Abstract
Bis(monoacylglycero)phosphate (BMP) assists lysosomal function by facilitating interaction of hydrolases and activator proteins with sphingolipid substrates. Impaired lysosomal degradation of the sphingolipid glucosylceramide (GC) occurs in Gaucher disease due to an inherited deficiency of acid β-glucosidase, with secondary BMP alterations. We investigated the nature of BMP accumulation and whether its correction reduced the storage burden in a THP-1 macrophage model of Gaucher disease. Using sucrose gradients and detergent solubility, 98% of BMP resided in the detergent-soluble membranes (DSM) rather than in the detergent-resistant membranes (DRM) where 73% of GC predominated. There was a 2-fold widespread elevation in BMP, including the saturated, mono- and polyunsaturated species. Linoleic acid in the culture media selectively reduced BMP from 4.2 nmol/mg to 0.49 nmol/mg (except 18:1/18:2) and prevented up to one third of GC, dihexosylceramide (DHC), and trihexosylceramide (THC) from accumulating. The 2-fold reduction in these sphingolipids occurred only in the DRM and did not reduce 18:1/16:0. However, once GC had accumulated, linoleic acid could not reverse it, DHC, or THC, despite effectively reducing BMP. These results imply a causative link for BMP in the pathobiology of Gaucher disease and demonstrate that linoleic acid can shield the cell from excessive substrate accumulation.
Keywords: detergent-resistant membranes, fatty acids, glucosylceramide, lysosomal disease, lysosomal dysfunction, lysosomal storage disorder, mass spectrometry, membrane microdomains
Bis(monoacylglycero)phosphate (BMP) belongs to the group of glycerophospholipids that consist of a glycerol-3-phosphate backbone whose sn1 and sn2 positions are esterified with fatty acids. The stereochemical configuration of BMP differs as each glycerol moiety is esterified through only the sn1 position to the phosphate and contains a single fatty acid (1). Evidence suggests that BMP is synthesized from its structural isomer, phosphatidylglycerol (2). The fatty acid from the sn2 position is removed with phospholipase A2 to produce lysophosphatidylglycerol, which then undergoes a transacylation reaction on the sn2 position of the glycerol head group (3, 4). The subsequent steps, which must remove the fatty acid from the sn1 position, rearrange the phosphoryl ester from the sn3 to the sn1 position and esterify the sn2 position to produce the final product, BMP, have not yet been adequately described. The synthesis of BMP is believed to occur in the lysosomal network, as BMP is highly enriched in the internal membranes of lysosomes (5).
The negative charge of BMP, coupled with its proposed cone-shaped structure, enables it to participate in the organization of the membranes of the lysosomal network, contributing to its multivesicular/multilamellar morphology (6, 7). These properties of BMP also facilitate lysosomal lipid degradation, which takes place on the surface of the inner membranes of lysosomes. At the acidic pH of the lysosome, BMP contributes its negative charge to enhance adherence of the polycationic enzymes and activator proteins, aiding in lipid hydrolysis (8). Together, the high BMP and low cholesterol content of the internal lysosomal membrane ensures the selective degradation of lipids destined for lysosomal degradation without affecting the limiting membrane of the lysosome (9).
BMP has been shown to be elevated in certain lysosomal diseases, which arise due to an inherited deficiency of a hydrolase or protein involved in lysosomal degradation (10, 11). Although the primary biochemical consequence is the accumulation of material in the lysosomes of affected cells due to the alteration of degradation pathways, in the majority of instances, the diseases are also associated with expansion of the lysosomal network and a corresponding increase in BMP (12). More recently, we have shown that the BMP profile is altered in cultured fibroblasts from lysosomal storage disorders regardless of the degree of BMP elevation. With the exception of Fabry disease, another seven disorders showed a loss of polyunsaturated BMP species relative to monounsaturated species (13). These changes in BMP composition are likely to alter the organization of the lysosomal membrane domains and interfere with lysosomal function. A role for BMP in lysosomal diseases is indicative, but the pathological significance is not known (14).
Gaucher disease, the most common of the lysosomal diseases, is the result of an inherited deficiency in acid β-glucosidase, the enzyme responsible for cleaving the β-glucosidase linkage in glucosylceramide (GC) to give glucose and ceramide (15). Consequently GC accumulates in affected cells. Although acid β-glucosidase is ubiquitous, the enzyme deficiency manifests primarily in the macrophage due to the acquisition of exogenously derived lipids from ingested senescent and apoptotic blood cells (16). Here we show that BMP accumulates in different membrane microdomains from the primary substrate, GC, in a Gaucher THP-1 macrophage model and that BMP could be lowered by manipulating its synthesis with an excessive amount of linoleic acid added to the culture media. This enables control over the fatty acids incorporated into BMP, aimed at preserving the integrity of the lysosomal membrane and facilitating lysosomal function. Furthermore, reducing BMP with linoleic acid reduced the amount of GC that accumulated in this model, thereby partially alleviating the primary storage burden. This study shows that it is possible to reduce the cellular consequences of Gaucher disease by correcting one aspect of this complex lysosomal disease even if the primary biochemical defect remains.
MATERIALS AND METHODS
Materials
Cell culture media and reagents, linoleic acid, oleic acid, and essentially fatty acid free BSA were purchased from Sigma (St. Louis, MO). FBS was purchased from In Vitro Technologies (Noble Park, Vic, Australia). Lipoprotein-deficient serum was purchased from Biomedical Technologies Inc. (Stoughton, MA). Conduritol B epoxide was purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). The internal standards ceramide (Cer) 18:1/17:0 {N-heptadecanoyl-D-erythro-sphingosine}; BMP 14:0/14:0 {bis(monomyristoylglycero)phosphate (S,R isomer) (ammonium salt)}; phosphatidylethanolamine (PE) 17:0/17:0 {1,2-diheptadecanoyl-sn-glycero-3-phosphoethanolamine}; phosphatidylglycerol (PG) 14:0/14:0 {1,2-dimyristoyl-sn-glycero-3-phospho-(1′-rac-glycerol) (sodium salt)}; and phosphatidylserine (PS) 17:0/17:0 {1,2-diheptadecanoyl-sn-glycero-3-phospho-L-serine (sodium salt)} were purchased from Avanti Polar Lipids (Alabaster, AL); dihexosylceramide (DHC) 18:1/16:0 (d3) {N-palmitoyl-d3-lactosylceramide}; GC 18:1/16:0 (d3) {N-palmitoyl-d3-glucopsychosine}; phosphatidylinositol (PI) 16:0/16:0 {phosphatidylinositol, dipalmitoyl (NH4+ salt)}; and trihexosylceramide (THC) 18:1/17:0 {N-heptadecanoyl ceramide trihexoside} were purchased from Matreya LLC (Pleasant Gap, PA); and phosphatidylcholine (PC) 14:0/14:0 {1,2-dimyristoyl-sn-glycero-3-phosphocholine} was purchased from Sigma (St. Louis, MO). All solvents were of HPLC grade, except chloroform, which contained 1% ethanol and was reagent grade, and were used without further purification.
Cell culture
THP-1 cells (human monocytic cell line) were differentiated into macrophages, and the Gaucher disease phenotype was induced by chemical inhibition of acid β-glucosidase with conduritol B epoxide to generate a Gaucher THP-1 macrophage model as described previously (17). These cells were cultured for 10 days in RPMI media supplemented with 10% FBS in the presence of 100 μM linoleic acid during induction of the Gaucher phenotype. When fatty acids (100 μM linoleic or oleic acid) were added for 24 h (following induction of the Gaucher phenotype), 10% FBS, 2% lipoprotein-deficient serum/0.4% essentially fatty acid free BSA, or serum free medium was used. Skin fibroblasts from two Gaucher patients (18) were grown to confluence before the addition of 100 μM linoleic acid for 24 h. The Women's and Children's Hospital Research Ethics Committee (Adelaide, Australia) approved the use of fibroblasts for this study. The addition of ethanol (0.1%, v/v) was substituted for the fatty acid and served as a control. Cells were harvested and washed twice with PBS before preparing homogenates by resuspending pellets in 200 μl 0.02 M Tris (pH 7.0) containing 0.5 M NaCl and 0.1% (v/v) nonidet P-40 and then sonicating for 20 s. Total cell protein was determined by the method of Lowry et al. (19).
Detergent-resistant membrane and detergent-soluble membrane isolation
Detergent-resistant membrane (DRM) and detergent-soluble membrane (DSM) were prepared from four T75 flasks of THP-1 macrophages with and without linoleic acid supplementation, according to the method of Lisanti et al. (20). Briefly, cell pellets were resuspended in 2 ml MES-buffered saline [MBS; 25 mM MES (pH 6.5) and 0.15 M NaCl] containing 1% (v/v) Triton X-100 and 1 mM PMSF, and then homogenized with 12 strokes of a Dounce homogenizer and incubated on ice for 30 min. Following incubation, the samples were centrifuged at 425 g for 5 min at 4°C to remove any cellular debris. A 50 μl aliquot of the supernatant was removed for total cell protein determination (19). The supernatant was placed in the bottom of a Beckman (Palo Alto, CA) centrifuge tube (14 mm × 95 mm), and the sucrose concentration adjusted to 40% (w/v) by the addition of 2 ml of 80% (w/v) sucrose in MBS containing 1% (v/v) Triton X-100 and 1 mM PMSF. The sample was overlaid with 5 ml of 30% (w/v) sucrose in MBS (without Triton X-100), followed by 3 ml of 5% (w/v) sucrose in MBS (without Triton X-100). Samples were centrifuged at 270,500 g for 16–20 h at 4°C using a swing-out rotor. Twelve fractions (each containing 1 ml) were collected from the top of the gradient.
Lipid extractions and mass spectrometric quantification
Lipids were extracted from 100 μg of total protein from cell homogenates according to the method of Folch et al. (21) or from 750 μl of each DRM and DSM fraction according to the method of Bligh and Dyer (22) with the inclusion of 400 pmol of BMP 14:0/14:0, Cer 18:1/17:0, DHC 18:1/16:0 (d3), GC 18:1/16:0 (d3), PC 14:0/14:0, PE 17:0/17:0, PG 14:0/14:0, PI 16:0/16:0, PS 17:0/17:0, and THC 18:1/17:0 as internal standards. Dried lipid extracts were resuspended in 200 μl of CH3OH containing 5 mM NH4COOH, and lipids were resolved using HPLC (Agilent) by injecting 20 µl onto a C18 (3 μm, 50 × 2.1 mm) column (Alltech) at a flow rate of 200 μl/min. The column was equilibrated in 70% mobile phase A (30% tetrahyrdofuran/20% CH3OH/50% H2O in 5 mM NH4COOH) and then linearly converted to 100% mobile phase B (70% tetrahyrdofuran/20% CH3OH/10% H2O in 5 mM NH4COOH) over 7 min and maintained there for 3 min. Reequilibration at 70% mobile phase A was performed for 3 min prior to the next injection. A Valco 10-port post column valve diverted column flow to waste for the first 1.6 min. Following chromatography, individual species of BMP, Cer, DHC, GC, PC, PE, PG, PI, PS, and THC were quantified using multiple reaction monitoring on a SCIEX API 4000 triple quadrupole mass spectrometer (see supplementary Table I) (13, 23). Concentrations of each molecular species were calculated by relating the peak areas of each species to the peak area of the corresponding internal standard using Analyst 1.4.2 software. To verify the accuracy of quantification, BMP, GC, and PE quantification was also performed against an eight-point calibration curve (see supplementary Table II).
RESULTS
BMP and phospholipids in the Gaucher THP-1 macrophage model
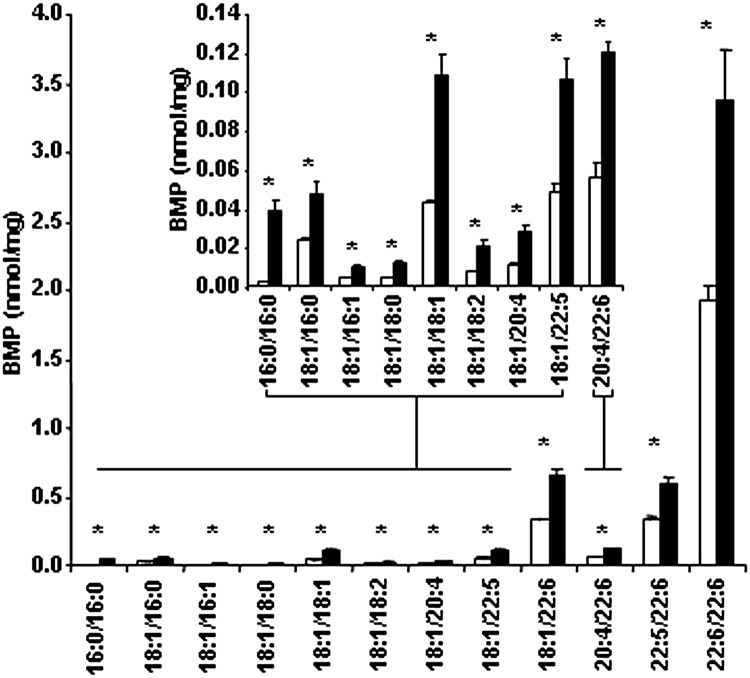
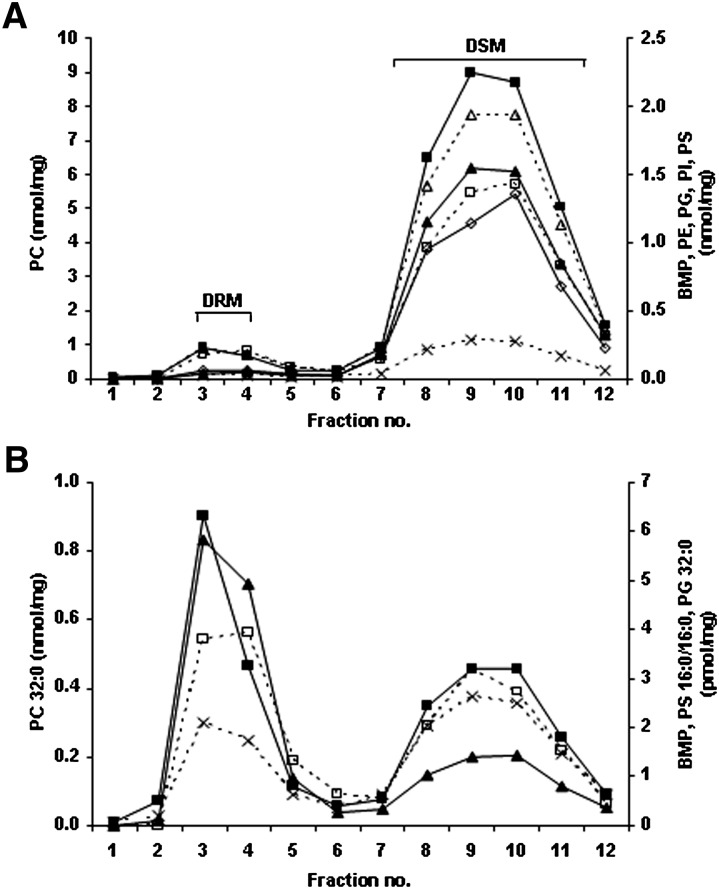
Fig. 1 shows that in the Gaucher THP-1 macrophage model all BMP species were significantly elevated, including the fully saturated fatty acid species (e.g., palmitic acid) as well as some monounsaturated (e.g., oleic acid) and polyunsaturated species (e.g., docosahexaenoic acid). PG, PC, PE, PI, and PS species gave similar concentrations in both the Gaucher THP-1 macrophage model and the THP-1 macrophages (data not shown). With the exception of the fully saturated BMP 16:0/16:0, PC 32:0, PG 32:0, and PS 16:0/16:0, which did not correlate, the remaining species measured in the membrane microdomains correlated (Pearson >0.8) and were therefore summed. Fig. 2A shows that the summed phospholipids (including BMP) were found almost exclusively (>91%) in the DSM (fractions 7 to 12). The fully saturated phospholipid species distributed differently, with 30–60% residing in the DRM (fractions 3 and 4) (Fig. 2B). THP-1 macrophages showed a similar distribution (data not shown).
Fig. 1.
BMP in the Gaucher THP-1 macrophage model. THP-1 macrophages were grown for 10 days, harvested, and then lipids extracted. Individual BMP species were measured by ESI-MS/MS and are shown in THP-1 macrophages (open bars) and the Gaucher THP-1 macrophage model (solid bars). Results are reported as nanomoles of each individual BMP species per milligram of total cell protein and expressed as the mean and standard deviation (n = 3). *Significant at P < 0.05 (Student t-test).
Fig. 2.
BMP and phospholipid distribution in DRMs and DSMs. Lipids in each fraction from the DRM and DSM isolation of the Gaucher THP-1 macrophage model were extracted and measured by ESI-MS/MS. (A) Individual species of BMP (solid triangles) (excluding BMP 16:0/16:0), PC (solid squares) (excluding PC 32:0), PE (open triangles), PG (crosses) (excluding PG 32:0), PI (open diamonds), and PS (open squares) (excluding PS 16:0/16:0) were summed in each of the membrane microdomain fractions to give total amounts. (B) Distribution of the fully saturated species, BMP 16:0/16:0 (solid triangles), PC 32:0 (solid squares), PG 32:0 (crosses), and PS 16:0/16:0 (open squares). Results are expressed as nanomoles or picomole of phospholipid per milligram of total protein loaded onto the gradient prior to fractionation.
BMP and other phospholipids with linoleic acid
We hypothesized that by maintaining normal BMP levels (by regulating fatty acid supply for synthesis), the integrity of the lysosomal membrane would be preserved. This should allow the lysosome to function more efficiently and prevent the excessive buildup of lipids, even though the primary metabolic defect remains uncorrected. Linoleic acid was included in the THP-1 macrophage culture media and maintained for the 10 days in culture during the induction of the Gaucher THP-1 macrophage model (see Materials and Methods). With the expected exception of 18:1/18:2, all species of BMP correlated (Pearson >0.8) and were summed. Table 1 shows that linoleic acid supplementation reduced BMP 9-fold and that this reduction was dose dependent (see supplementary Fig. I). As well as increased BMP 18:1/18:2 in the Gaucher THP-1 macrophage model, linoleic acid resulted in increases in some of the PC, PE, PG, and PS species, namely, PC 32:0, 34:2, and 36:2; PE 18:0/18:2 and 18:1/18:2; PG 36:2, 36:3, and 40:8; and PS 16:0/16:0 and 18:0/18:2. As these species correlated (Pearson >0.8) (except PC 32:0), they were summed and their increased concentrations are reported in Table 1. The remaining species of PC, PE, PG, PI, and PS measured also correlated (Pearson >0.8) (except PG 34:2, which was unaffected by linoleic acid) and were summed, but unlike the other species, there was a reduction in their concentration with linoleic acid. Although the phospholipid changes were notable in the DRM, they were more pronounced in the DSM as this is where the majority of the phospholipids reside (Table 1). Table 1 also shows that linoleic acid had the same effect on THP-1 macrophages as the Gaucher THP-1 macrophage model.
TABLE 1.
BMP and phospholipids, GC and sphingolipids, in the Gaucher THP-1 macrophage model with linoleic acid
| Cellsb | DRMc | DSMc | ||||||||||
| Analytea | Control | Control + LAd | Gaucher | Gaucher + LAd | Control | Control + LAd | Gaucher | Gaucher + LAd | Control | Control + LAd | Gaucher | Gaucher + LAd |
| BMP | 2.6 (0.23) | 0.33* (0.02) | 4.2 (0.20) | 0.49* (0.04) | 0.04 (0.01) | 0* (0) | 0.12 (0.02) | 0.01* (0) | 5.7 (1.1) | 0.66* (0.18) | 7.3 (0.13) | 0.82* (0.33) |
| BMP 18:1/18:2 | 0.01 (0) | 0.05** (0) | 0.02 (0) | 0.10** (0.01) | 0 (0) | 0** (0) | 0 (0) | 0** (0) | 0.01 (0) | 0.08** (0.01) | 0.02 (0) | 0.15** (0.02) |
| PC 32:0 | 3.9 (0.25) | 6.1** (0.46) | 3.5 (0.21) | 5.0** (0.36) | 2.0 (0.16) | 1.1 (0.57) | 1.9 (0.50) | 0.87* (0.26) | 2.4 (0.79) | 5.5** (0.68) | 2.1 (0.18) | 5.1** (0.51) |
| PC 32:1, 34:1 | 29 (0.84) | 5.2* (0.29) | 26 (1.9) | 4.3* (0.39) | 1.7 (0.19) | 0.17* (0.07) | 1.8 (0.34) | 0.16* (0.04) | 30 (4.9) | 4.9* (0.85) | 27 (1.6) | 4.6* (1.1) |
| PC 34:2, 36:2 | 10 (0.02) | 35** (0.82) | 9.3 (0.60) | 31** (2.0) | 0.33 (0.04) | 0.69** (0.23) | 0.38 (0.06) | 0.64** (0.10) | 16 (2.3) | 35** (4.7) | 15 (0.76) | 34** (1.9) |
| PE | 8.6 (0.79) | 2.3* (0.24) | 7.7 (0.76) | 2.1* (0.26) | 0.55 (0.07) | 0.08* (0.02) | 0.53 (0.03) | 0.07* (0.02) | 10 (1.4) | 2.2* (0.26) | 8.8 (0.61) | 2.0* (0.34) |
| PE 18:0/18:2, 18:1/18:2 | 0.32 (0.03) | 4.3** (0.41) | 0.33 (0.04) | 3.9** (0.44) | 0.02 (0) | 0.14** (0.03) | 0.02 (0) | 0.12** (0.03) | 0.50 (0.07) | 4.5** (0.88) | 0.46 (0.03) | 4.2** (0.13) |
| PG | 1.3 (0.04) | 0.28* (0.02) | 1.2 (0.05) | 0.21* (0.02) | 0.06 (0.01) | 0.01* (0) | 0.06 (0.01) | 0.01* (0) | 1.3 (0.24) | 0.25* (0.03) | 1.2 (0.04) | 0.24* (0.05) |
| PG 36:2, 36:3, 40:8 | 0.09 (0.01) | 0.36** (0.01) | 0.10 (0.01) | 0.31** (0.02) | 0 (0) | 0.01** (0) | 0 (0) | 0.01** (0) | 0.16 (0.04) | 0.38** (0.05) | 0.15 (0) | 0.37** (0.03) |
| PG 34:2 | 0.05 (0) | 0.06** (0) | 0.05 (0) | 0.06 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.08 (0.01) | 0.07 (0.01) | 0.07 (0) | 0.07 (0.01) |
| PI | 5.3 (0.23) | 3.9* (0.10) | 4.6 (0.47) | 3.5* (0.51) | 0.18 (0.01) | 0.05* (0.02) | 0.17 (0.04) | 0.05* (0.01) | 7.5 (1.1) | 3.2* (0.72) | 6.2 (0.29) | 3.0* (0.80) |
| PS | 5.9 (0.70) | 1.1* (0.04) | 5.7 (0.74) | 0.96* (0.16) | 0.44 (0.22) | 0.08* (0.01) | 0.52 (0.14) | 0.05* (0.02) | 6.5 (0.92) | 0.97* (0.21) | 6.2 (0.23) | 0.98* (0.27) |
| PS 16:0/16:0, 18:0/18:2 | 0.51 (0.04) | 5.7** (0.18) | 0.53 (0.07) | 5.5** (0.78) | 0.03 (0.01) | 0.24** (0.05) | 0.05 (0.01) | 0.22** (0.03) | 0.80 (0.11) | 6.3** (1.2) | 0.82 (0.05) | 6.3** (0.67) |
| GC | 3.2 (0.11) | 1.0* (0.11) | 26 (1.6) | 11* (0.92) | 1.3 (0.40) | 0.22* (0.13) | 9.7 (0.79) | 4.3* (0.64) | 0.84 (0.39) | 0.44 (0.10) | 3.5 (0.64) | 2.8 (0.41) |
| GC 18:1/16:0 | 0.95 (0.04) | 1.3** (0.05) | 9.0 (0.59) | 11** (0.68) | 0.46 (0.18) | 0.27 (0.17) | 3.4 (0.60) | 3.9 (0.49) | 0.24 (0.12) | 0.43 (0.14) | 1.4 (0.28) | 2.9** (0.09) |
| Cer | 1.0 (0.03) | 0.36* (0.01) | 0.84 (0.11) | 0.31* (0.03) | 0.40 (0.06) | 0.10* (0.03) | 0.40 (0.10) | 0.12* (0.01) | 1.1 (0.17) | 0.33* (0.08) | 0.82 (0.03) | 0.31* (0.10) |
| Cer 18:1/16:0 | 0.53 (0.03) | 0.74** (0.04) | 0.46 (0.09) | 0.61 (0.06) | 0.29 (0.15) | 0.20 (0.10) | 0.26 (0.14) | 0.22 (0.04) | 1.4 (0.39) | 1.1 (0.23) | 1.0 (0.12) | 0.97 (0.22) |
| DHC | 0.37 (0.02) | 0.18* (0) | 0.63 (0.04) | 0.28* (0.03) | 0.17 (0.04) | 0.04* (0.02) | 0.33 (0.07) | 0.09* (0.02) | 0.13 (0.05) | 0.07 (0.02) | 0.14 (0.03) | 0.12 (0) |
| DHC 18:1/16:0 | 0.13 (0.01) | 0.24** (0) | 0.18 (0.01) | 0.31** (0.03) | 0.05 (0.02) | 0.04 (0.02) | 0.08 (0.03) | 0.08 (0.02) | 0.05 (0.02) | 0.08 (0.02) | 0.06 (0.01) | 0.14** (0.01) |
| THC | 0.70 (0.02) | 0.29* (0.02) | 0.94 (0.04) | 0.42* (0.02) | 0.25 (0.05) | 0.07* (0.04) | 0.47 (0.13) | 0.15* (0.05) | 0.22 (0.09) | 0.15 (0.03) | 0.25 (0.06) | 0.22 (0.01) |
| THC 18:1/16:0 | 0.23 (0.01) | 0.36** (0.02) | 0.27 (0.02) | 0.40** (0.01) | 0.07 (0.02) | 0.06 (0.04) | 0.11 (0.04) | 0.10 (0.03) | 0.08 (0.03) | 0.14 (0.03) | 0.09 (0.02) | 0.20** (0.02) |
Significantly reduced with linoleic acid compared with the untreated THP-1 macrophage model, P < 0.05 (Student t-test). **Significantly elevated with linoleic acid compared with the untreated THP-1 macrophage model, P < 0.05 (Student t-test). LA, linoleic acid.
Total lipids were determined by summing the individual lipid species which correlated (Pearson's >0.8). The lipid species which did not correlate are listed separately.
Results reported as nanomoles of lipid per milligram of total cell protein and expressed as the mean and standard deviation (n = 3).
Results reported as nanomoles of lipid in the DRM (fractions 3 and 4) or DSM (fractions 7 to 12) and expressed as the mean and standard deviation (n = 3).
Linoleic acid (100 μM) was included in the culture media for 10 days during the induction of the Gaucher THP-1 macrophage model.
Effect of linoleic acid on GC and secondary sphingolipids
We next determined whether the reduction in BMP with linoleic acid had any effect on the primary storage of GC and secondary sphingolipid accumulation. Apart from the 18:1/16:0, the individual species of Cer, GC, DHC, and THC correlated (Pearson >0.8) and were therefore summed. Table 1 shows a 2-fold reduction in the amount of GC, DHC, and THC that accumulated in the presence of linoleic acid; this reduction occurred only in the DRM with no change in the DSM. The reduction in GC with linoleic acid was found to be dose dependent (see supplementary Fig. I). The 18:1/16:0 species increased with linoleic acid; however, unlike the other species, this increase was seen in the DSM with no change in the DRM (Table 1). Although no Cer accumulation was observed in the Gaucher THP-1 macrophage model, a 3-fold reduction (excluding 18:1/16:0) was observed with linoleic acid (Table 1). Unlike GC, DHC, and THC, Cer concentrations were significantly lower in both the DRM and DSM, whereas Cer 18:1/16:0 showed no change in the Gaucher THP-1 macrophage model with linoleic acid.
Fatty acid supplementation post GC accumulation
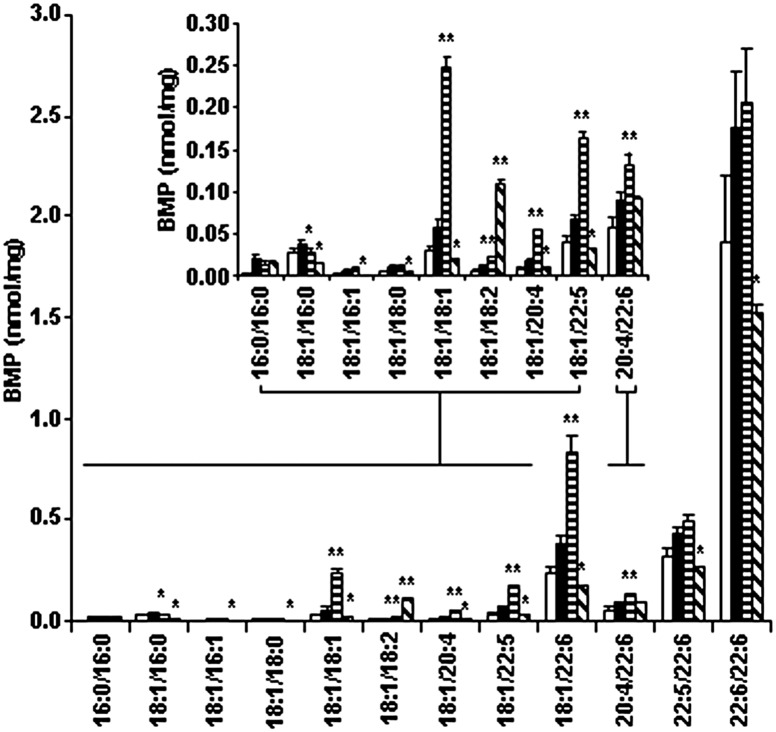
The question then arose as to whether linoleic acid could reverse the lipid changes once they were present. In this instance, we added linoleic acid for 24 h once GC had already accumulated 6-fold (i.e., following induction of the Gaucher phenotype). Fig. 3 shows that the results for BMP were similar as when linoleic acid had been present continuously; all species of BMP reduced substantially except BMP 18:1/18:2, which increased 9-fold, and BMP 20:4/22:6, which remained unchanged. Even with this increase in 18:1/18:2, the total amount of BMP in the Gaucher THP-1 macrophage model reduced from 3.6 nmol/mg to 2.3 nmol/mg, which is similar to the 2.6 nmol/mg seen in the control (THP-1 macrophages). However, there was no reduction in any of the individual species of GC or in the secondary storage of DHC and THC (data not shown).
Fig. 3.
BMP in the Gaucher THP-1 macrophage model with the addition of oleic and linoleic acid. Gaucher THP-1 macrophages were grown in 10% FBS in the presence of 100 μM oleic or linoleic acid for 24 h. Cells were harvested and BMP was analyzed by ESI-MS/MS. The concentration of individual BMP species is shown in THP-1 macrophages (open bars), Gaucher THP-1 macrophage model without (solid bars) or with oleic acid (horizontal stripes) or linoleic acid (diagonal stripes). Results are reported as nanomoles of BMP per milligram of total cell protein and expressed as the mean and standard deviation (n = 3). *Significantly reduced (linoleic/oleic acid compared with the Gaucher THP-1 macrophage model), P < 0.05 (Student t-test). **Significantly increased (linoleic/oleic acid compared with the Gaucher THP-1 macrophage model), P < 0.05 (Student t-test).
We also supplemented the culture media with a monounsaturated fatty acid (oleic acid) for 24 h, and although a reduction in BMP 18:1/16:0 was observed, increases in BMP 18:1/18:1, 18:1/18:2, 18:1/20:4, 18:1/22:5, 18:1/22:6, and 20:4/22:6 resulted in an increase in total BMP to 4.6 nmol/mg (Fig. 3). It is worth noting that the fatty acid supplementation shown in Fig. 3 was performed in the presence of 10% FBS; however, we observed the same increase in BMP 18:1/18:2 and decreases in the other BMP species when linoleic acid was added in 2% lipoprotein-deficient serum/0.4% essentially fatty acid free BSA or serum free media, all of which reduced total BMP 2-fold (data not shown).
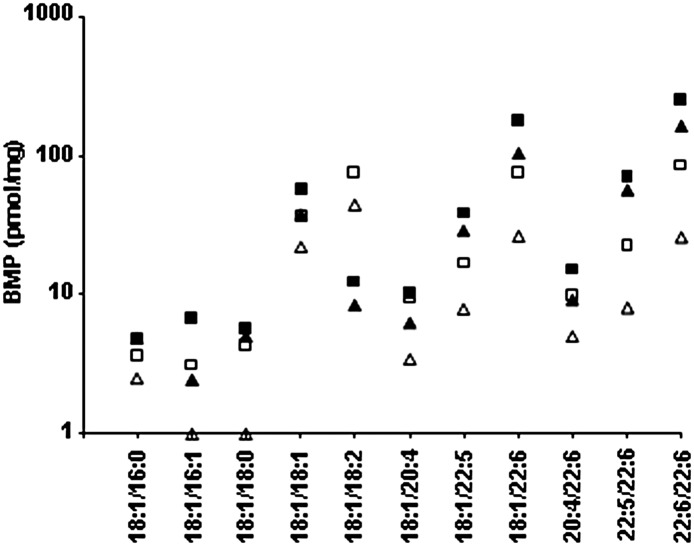
To verify that the effect of linoleic acid was not restricted to THP-1 macrophages, we supplemented the media of cultured skin fibroblasts from two Gaucher patients [previously shown to accumulate GC (18)] with linoleic acid for 24 h. Fig. 4 shows that, in cultured skin fibroblasts from patients with Gaucher disease, the results were similar, with an increase in BMP 18:1/18:2 and a reduction in the other species. A 2- to 3-fold reduction in total BMP concentration in the Gaucher fibroblasts was observed, which was similar to the Gaucher THP-1 macrophage model (2-fold reduction).
Fig. 4.
BMP in cultured Gaucher skin fibroblasts with linoleic acid. Cultured fibroblasts were grown to confluence before being grown in the presence of 100 μM linoleic acid for 24 h. Cells were harvested and BMP was analyzed by ESI-MS/MS. The concentration of individual BMP species is shown in cultured skin fibroblasts from two Gaucher patients (squares and triangles) without (solid symbols) and with linoleic acid (open symbols). Results are reported as picomoles of each individual BMP species per milligram of total cell protein.
DISCUSSION
In our model, we found that the majority of BMP, as with the other phospholipids measured, resided in the DSM (>91%), which is consistent with an earlier report that located BMP in the soluble domains of late endosomes (24). The DRM, commonly characterized by the presence of fully saturated lipids, was where BMP 16:0/16:0 and the other fully saturated phospholipid species resided. As there were no obvious alterations in the fatty acid species, this increase in BMP may not disrupt the actual structure of the lysosomal membranes, as it has been implied that the fatty acid composition dictates the structural integrity of membranes (25). Rather, the increase in BMP is most likely due to an expansion of the lysosomal membranes as the lysosomes struggle to cope with the increased GC load. As BMP accumulates primarily in the DSM rather than in the DRM where GC predominates (23), elevations in BMP may be refractive to conventional therapies directed at reducing the GC storage burden alone, and we would therefore predict incomplete reversal of disease unless BMP was normalized.
We hypothesized that if the concentration of BMP in the lysosomal membranes could be maintained, damage to the lysosomal system would be minimized and that this in turn would prevent at least some of the accumulation of primary and secondary sphingolipids. Linoleic acid, a polyunsaturated fatty acid, when remodeled with the assistance of desaturases and elongases for the synthesis of BMP (26), successfully reduced BMP in the Gaucher THP-1 macrophage model. Even with the expected exception of the 18:1/18:2, the other fatty acid species of BMP reduced 9-fold, bringing the total BMP concentration to 0.6 nmol/mg, which was actually lower than control THP-1 macrophages. However, with 24 h linoleic acid, total BMP reduced to 2.3 nmol/mg, which was similar to control cells. This, together with our finding that the degree of reduction in BMP is dose dependent (see supplementary Fig. I), suggests that the dose of linoleic acid may be important. Increases in the other phospholipids were also seen in response to linoleic acid when the 18:2 fatty acid was present, although this could not be confirmed for PC as it was not possible to fragment PC in negative ion mode to give the 18:2 fatty acid as a fragment ion. We could confirm the presence of the 18:2 fatty acid in PG by measuring the 18:2 as a product ion in negative ion MS/MS (data not shown). There were also increases in some of the other phospholipids that contained a 16:0, which may be a flow-on effect from the excess 18:2, and minor decreases in others; further work will be required to determine the specificity of linoleic acid.
The finding that the inclusion of linoleic acid in the culture media also prevented the accumulation of one third of GC, DHC, and THC (or three fifths, excluding the 18:1/16:0 species, which increase with linoleic acid) in the DRM presumably occurs as the membrane remodels. Membrane remodeling will occur when multivesicular BMP-rich endolysosomes containing GC, DHC, and THC (destined for lysosomal degradation) fuse with BMP-enriched lysosomes for digestion. The 18:1/16:0 species of GC increased in response to linoleic acid, most likely explained by the lack of an enzyme to shorten the 18:0 fatty acid to a 16:0 derivative. Importantly, supplementing the culture media of the Gaucher THP-1 macrophage model with linoleic acid for 24 h had no effect on GC, despite reducing BMP concentrations (Fig. 3). Similarly, treatment of cultured skin fibroblasts from two Gaucher patients with linoleic acid induced analogous alterations in BMP, but the decreases in the other phospholipids were not as great in the fibroblasts compared with the Gaucher THP-1 macrophage model (Fig. 4 and data not shown). This is most likely due to differences in cell type, as Gaucher disease is primarily a disease of macrophages (16).
The exact mechanism by which linoleic acid alters the membranes is not known, but our data are in agreement with others who have postulated a role for BMP in controlling the storage capacity of the lysosomal system (27). One of the limitations of this study is that a comprehensive analysis of the effect of linoleic acid on other lipids has not been determined, and the functional consequences have not been addressed. Ideally these experiments need to be performed in an animal model, and future work would involve dietary supplementation of chow with linoleic acid to the Gaucher mouse model to assess whether any pathology is alleviated (28). To this end, it will be important to determine the dose and concentration of linoleic acid, as we have shown that the reduction in BMP and GC is dose dependent (see supplementary Fig. I). Here we found that the concentrations of some BMP species appeared overcompensated with 100 µM linoleic acid in the culture media, and it is unclear what this equates to when given to mice in their chow.
Supplementary Material
Acknowledgments
The authors thank Kathryn Hattersley, Alison Whittle, and Troy Stomski for technical assistance.
Footnotes
Abbreviations:
- BMP
- bis(monoacylglycero)phosphate
- Cer
- ceramide
- DHC
- dihexosylceramide
- DRM
- detergent-resistant membrane
- DSM
- detergent-soluble membrane
- GC
- glucosylceramide
- MBS
- MES-buffered saline
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- THC
- trihexosylceramide
This work was supported in part by the Women's and Children's Hospital Foundation of South Australia.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure, and two tables.
REFERENCES
- 1.Heravi J., Waite M. 1999. Transacylase formation of bis(monoacylglycerol)phosphate. Biochim. Biophys. Acta. 1437: 277–286 [DOI] [PubMed] [Google Scholar]
- 2.Hullin-Matsuda F., Kawasaki K., Delton-Vandenbroucke I., Xu Y., Nishijimi M., Lagarde M., Schlame M., Kobayashi T. 2007. De novo biosynthesis of the late endosome lipid, bis(monoacylglycero)phosphate. J. Lipid Res. 48: 1997–2008 [DOI] [PubMed] [Google Scholar]
- 3.Ito M., Tchoua U., Okamoto M., Tojo H. 2002. Purification and properties of a phospholipase A2/lipase preferring phosphatidic acid, bis(monoacylglycerol)phosphate, and monoacylglycerol from rat testis. J. Biol. Chem. 277: 43674–43681 [DOI] [PubMed] [Google Scholar]
- 4.Amidon B., Brown A., Waite M. 1996. Transacylase and phospholipases in the synthesis of bis(monoacylglycero)phosphate. Biochemistry. 35: 13995–14002 [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi T., Beuchat M. H., Chevallier J., Makino A., Mayran N., Escola J. M., Lebrand C., Cosson P., Gruenberg J. 2002. Separation and characterisation of late endosomal membrane domains. J. Biol. Chem. 277: 32157–32164 [DOI] [PubMed] [Google Scholar]
- 6.Gruenberg J. 2001. The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2: 721–730 [DOI] [PubMed] [Google Scholar]
- 7.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N. S., Matile S., Dubochet J., Sadoul R., et al. 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 303: 531–534 [DOI] [PubMed] [Google Scholar]
- 8.Wilkening G., Linke T., Sandhoff K. 1998. Lysosomal degradation on vesicular membrane surfaces. Enhanced glucosylceramide degradation by lysosomal anionic lipids and activators. J. Biol. Chem. 273: 30271–30278 [DOI] [PubMed] [Google Scholar]
- 9.Schulze H., Kolter T., Sandhoff K. 2009. Principles of lysosomal membrane degradation. Cellular topology and biochemistry of lysosomal membrane degradation. Biochim. Biophys. Acta. 1793: 674–683 [DOI] [PubMed] [Google Scholar]
- 10.Futerman A. H., van Meer G. 2004. The cell biology of lysosomal storage disorders. Nat. Rev. Mol. Cell Biol. 5: 554–565 [DOI] [PubMed] [Google Scholar]
- 11.Kahma K., Brotherus J., Haltia M., Renkonen O. 1976. Low and moderate concentrations of lysobisphosphatidic acid in brain and liver of patients affected by some storage diseases. Lipids. 11: 539–544 [DOI] [PubMed] [Google Scholar]
- 12.Brotherus J., Niinioja T., Sandelin K., Renkonen O. 1977. Experimentally caused proliferation of lysosomes in cultured BHK cells involving an increase of bisphosphatidic acids and triglycerides. J. Lipid Res. 18: 379–388 [PubMed] [Google Scholar]
- 13.Meikle P. J., Duplock S., Blacklock D., Whitfield P. D., MacIntosh G., Hopwood J. J., Fuller M. 2008. Effect of lysosomal storage on bis(monoacylglycero)phosphate. Biochem. J. 411: 71–78 [DOI] [PubMed] [Google Scholar]
- 14.Hullin-Matsuda F., Luquain-Costaz C., Bouvier J., Delton-Vandenbroucke I. 2009. Bis(monoacylglycero)phosphate, a peculiar phospholipid to control the fate of cholesterol: implications in pathology. Prostaglandins Leukot. Essent. Fatty Acids. 81: 313–324 [DOI] [PubMed] [Google Scholar]
- 15.Beutler E., Grabowski G. A. 2001. Gaucher disease. In The Metabolic and Molecular Basis of Inherited Disease. C. R. Scriver, A. C. Beaudet, W. S. Sly, and D. Valle, editors. McGraw-Hill, New York, NY. 3635–3668. [Google Scholar]
- 16.Kattlove H. E., Williams J. C., Gaynor E., Spivack M., Bradley R. M., Brady R. O. 1969. Gaucher cells in chronic myelocytic leukemia: an acquired abnormality. Blood. 33: 379–390 [PubMed] [Google Scholar]
- 17.Hein L. K., Meikle P. J., Hopwood J. J., Fuller M. 2007. Secondary sphingolipid accumulation in a macrophage model of Gaucher disease. Mol. Genet. Metab. 92: 336–345 [DOI] [PubMed] [Google Scholar]
- 18.Fuller M., Rozaklis T., Lovejoy M., Zarrinkalam K., Hopwood J. J., Meikle P. J. 2008. Glucosylceramide accumulation is not confined to the lysosome in fibroblasts from patients with Gaucher disease. Mol. Genet. Metab. 93: 437–443 [DOI] [PubMed] [Google Scholar]
- 19.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193: 265–275 [PubMed] [Google Scholar]
- 20.Lisanti M. P., Tang Z. L., Scherer P. E., Sargiacomo M. 1995. Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol. 250: 655–668 [DOI] [PubMed] [Google Scholar]
- 21.Folch J., Lee M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226: 497–509 [PubMed] [Google Scholar]
- 22.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917 [DOI] [PubMed] [Google Scholar]
- 23.Hein L. K., Duplock S., Hopwood J. J., Fuller M. 2008. Lipid composition of microdomains is altered in a cell model of Gaucher disease. J. Lipid Res. 49: 1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobo K., Chevallier J., Parton R. G., Gruenberg J., van der Goot F. G. 2007. Diversity of raft-like domains in late endosomes. PLoS ONE. 2: e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wassall S. R., Stillwell W. 2009. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim. Biophys. Acta. 1788: 24–32 [DOI] [PubMed] [Google Scholar]
- 26.Huterer S., Wherrett J. R. 1986. Incorporation of polyunsaturated fatty acids into bis(monoacylglycero)phosphate and other lipids of macrophages and of fibroblasts from control and Niemann-Pick patients. Biochim. Biophys. Acta. 876: 318–326 [DOI] [PubMed] [Google Scholar]
- 27.Chevallier J., Chamoun Z., Jiang G., Prestwich G., Sakai N., Matile S., Parton R. G., Gruenberg J. 2008. Lysobisphosphatidic acid controls endosomal cholesterol levels. J. Biol. Chem. 283: 27871–27880 [DOI] [PubMed] [Google Scholar]
- 28.Sinclair G. B., Jevon G., Colobong K. E., Randall D. R., Choy F. Y. M., Clarke L. A. 2007. Generation of a conditional knockout of murine glucocerebrosidase: utility for the study of Gaucher disease. Mol. Genet. Metab. 90: 148–156 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.