | −− | ++ | + | − |
| 12(13)-EpOME | LMFA02000038 | CYP | 18:2n-6 (LA) | 8 | 2.6 | 1.0 | 8 | 2.7 | 1.0 | 8 | 2.5 | 1.4 | (−) | − | (+) | + | −− |
| 12,13-DiHOME | LMFA01050351 | CYP | 18:2n-6 (LA) | 8 | 8.2 | 1.7 | 8 | 7.4 | 1.6 | 8 | 8.4 | 2.3 | −− | (+) | ++ | − | |
| 9-HODE | LMFA01050278 | LOX | 18:2n-6 (LA) | 8 | 27.5 | 7.2 | 8 | 23.4 | 5.5 | 8 | 30.3 | 7.3 | (−) | + | |||
| 13-HODE | LMFA01050349 | LOX | 18:2n-6 (LA) | 8 | 55.9 | 18.1 | 8 | 49.9 | 16.6 | 8 | 60.4 | 20.0 | |||||
| 13-KODE | LMFA02000016 | LOX | 18:2n-6 (LA) | 8 | 3.6 | 1.2 | 8 | 2.9 | 0.8 | 8 | 4.3 | 1.7 | |||||
| 9-HOTrE | LMFA02000024 | LOX | 18:3n-3 (ALA) | 8 | 1.1 | 0.3 | 8 | 1.0 | 0.3 | 8 | 1.3 | 0.4 | |||||
| PGF1α | LMFA03010137 | COX | 20:3n-6 (DGLA) | 8 | 2.0 | 0.9 | 8 | 1.8 | 0.3 | 8 | 1.9 | 0.6 | |||||
| PGF2α | LMFA03010002 | COX | 20:4n-6 (ARA) | 8 | 3.5 | 1.4 | 8 | 4.3 | 2.8 | 8 | 2.8 | 0.6 | |||||
| PGE2 | LMFA03010003 | COX | 20:4n-6 (ARA) | 8 | 0.9 | 0.6 | 8 | 0.9 | 0.9 | 8 | 0.9 | 0.3 | (−) | −− | + | ||
| 13,14-dihydro-15-keto-PGF2α | LMFA03010027 | COX | 20:4n-6 (ARA) | 6 | 1.2 | 0.3 | 7 | 1.5 | 0.3 | 7 | 1.1 | 0.3 | (+) | − | (−) | + | (−) |
| TXB2 | LMFA03030002 | COX | 20:4n-6 (ARA) | 8 | 3.1 | 1.1 | 8 | 5.5 | 3.9 | 8 | 2.5 | 0.8 | ++ | −− | |||
| 11-HETE | LMFA03060028 | COX | 20:4n-6 (ARA) | 8 | 1.8 | 0.7 | 8 | 2.3 | 1.8 | 8 | 1.5 | 0.4 | |||||
| 5,6-DiHETrE | LMFA03050004 | CYP | 20:4n-6 (ARA) | 8 | 1.8 | 0.5 | 8 | 2.0 | 1.2 | 8 | 1.8 | 1.0 | |||||
| 11,12-DiHETrE | LMFA03050008 | CYP | 20:4n-6 (ARA) | 8 | 1.5 | 0.5 | 8 | 1.2 | 0.3 | 8 | 1.4 | 0.5 | |||||
| 14,15-DiHETrE | LMFA03050010 | CYP | 20:4n-6 (ARA) | 8 | 3.9 | 1.4 | 8 | 3.4 | 1.1 | 8 | 3.5 | 0.8 | ++ | ||||
| 5-HETE | LMFA03060002 | LOX | 20:4n-6 (ARA) | 7 | 2.1 | 1.0 | 8 | 2.1 | 1.2 | 8 | 1.8 | 0.5 | |||||
| 8-HETE | LMFA03060006 | LOX | 20:4n-6 (ARA) | 8 | 22.0 | 24.6 | 8 | 29.0 | 46.1 | 8 | 17.4 | 8.9 | |||||
| 12-HETE | LMFA03060088 | LOX | 20:4n-6 (ARA) | 8 | 3.0 | 0.9 | 8 | 2.9 | 1.6 | 8 | 2.7 | 0.8 | |||||
| 15-HETE | LMFA03060001 | LOX | 20:4n-6 (ARA) | 8 | 3.2 | 2.1 | 8 | 3.3 | 3.4 | 8 | 2.5 | 0.7 | |||||
| PGF3α | LMFA03010138 | COX | 20:5n-3 (EPA) | 8 | 6.9 | 3.5 | 7 | 8.8 | 5.8 | 7 | 7.8 | 5.0 | |||||
| 17,18-DiHETE | LMFA03060078 | CYP | 20:5n-3 (EPA) | 8 | 31.6 | 13.4 | 8 | 33.7 | 13.8 | 8 | 47.9 | 21.8 | ++* | ||||
| 5(S)-HEPE | LMFA03070010 | LOX | 20:5n-3 (EPA) | 8 | 0.6 | 0.2 | 8 | 1.0 | 0.9 | 8 | 1.0 | 0.4 | ++ | ||||
| 19(20)-EpDPE | LMFA04000038 | CYP | 22:5n-3 (DHA) | 8 | 84.4 | 113.8 | 8 | 116.7 | 197.9 | 8 | 90.9 | 47.5 | − | ||||
| 19,20-DiHDPA | LMFA04000043 | CYP | 22:5n-3 (DHA) | 8 | 11.7 | 4.1 | 8 | 12.9 | 4.2 | 8 | 19.2 | 7.2 | ++* | ||||
Lipid classification according to LIPID MAPS Structure Database (33).
Significances by linear mixed modeling methodology for dose-response effect of dietary ARA or DHA and gender (interaction) effects on fatty acids. +/−, positive/negative term in the regression model; ++/−−, significant at 95% confidence level; +/−, significant at 90% confidence level; (+)/(−), other terms; *, significant after FDR correction.
Effect of ARA intake on plasma oxylipins
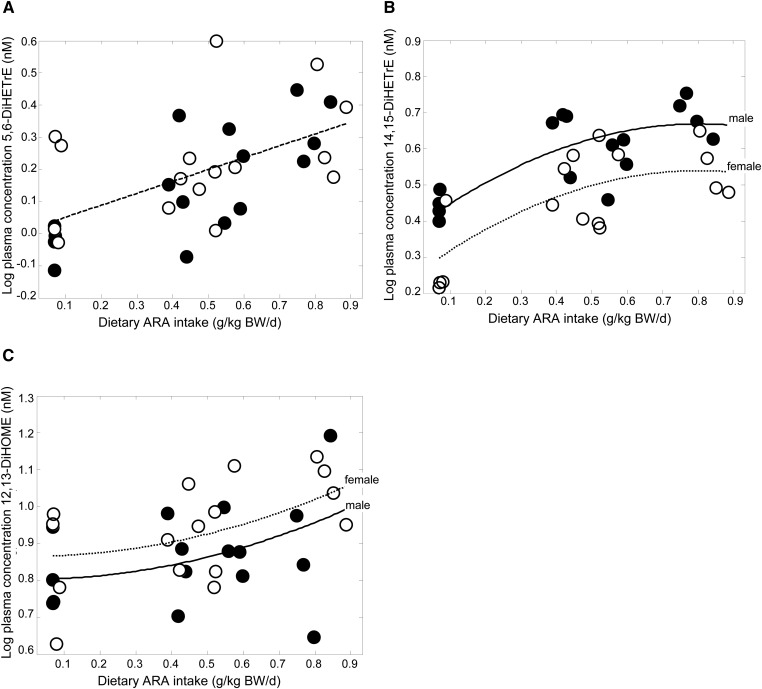
Regression analysis demonstrated that incremental ARA intake significantly increased plasma concentrations of the CYP/sEH-derived ARA metabolites 5,6-DiHETrE and 14,15-DiHETrE after correcting for multiple testing (Table 2 and Fig. 1A, B). The coefficients, P values, and FDRs of the mixed modeling are shown in supplementary Table II. Incremental ARA intake by the piglets did not significantly affect any of the detected ARA-derived LOX- or COX-synthesized eicosanoids (5-HETE, 8-HETE, 11-HETE, 12-HETE, 15-HETE, TXB2, PGF2α, PGE2, and 13,14-dihydro-15-keto-PGF2α).
Fig. 1.
Significant relationships between dietary ARA at constant DHA and (A) ARA-derived 5,6-DiHETrE, (B) ARA-derived 14,15-DiHETrE, and (C) linoleic acid-derived 12,13-DiHOME. Filled circle, male; open circle, female.
Increasing ARA intake elevated plasma 12,13-DiHOME, a CYP/sEH-derived metabolite of linoleic acid (Fig. 1C and Table 2). No significant effects of ARA intake were observed on DHA, α-linolenic acid, or dihomo-γ-linolenic acid derivatives following multiple comparison correction.
Effect of DHA intake on plasma oxylipins
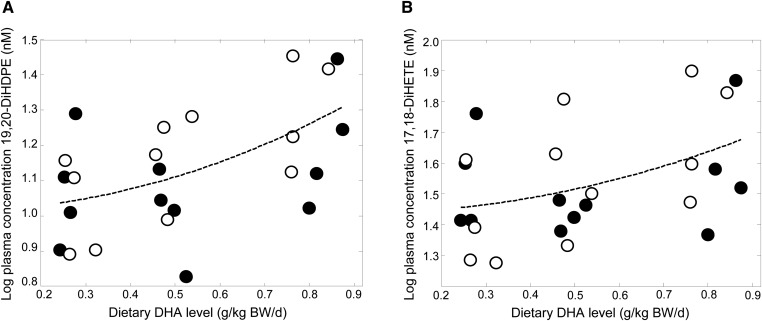
DHA intake dose-dependently increased the plasma CYP/sEH-catalyzed DHA metabolite 19,20-DiHDPE after the multiple comparison procedure (Table 3 and Fig. 2A), although no effect of DHA was apparent on the precursor metabolite 19(20)-EpDPE. Dietary DHA also increased the CYP/sEH-catalyzed EPA metabolite 17,18-DiHETE (Fig. 2B), but the precursor 17(18)-EpETE was not detected in plasma. No significant effects were observed of increasing DHA intake on oxylipins derived from α-linolenic acid, ARA, DGLA, or linoleic acid after correcting for multiple comparisons. The coefficients, P values, and FDRs are shown in supplementary Table III.
Fig. 2.
Significant relationship between dietary DHA at constant ARA and (A) DHA-derived 19,20-DiHDPE and (B) EPA-derived 17,18-DiHETE. Filled circle, male; open circle, female.
DISCUSSION
Pigs are an accepted model of human nutrition since they are very similar to humans with respect to anatomy, physiology (34, 35), and fatty acid metabolism (36). Based on the high homology between the pig and human genome, high sequence homology between human and porcine COX and LOX enzymes can be expected. Some porcine CYP enzymes showed about 70–83% homology to human forms and metabolized the same test substrates (37). This is the first study to report on the dose responsiveness of fatty acid-derived oxylipins to dietary intake of ARA and DHA during early postnatal development in piglets. The use of metabolomics technology in this study allowed us to characterize the detectable piglet oxylipin metabolome. The data show that some endogenous n-6 and n-3 oxylipin levels can be dose-dependently modulated by dietary DHA and ARA levels. Most oxylipin curves did not follow Michaelis-Menten enzyme kinetics, which could be explained by the involvement of multiple downstream enzymes in their production and the nonclassical enzyme kinetics as described for some CYP enzymes (38). The extent to which these oxylipins may mediate beneficial health effects remains to be explored. These data help to focus such studies on particular oxylipin species in various dietary circumstances of ARA/DHA intake during early development.
Arachidonic acid metabolites
Eicosanoids generated via COX and LOX exert many important functions, as exemplified by various pathologies manifested in mice lacking LOX and COX expression (39, 40). Despite the traditional belief that ARA-derived eicosanoids generated via the COX or LOX pathway contribute to the initiation of inflammation, emerging data suggest that these eicosanoids also contribute to the resolution of inflammation (23, 41–44). In perinatal development, COX-catalyzed PGE2 is an important lipid metabolite not only in immune cell homeostasis (45) but also in intestinal crypt proliferation (45) and preservation of neural function (46). Results of this study in growing piglets demonstrate that increasing dietary ARA levels do not affect plasma concentrations of the LOX- or COX-synthesized ARA metabolites, including PGE2, in spite of the increased levels of ARA in plasma and tissue (18). Also, plasma inflammation markers remained unchanged by increasing dietary ARA, as was previously reported (47). Although DHA may compete with ARA for membrane phospholipid incorporation and subsequent eicosanoid formation, dietary DHA also did not influence any of LOX- or COX-synthesized ARA metabolites, In several studies, dietary n-3 LCPUFA was shown to reduce ARA-derived LOX and COX products from ex vivo immune cells in the presence of an inflammatory stimulus (48–51). Our data provide no evidence that ARA-rich or DHA-rich formula influence circulating COX or LOX eicosanoids in healthy growing piglets. As physiologically comparable levels of ARA are found in human breast milk and infant formula, comparable effects in humans may be inferred. The LC-MS/MS method employed is sufficiently sensitive to detect multiple LOX- or COX-synthesized eicosanoids from ARA. The COX- and LOX-derived metabolites are rapidly inactivated and excreted, and some of these inactivation products are released to and/or created in the circulatory system. At continuous elevated production of eicosanoids, stable metabolites may accumulate to detectable levels in peripheral blood. However, a low-level production of mediators in peripheral tissues may cause only a transient or nondetectable change in circulating concentrations due to dilution in the greater volume of blood.
Increasing ARA intake dose-dependently raised plasma ARA levels in parallel with plasma 5,6-DiHETrE and 14,15-DiHETrE concentrations. The dose-dependent increases in these vicinal diols suggest increased conversion of ARA via the CYP/sEH enzymatic pathway. The precursor epoxides 5(6)-EpETrE and 14(15)-EpETrE were however not detected. EpETrEs may be difficult to detect; most EpETrE are esterified into phospholipids, whereas nonesterified EpETrEs are rapidly metabolized by sEH to their vicinal diols (26). In humans, 14(15)-EpETrE concentrations were on par with their corresponding vicinal diols (52). However, the analysis we employed had a one-third lower sensitivity for epoxides than for diols (27). Therefore, changes in the circulating concentrations of ARA-derived epoxides cannot be ruled out.
The epoxygenases of the CYP2 gene family have prominent roles in vascular regulation and generate the epoxides 5,6-, 8,9-, 11,12-, and 14,15-EpETrE from ARA (53). EpETrEs are produced by several tissues, including neural (54) and vascular tissues (55). Data from in vitro and in vivo studies demonstrate manifold biological activities of EpETrEs, including anti-inflammatory, neuroprotective, and vascular-protective effects like vasodilation (reviewed in Refs. 56, 57). Little is known about CYP metabolism in pigs, but porcine ovary was found to highly express CYP2J and sEH and produce EpETrEs and EpOMEs, as well as DiHETrEs and DiHOMEs during ovulation (58). In cultured endothelial cells of porcine origin, generation of 8,9-, 11,12-, and 14,15- EpETrE has also been observed (59, 60). In growing pigs, EpETrEs may play an important role in vascular formation, as they were shown to be involved in proliferation of porcine endothelial cells (61). These EpETrE metabolites are further metabolized by sEH to their corresponding vicinal diols (DiHETrEs), which have less biological activity than their precursor metabolites when sensitive endpoints are considered (62).
Linoleic acid metabolites
Another plasma metabolite that dose-dependently and curvilinearly increased with piglet intake of ARA was 12,13-DiHOME, while a nonsignificant trend was observed for 9,10-DiHOME. Linoleic acid epoxidation by CYP results in the 9(10)- and 12(13)-EpOME regiomers, which are rapidly hydrolyzed by sEH in other tissues to their corresponding diols, 9,10- and 12,13-DiHOME (63). Epoxides and diols of linoleic acid produced by activated inflammatory leukocytes have neutrophil chemotactic activity (64). A trend toward increasing plasma linoleic acid with increasing ARA intake was not significant; thus, increased conversion of linoleic acid to 12,13-DiHOME via the CYP/sEH pathway may be regulated by physiological demand due to ARA and not the level of substrate availability.
Eicosapentaenoic acid metabolites
In the current study, dietary DHA significantly increased EPA levels in tissue phospholipids, although this was not reflected in the plasma free fatty acid pool. Dietary DHA also increased the EPA-derived vicinal diol 17,18-DiHETE metabolized in the CYP/sEH pathway, suggesting that DHA retroconversion to EPA may occur to some extent. Due to competitive metabolic effects between n-3 and n-6 fatty acids, the effect of DHA on retroconversion to EPA and subsequent metabolites may be less pronounced when ARA is concomitantly present in the diet, as has been reported for pigs fed both DHA and ARA (65). Although little is known about the role of 17,18-DiHETE, it has been described to have anti-inflammatory properties (66). The EPA-derived COX-2-mediated PGF3α and LOX-5-mediated 5(s)-HEPE appeared unresponsive to dietary DHA changes and may require a stimulus for production.
Docosahexaenoic acid CYP metabolites
The most obvious finding was the dose-responsiveness of plasma 19,20-DiHDPE to dietary DHA intake of the piglets. This is similar to increased plasma 19,20-DiHDPE observed in healthy humans who consumed EPA and DHA from concentrated fish oil (31). However, in the latter study, a much broader range of oxylipins was significantly affected by EPA and DHA than in our study, possibly due to the much larger group sizes used. Little is known about this DHA-derived 19,20-DiHDPE diol, but recent research in sEH knockout mice showed that sEH metabolites, particularly 19,20-DiHDPE, play an important role in normal retinal vascularization (67). To what extent this metabolite is involved in the maturation of the retina remains to be established.
This study investigated the dose responsiveness of different plasma oxylipins to incremental dietary intakes of ARA and DHA in growing piglets. We considered the pig model to be a relatively representative model of the human because the LCPUFA metabolism and enzymes are highly comparable (36, 37, 65). Our data revealed no significant change in circulating COX or LOX products with increasing ARA or DHA in the diet. In general, constitutively expressed COX-1 is the source of prostanoids that serve housekeeping functions, whereas COX-2, induced by inflammatory stimuli and other factors, is the dominant source of prostanoid formation in inflammation (68). Prostanoids produced by COX-1 (e.g., PGE2, TXB2) are responsive to ARA substrate availability (69); however, circulating levels are generally very low to nondetectable unless COX-2 prostanoids are induced by an inflammatory stimulus (48, 68). The profile of prostanoid production is mostly determined by the regulated expression of COX-2 enzymes within cells present at sites of inflammation (68). The thromboids are also generally low in the circulation unless changes in platelet degranulation or blood pressure/volume are induced. In human studies, the LOX-dependent HETEs and CYP-dependent metabolite profiles were generally (but not exclusively) reflective of the parent lipid (i.e., substrate) distribution (19, 58), suggesting that most of the CYP-epoxygenases accept DHA and ARA as equally efficient substrates (70). Therefore, circulating concentrations of CYP-dependent epoxides and their hydrolysis products appear to be most responsive to changes in dietary PUFAs and appear to reflect changes in circulating PUFA concentrations.
In summary, results from the present study demonstrate the dose responsiveness of several circulating vicinal diols to dietary ARA and DHA levels in growing neonatal pigs. These compounds may be markers for active epoxide precursors involved in a variety of homeostatic functions. Dietary ARA levels within physiological ranges did not produce detectable changes in plasma concentrations of eicosanoids synthesized through the LOX- or COX-dependent pathway. Further investigations are warranted to probe the biological implication of dietary modification of these oxylipins as efforts continue to optimize ARA and DHA levels in infant formulas.
Supplementary Material
Footnotes
Abbreviations:
- ALA
- α-linolenic acid
- ARA
- arachidonic acid
- BW
- body weight
- COX
- cyclooxygenase
- CYP
- cytochrome P450 epoxygenase
- DGLA
- dihomo-γ-linolenic acid
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- DiHDPE
- dihydroxydocosapentaenoic acid
- DiHETE
- dihydroxyeicosatetraenoic acid
- DiHETrE
- dihydroxyeicosatrienoic acid
- DiHOME
- dihydroxyoctadeca(mono)enoic acid
- EpDPE
- epoxydocosapentaenoic acid
- EpETrE
- epoxyeicosatrienoic acid
- EpOME
- epoxyoctadecamonoenoic acid
- FDR
- false discovery rate
- HEPE
- hydroxyeicosapentaenoic acid
- HETE
- hydroxyeicosatetraenoic acid
- HHTrE
- hydroxy-heptadecatrienoic acid
- HODE
- hydroxyoctadecadienoic acid
- HOTrE
- hydroxyoctatrienoic acid
- KODE
- ketooctadecadienoic acid
- LA
- linoleic acid
- LOX
- lipoxygenase
- PG
- prostaglandin
- LCPUFA
- long-chain polyunsaturated fatty acid
- sEH
- soluble epoxide hydrolase
- TX
- thromboxane
This work was supported by DSM Biotechnology Center (Delft, The Netherlands, and Columbia, MD) (C.T. and J.T.B.) and by US Department of Agriculture (USDA), Agricultural Research Service (ARS), CRIS project 5306-51530-019-00D (J.W.N.). The plasma and statistical analyses were (co)financed by the Netherlands Metabolomics Centre (NMC), which is a part of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (R.J.V. and K.S.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and three tables.
REFERENCES
- 1.Brenna J. T., Varamini B., Jensen R. G., Diersen-Schade D. A., Boettcher J. A., Arterburn L. M. 2007. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 85: 1457–1464 [DOI] [PubMed] [Google Scholar]
- 2.Martinez M. 1992. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 120: S129–S138 [DOI] [PubMed] [Google Scholar]
- 3.Pawlosky R. J., Lin Y. H., Llanos A., Mena P., Uauy R., Salem N., Jr 2006. Compartmental analyses of plasma 13C- and 2H-labeled n-6 fatty acids arising from oral administrations of 13C-U-18:2n-6 and 2H5–20:3n-6 in newborn infants. Pediatr. Res. 60: 327–333 [DOI] [PubMed] [Google Scholar]
- 4.Salem N., Jr, Wegher B., Mena P., Uauy R. 1996. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc. Natl. Acad. Sci. USA. 93: 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y. H., Llanos A., Mena P., Uauy R., Salem N., Jr, Pawlosky R. J. 2010. Compartmental analyses of 2H5-alpha-linolenic acid and C-U-eicosapentaenoic acid toward synthesis of plasma labeled 22:6n-3 in newborn term infants. Am. J. Clin. Nutr. 92: 284–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koletzko B., Decsi T., Demmelmair H. 1996. Arachidonic acid supply and metabolism in human infants born at full term. Lipids. 31: 79–83 [DOI] [PubMed] [Google Scholar]
- 7.O'Connor D. L., Hall R., Adamkin D., Auestad N., Castillo M., Connor W. E., Connor S. L., Fitzgerald K., Groh-Wargo S., Hartmann E. E., et al. 2001. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics. 108: 359–371 [DOI] [PubMed] [Google Scholar]
- 8.Westerberg A. C., Schei R., Henriksen C., Smith L., Veierod M. B., Drevon C. A., Iversen P. O. 2010. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta Paediatr. 99: 556–562 [DOI] [PubMed] [Google Scholar]
- 9.Birch E. E., Hoffman D. R., Castaneda Y. S., Fawcett S. L., Birch D. G., Uauy R. D. 2002. A randomized controlled trial of long-chain polyunsaturated fatty acid supplementation of formula in term infants after weaning at 6 wk of age. Am. J. Clin. Nutr. 75: 570–580 [DOI] [PubMed] [Google Scholar]
- 10.Birch E. E., Hoffman D. R., Uauy R., Birch D. G., Prestidge C. 1998. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr. Res. 44: 201–209 [DOI] [PubMed] [Google Scholar]
- 11.Birch E. E., Garfield S., Hoffman D. R., Uauy R., Birch D. G. 2000. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 42: 174–181 [DOI] [PubMed] [Google Scholar]
- 12.Hoffman D. R., Birch E. E., Birch D. G., Uauy R., Castaneda Y. S., Lapus M. G., Wheaton D. H. 2000. Impact of early dietary intake and blood lipid composition of long-chain polyunsaturated fatty acids on later visual development. J. Pediatr. Gastroenterol. Nutr. 31: 540–553 [DOI] [PubMed] [Google Scholar]
- 13.Arterburn L. M., Hall E. B., Oken H. 2006. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 83: 1467S–1476S [DOI] [PubMed] [Google Scholar]
- 14.Abedin L., Lien E. L., Vingrys A. J., Sinclair A. J. 1999. The effects of dietary alpha-linolenic acid compared with docosahexaenoic acid on brain, retina, liver, and heart in the guinea pig. Lipids. 34: 475–482 [DOI] [PubMed] [Google Scholar]
- 15.Diau G. Y., Hsieh A. T., Sarkadi-Nagy E. A., Wijendran V., Nathanielsz P. W., Brenna J. T. 2005. The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med. 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harbige L. S. 2003. Fatty acids, the immune response, and autoimmunity: a question of n-6 essentiality and the balance between n-6 and n-3. Lipids. 38: 323–341 [DOI] [PubMed] [Google Scholar]
- 17.Hess H. A., Corl B. A., Lin X., Jacobi S. K., Harrell R. J., Blikslager A. T., Odle J. 2008. Enrichment of intestinal mucosal phospholipids with arachidonic and eicosapentaenoic acids fed to suckling piglets is dose and time dependent. J. Nutr. 138: 2164–2171 [DOI] [PubMed] [Google Scholar]
- 18.Tyburczy C., Kothapalli K. S., Park W. J., Blank B. S., Bradford K. L., Zimmer J. P., Butt C. M., Salem N., Jr, Brenna J. T. 2011. Heart arachidonic acid is uniquely sensitive to dietary arachidonic acid and docosahexaenoic acid content in domestic piglets. Prostaglandins Leukot. Essent. Fatty Acids. 85: 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shearer G. C., Harris W. S., Pedersen T. L., Newman J. W. 2010. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J. Lipid Res. 51: 2074–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buczynski M. W., Dumlao D. S., Dennis E. A. 2009. Thematic Review Series: Proteomics. An integrated omics analysis of eicosanoid biology. J. Lipid Res. 50: 1015–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenson W. F. 2007. Prostaglandins and epithelial response to injury. Curr. Opin. Gastroenterol. 23: 107–110 [DOI] [PubMed] [Google Scholar]
- 22.Wray J., Bishop-Bailey D. 2008. Epoxygenases and peroxisome proliferator-activated receptors in mammalian vascular biology. Exp. Physiol. 93: 148–154 [DOI] [PubMed] [Google Scholar]
- 23.Stables M. J., Gilroy D. W. 2011. Old and new generation lipid mediators in acute inflammation and resolution. Prog. Lipid Res. 50: 35–51 [DOI] [PubMed] [Google Scholar]
- 24.Serhan C. N. 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 25: 101–137 [DOI] [PubMed] [Google Scholar]
- 25.Serhan C. N. 2005. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr. Opin. Clin. Nutr. Metab. Care. 8: 115–121 [DOI] [PubMed] [Google Scholar]
- 26.Spector A. A., Fang X., Snyder G. D., Weintraub N. L. 2004. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog. Lipid Res. 43: 55–90 [DOI] [PubMed] [Google Scholar]
- 27.Strassburg K., Huijbrechts A. M., Kortekaas K. A., Lindeman J. H., Pedersen T. L., Dane A., Berger R., Brenkman A., Hankemeier T., van Duynhoven J., et al. 2012. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: application in cardiac surgery. Anal. Bioanal. Chem. 404: 1413–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arita M., Yoshida M., Hong S., Tjonahen E., Glickman J. N., Petasis N. A., Blumberg R. S., Serhan C. N. 2005. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA. 102: 7671–7676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingraham R. H., Gless R. D., Lo H. Y. 2011. Soluble epoxide hydrolase inhibitors and their potential for treatment of multiple pathologic conditions. Curr. Med. Chem. 18: 587–603 [DOI] [PubMed] [Google Scholar]
- 30.Balvers M. G., Verhoeckx K. C., Bijlsma S., Rubingh C. M., Meijerink J., Wortelboer H. M., Witkamp R. F. 2012. Fish oil and inflammatory status alter the n-3 to n-6 balance of the endocannabinoid and oxylipin metabolomes in mouse plasma and tissues. Metabolomics. 8: 1130–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keenan A. H., Pedersen T. L., Fillaus K., Larson M. K., Shearer G. C., Newman J. W. 2012. Basal omega-3 fatty acid status affects fatty acid and oxylipin responses to high-dose n3-HUFA in healthy volunteers. J. Lipid Res. 53: 1662–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legler D. F., Bruckner M., Uetz-von Allmen E., Krause P. 2010. Prostaglandin E2 at new glance: novel insights in functional diversity offer therapeutic chances. Int. J. Biochem. Cell Biol. 42: 198–201 [DOI] [PubMed] [Google Scholar]
- 33.LIPID MAPS Lipidomics Gateway 2012. Available at: http://www.lipidmaps.org/
- 34.Dodds W. J. 1982. The pig model for biomedical research. Fed. Proc. 41: 247–256 [Google Scholar]
- 35.Miller E. R., Ullrey D. E. 1987. The pig as a model for human nutrition. Annu. Rev. Nutr. 7: 361–382 [DOI] [PubMed] [Google Scholar]
- 36.Innis S. M. 1993. The colostrum-deprived piglet as a model for study of infant lipid nutrition. J. Nutr. 123: 386–390 [DOI] [PubMed] [Google Scholar]
- 37.Skaanild M. T. 2006. Porcine cytochrome P450 and metabolism. Curr. Pharm. Des. 12: 1421–1427 [DOI] [PubMed] [Google Scholar]
- 38.Atkins W. M. 2005. Non-Michaelis-Menten kinetics in cytochrome P450-catalyzed reactions. Annu. Rev. Pharmacol. Toxicol. 45: 291–310 [DOI] [PubMed] [Google Scholar]
- 39.Hu C., Dandapat A., Sun L., Chen J., Marwali M. R., Romeo F., Sawamura T., Mehta J. L. 2008. LOX-1 deletion decreases collagen accumulation in atherosclerotic plaque in low-density lipoprotein receptor knockout mice fed a high-cholesterol diet. Cardiovasc. Res. 79: 287–293 [DOI] [PubMed] [Google Scholar]
- 40.Langenbach R., Loftin C., Lee C., Tiano H. 1999. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem. Pharmacol. 58: 1237–1246 [DOI] [PubMed] [Google Scholar]
- 41.Harris S. G., Padilla J., Koumas L., Ray D., Phipps R. P. 2002. Prostaglandins as modulators of immunity. Trends Immunol. 23: 144–150 [DOI] [PubMed] [Google Scholar]
- 42.Scher J. U., Pillinger M. H. 2009. The anti-inflammatory effects of prostaglandins. J. Investig. Med. 57: 703–708 [DOI] [PubMed] [Google Scholar]
- 43.Das U. N. 2011. Lipoxins as biomarkers of lupus and other inflammatory conditions. Lipids Health Dis. 10: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bannenberg G., Serhan C. N. 2010. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys. Acta. 1801: 1260–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan Y. Y., Monk J. M., Hou T. Y., Callaway E., Vincent L., Weeks B., Yang P., Chapkin R. S. 2012. Characterization of an arachidonic acid-deficient (Fads1 knock-out) mouse model. J. Lipid Res. 53: 1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Najarian T., Hardy P., Hou X., Lachapelle J., Doke A., Gobeil F., Jr, Roy M. S., Lachapelle P., Varma D. R., Chemtob S. 2000. Preservation of neural function in the perinate by high PGE(2) levels acting via EP(2) receptors. J. Appl. Physiol. 89: 777–784 [DOI] [PubMed] [Google Scholar]
- 47.Tyburczy C., Kothapalli K. S., Park W. J., Blank B. S., Liu Y. C., Nauroth J. M., Zimmer J. P., Salem N., Jr, Brenna J. T. 2012. Growth, clinical chemistry and immune function in domestic piglets fed varying ratios of arachidonic acid and DHA. Br. J. Nutr. 107: 809–816 [DOI] [PubMed] [Google Scholar]
- 48.Peterson L. D., Jeffery N. M., Thies F., Sanderson P., Newsholme E. A., Calder P. C. 1998. Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids. 33: 171–180 [DOI] [PubMed] [Google Scholar]
- 49.Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. 1985. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N. Engl. J. Med. 312: 1217–1224 [DOI] [PubMed] [Google Scholar]
- 50.Meydani S. N., Endres S., Woods M. M., Goldin B. R., Soo C., Morrill-Labrode A., Dinarello C. A., Gorbach S. L. 1991. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J. Nutr. 121: 547–555 [DOI] [PubMed] [Google Scholar]
- 51.Calder P. C. 2013. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br. J. Clin. Pharmacol. 75: 645–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Psychogios N., Hau D. D., Peng J., Guo A. C., Mandal R., Bouatra S., Sinelnikov I., Krishnamurthy R., Eisner R., Gautam B., et al. 2011. The human serum metabolome. PLoS ONE. 6: e16957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fleming I. 2011. The cytochrome P450 pathway in angiogenesis and endothelial cell biology. Cancer Metastasis Rev. 30: 541–555 [DOI] [PubMed] [Google Scholar]
- 54.Zhang W., Otsuka T., Sugo N., Ardeshiri A., Alhadid Y. K., Iliff J. J., DeBarber A. E., Koop D. R., Alkayed N. J. 2008. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 39: 2073–2078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosolowsky M., Falck J. R., Willerson J. T., Campbell W. B. 1990. Synthesis of lipoxygenase and epoxygenase products of arachidonic acid by normal and stenosed canine coronary arteries. Circ. Res. 66: 608–621 [DOI] [PubMed] [Google Scholar]
- 56.Sudhahar V., Shaw S., Imig J. D. 2010. Epoxyeicosatrienoic acid analogs and vascular function. Curr. Med. Chem. 17: 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iliff J. J., Jia J., Nelson J., Goyagi T., Klaus J., Alkayed N. J. 2010. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 91: 68–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newman J. W., Stok J. E., Vidal J. D., Corbin C. J., Huang Q., Hammock B. D., Conley A. J. 2004. Cytochrome p450-dependent lipid metabolism in preovulatory follicles. Endocrinology. 145: 5097–5105 [DOI] [PubMed] [Google Scholar]
- 59.Fisslthaler B., Popp R., Michaelis U. R., Kiss L., Fleming I., Busse R. 2001. Cyclic stretch enhances the expression and activity of coronary endothelium-derived hyperpolarizing factor synthase. Hypertension. 38: 1427–1432 [DOI] [PubMed] [Google Scholar]
- 60.Fang X., Kaduce T. L., Weintraub N. L., Spector A. A. 1997. Cytochrome P450 metabolites of arachidonic acid: rapid incorporation and hydration of 14,15-epoxyeicosatrienoic acid in arterial smooth muscle cells. Prostaglandins Leukot. Essent. Fatty Acids. 57: 367–371 [DOI] [PubMed] [Google Scholar]
- 61.Webler A. C., Michaelis U. R., Popp R., Barbosa-Sicard E., Murugan A., Falck J. R., Fisslthaler B., Fleming I. 2008. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. Am. J. Physiol. Cell Physiol. 295: C1292–C1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Imig J. D., Navar L. G., Roman R. J., Reddy K. K., Falck J. R. 1996. Actions of epoxygenase metabolites on the preglomerular vasculature. J. Am. Soc. Nephrol. 7: 2364–2370 [DOI] [PubMed] [Google Scholar]
- 63.Konkel A., Schunck W. H. 2011. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta. 1814: 210–222 [DOI] [PubMed] [Google Scholar]
- 64.Thompson D. A., Hammock B. D. 2007. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J. Biosci. 32: 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Craig-Schmidt M. C., Huang M. C. 1998. Interaction of n-3 and n-6 fatty acids: implications for supplementation of infant formula with long-chain fatty acids. In Lipids in Infant Nutrition. M. C. Huang and A. J. Sinclair, editors. AOCS Press. 63–84. [Google Scholar]
- 66.Morin C., Sirois M., Echave V., Albadine R., Rousseau E. 2010. 17,18-epoxyeicosatetraenoic acid targets PPARgamma and p38 mitogen-activated protein kinase to mediate its anti-inflammatory effects in the lung: role of soluble epoxide hydrolase. Am. J. Respir. Cell Mol. Biol. 43: 564–575 [DOI] [PubMed] [Google Scholar]
- 67.Hu J., Popp R., Awwad K., Fromel T., Fleming I. 2012. 19,20-DiHDPA, a product of the soluble epoxide hydrolase, promotes angiogenesis by direct inhibition of Notch signalling. Clin. Res. Cardiol. 101(Suppl. 1): April 2012. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22481434 [Google Scholar]
- 68.Ricciotti E., FitzGerald G. A. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31: 986–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caughey G. E., Cleland L. G., Penglis P. S., Gamble J. R., James M. J. 2001. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J. Immunol. 167: 2831–2838 [DOI] [PubMed] [Google Scholar]
- 70.Arnold C., Markovic M., Blossey K., Wallukat G., Fischer R., Dechend R., Konkel A., von Schacky C., Luft F. C., Muller D. N., et al. 2010. Arachidonic acid-metabolizing cytochrome P450 enzymes are targets of {omega}-3 fatty acids. J. Biol. Chem. 285: 32720–32733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.