Abstract
Few studies have addressed the delivery of lipoprotein-derived cholesterol to the adrenals for steroid production in humans. While there is evidence against a role for low-density lipoprotein (LDL), it is unresolved whether high density lipoprotein (HDL) contributes to adrenal steroidogenesis. To study this, steroid hormone profiles in urine were assessed in male subjects suffering from functional mutations in ATP binding cassette transporter A1 (ABCA1) (n = 24), lecithin:cholesterol acyltransferase (LCAT) (n = 40), as well as in 11 subjects with low HDL cholesterol (HDL-C) without ABCA1/LCAT mutations. HDL-C levels were 39% lower in the ABCA1, LCAT, and low HDL-C groups compared with controls (all P < 0.001). In all groups with low HDL-C levels, urinary excretion of 17-ketogenic steroids was reduced by 33%, 27%, and 32% compared with controls (all P < 0.04). In seven carriers of either type of mutation, adrenocorticotropic hormone (ACTH) stimulation did not reveal differences from normolipidemic controls. In conclusion, this study shows that basal but not stimulated corticosteroid metabolism is attenuated in subjects with low HDL-C, irrespective of its molecular origin. These findings lend support to a role for HDL as a cholesterol donor for basal adrenal steroidogenesis in humans.
Keywords: steroid hormones, cortisol, dyslipidemia, hypoalphalipoproteinemia, high density lipoprotein cholesterol
The synthesis and secretion of adrenal steroid hormones for regulating stress responses, electrolyte homeostasis, and maintenance of secondary sexual characteristics depends upon the availability of the precursor cholesterol (1). To secure a continuous cholesterol supply, the adrenal glands can synthesize cholesterol, metabolize intracellular esterified cholesterol, or obtain cholesterol from circulating lipoproteins (1). Although exact data are lacking, plasma lipoproteins have been suggested to contribute more than 75% of all cholesterol required for adrenal steroidogenesis (2, 3), but the current literature gives little insight in associations between plasma lipoprotein levels and adrenal function in humans.
With respect to a role for low-density lipoprotein (LDL), it has been shown that LDL receptor-deficient patients suffering from familial hypercholesterolemia display normal urinary excretion of both 17 hydroxycorticosteroids (17-OHCS) and 17 ketogenic steroid metabolites (17-KS) indicative of unaffected adrenal function (4, 5). In addition, low or absent LDL cholesterol (LDL-C) in carriers of one or two defective APOB alleles respectively did not affect basal adrenal function either (6). Combined this suggests that LDL is probably not playing a major role in delivering cholesterol for steroid hormone production in humans. Associations with HDL cholesterol (HDL-C) have thus far only been studied in critically ill patients. In one study it was shown that low HDL-C in such patients was associated with attenuated adrenal responses to synthetic adrenocorticotropic hormone (ACTH) (7). In support of this, others reported a high incidence of adrenal failure in critically ill individuals with liver disease, with HDL-C being the only variable predictive of adrenal insufficiency (8).
Using adrenal cells, it has been suggested that HDL is the preferred lipoprotein for cholesterol delivery to the adrenal gland (9) and in accordance, scavenger receptor type B1 (SRB1)-mediated cholesterol uptake from HDL has been shown to be the predominant source of cholesterol in mice (10–13). In line, it has been shown that mice lacking SRB1 display an impaired adrenal glucocorticoid stress response (14, 15), lending support to a major role for HDL as a cholesterol donor in mice. In humans, we also showed that adrenal function was compromised in individuals with a functional mutation in SCARB1, the gene encoding SRB1 (16). While this study showed that cholesterol delivery to the adrenals via the HDL-SRB1 pathway is important for adrenal steroidogenesis in humans, it is unclear whether plasma HDL-C levels are associated with adrenal steroidogenesis in humans. To investigate this, we assessed basal and ACTH-stimulated adrenal cortical function in males with low HDL-C due to mutations in either ATP binding cassette transporter 1 (ABCA1) (17) or lecithin:cholesterol acyltransferase (LCAT) and in subjects with low HDL-C without mutations in ABCA1/LCAT, as well as in normolipidemic controls (18). We hypothesized that in subjects with low HDL-C levels, adrenal function would be compromised irrespective of the molecular origin of the low HDL-C.
METHODS
Recruitment of study participants
Male subjects with HDL-C levels below the fifth percentile were screened for mutations in ABCA1 and LCAT (17, 19), of which the functionality was assessed in previously published studies (17, 20, 21). For the current study, we enrolled 24 carriers of mutations in the ABCA1 gene. We furthermore enrolled 40 male carriers of mutations in the LCAT gene. In addition, subjects with similarly reduced HDL-C levels without mutations in ABCA1 and LCAT were included (n = 11). As a control group, normolipidemic age-matched male individuals were recruited by advertisement. None of the included individuals used medication interfering with steroid metabolism. The study was approved by the institutional review board of the Academic Medical Center, Amsterdam, The Netherlands and all participants provided written informed consent.
Questionnaire and biochemical measurements
Medical history, cardiovascular risk factors, use of medication, and family history of cardiovascular disease were assessed using a questionnaire. Brachial artery blood pressures were measured using an oscillometric blood pressure device (Omron 705IT, Hoofddorp, The Netherlands). Hypertension was defined as 1) use of antihypertensive medication, or 2) a systolic blood pressure at visit above 140 mm Hg and/or diastolic blood pressure above 90 mm Hg.
Plasma was obtained after an overnight fast and stored at −80°C. Total cholesterol, LDL-C, HDL-C, and triglyceride levels were analyzed using commercially available enzymatic methods (Randox, Antrim, UK and Wako, Neuss, Germany) on a Cobas Mira autoanalyzer (Roche, Basel, Switzerland). Free cholesterol and total cholesterol were measured before and after precipitation of apoB-containing lipoproteins using phosphotungstic acid (Sigma) and a commercially available enzymatic assay (Diasys) on a Selectra autoanalyzer (Sopachem). ACTH was determined by an immunoluminometric assay (Nichols Institute, Los Angeles, CA). Aldosterone was measured using a radioimmunoassay (Siemens, Los Angeles, CA).
Baseline adrenal steroidogenesis
Urinary excretion of steroid metabolites was analyzed by gas chromatography in 24 h urine samples as previously described (22, 23). Androsteron (A), etiocholanolon (E), dehydroepiandrosteron (D), 11-ketoandrosteron (KA), 11-ketoetiocholanolon (KE), 11-hydroxy-androsteron (HA), 11-hydroxy-etiocholanolon (HE), pregnaandiol (P2), pregnaantriol (P3), 11-deoxytetrahydrocortisol (THS), tetrahydrocortison (THE), tetrahydrocortisol (THF), and allo-tetrahydrocortisol (ALLO) were measured as readout of adrenal steroidogenesis. A, E, D, KA, KE, HA, and HE made up the total 17-KS, whereas THS, THE, THF, ALLO, and P3 were the constituents of the total 17-OHCS. In addition, urinary free cortisol was determined using solid-phase extraction liquid chromatography- tandem mass spectrometry on a Symbiosis Pharma (Spark Holland, Emmen, The Netherlands) Quattro Premier tandem mass spectrometer (Waters, Milford, MA) system. Solid phase extraction was performed on Oasis HLB cartridges (Waters), chromatographic separation was achieved on a Waters Sunfire C18 column (3.5 μm, 2.1 × 50 mm) using ammonium acetate mM with 0.1% formic acid as mobile phase and acetonitrile as mobile phase B. Limit of detection 5 nmol/l, intra-assay variation <4%, total assay variation <7%.
Stimulated adrenal steroidogenesis
Random subgroups of seven ABCA1 and seven LCAT mutation carriers consented to an ACTH stimulation study (cosyntropin or tetracosactin, 0.25 mg/ml; Novartis Pharma B.V., Arnhem, The Netherlands). After an overnight fast, participants underwent cosyntropin testing at 0900 h. Two baseline blood samples were obtained, 15 min and 1 min before administration of the 1 μg cosyntropin bolus. Subsequent blood samples were drawn 30 min and 60 min after cosyntropin administration. Plasma cortisol levels were measured by enzyme immunoassay (Siemens Medical Solutions, Los Angeles, CA), and cortisol binding globulin (CBG) levels were measured with a commercial radioimmunoassay (Siemens Medical Solutions). Free cortisol levels were calculated using the method described by Coolens, van Baelen, and Heyns (24).
Statistical analysis
Unpaired Student's t-test was performed for analysis of continuous data with a normal distribution. In case of a skewed distribution, data were log-transformed prior to t-testing. Categorical data were assessed by χ2 testing. A P value of <0.05 was considered statistically significant. A linear regression model was used to correct for differences in LDL-C and statin use.
RESULTS
Population characteristics
We enrolled 24 and 40 carriers of loss-of-function mutations in either ABCA1 or LCAT. Two of the ABCA1 mutation carriers were compound heterozygotes while three of the LCAT mutation carriers were homozygotes. None of the participants were referred to our clinic for symptoms of adrenal dysfunction. Individuals were matched to male controls for age. In parallel, we also included subjects with equally low HDL-C levels without mutations in ABCA1 or LCAT. Demographic, clinical, and biochemical characteristics of all low HDL-C groups as well as controls are listed in Table 1. As expected, HDL-C levels were 39% lower in carriers of ABCA1 or LCAT mutations and in the low HDL-C group, compared with normolipidemic controls (P < 0.001). LDL-C levels were 25% lower in carriers of ABCA1 mutations (P = 0.003), 11% lower in carriers of LCAT mutations (n.s.), and 12% lower in the low HDL-C group (P = 0.34 and P = 0.39) compared with controls. Detailed analysis of the concentrations of cholesteryl esters and free cholesterol in the total cholesterol fraction and the isolated HDL-C fraction, in a subset of patients, is provided in Table 2.
TABLE 1.
Characteristics of male ABCA1 and LCAT mutation carriers and matched male controls
| ABCA1 |
LCAT |
|||||||||
| Characteristics | Carriers (n = 24) | Controls (n = 24) | P | Low HDL-C (n = 11) | P | Carriers (n = 40) | Controls (n = 40) | P | Low HDL-C (n = 11) | P |
| Demographic | ||||||||||
| Age, years | 45.2 ± 20.6 | 45.5 ± 18.5 | 0.96 | 44.6 ± 12.0 | 0.91 | 46.2 ± 12.8 | 45.2 ± 14.9 | 0.84 | 44.6 ± 12.0 | 0.71 |
| BMI, kg/m2 | 25.3 ± 3.3 | 25.1 ± 5.2 | 0.92 | 26.3 ± 8.8 | 0.81 | 26.3 ± 2.9 | 24.9 ± 4.5 | 0.17 | 26.3 ± 8.8 | 0.99 |
| Current smoker, n (%) | 8 (33.3) | 4 (17.4) | 0.21a | 3 (27) | 0.72a | 7 (18.4) | 4 (10.8) | 0.35a | 3 (27) | 0.52a |
| Alcohol users, n (%) | 17 (71) | 19 (79) | 0.51a | 9 (82) | 0.49a | 36 (90) | 33 (83) | 0.33a | 9 (82) | 0.46a |
| Clinical | ||||||||||
| Coronary artery disease, n (%) | 7 (29.2) | 1 (4.8) | — | 4 (46) | — | 9 (23.7) | 1 (2.9) | — | 4 (36) | — |
| Diabetes mellitus, n (%) | 3 (13.6) | 0 (0) | 0.07a | 1 (9) | 0.71a | 1 (2.6) | 0 (0) | 0.32a | 1 (9) | 0.34a |
| Hypertension, n (%) | 7 (29.2) | 3 (12.5) | 0.16a | 1 (9) | 0.19a | 13 (32.5) | 4 (10) | 0.01a | 19(8) | 0.12a |
| Statin use, n (%) | 8 (33) | 2 (8) | 0.02a | 4 (33) | 0.84a | 15 (38) | 2 (5) | <0.001a | 4 (36) | 0.80a |
| Blood pressure, mm Hg | ||||||||||
| Systolic | 136 ± 16 | 132 ± 13 | 0.34 | 127 ± 11 | 0.29 | 139 ± 17 | 132 ± 13 | 0.14 | 127 ± 11 | 0.19 |
| Diastolic | 79 ± 12 | 80 ± 10 | 0.75 | 78 ± 11 | 0.90 | 81 ± 9 | 80 ± 9 | 0.81 | 78 ± 11 | 0.57 |
| Biochemical | ||||||||||
| Aldosterone, nmol/l | 0.20 ± 0.14 | 0.23 ± 0.12 | 0.55 | 0.22 ± 0.19 | 0.87 | 0.19 ± 0.11 | 0.24 ± 0.14 | 0.12 | 0.22 ± 0.19 | 0.68 |
| ACTH, ng/l | 23.11 ± 12.62 | 21.64 ± 15.10 | 0.25b | n.a. | 22.00 ± 14.25 | 24.57 ± 23.08 | 0.81b | n.a. | ||
| Total cholesterol, mmol/l | 3.9 ± 1.2 | 5.2 ± 1.3 | 0.001 | 4.5 ± 2.1 | 0.27 | 4.4 ± 1.1 | 5.3 ± 1.2 | 0.001 | 4.5 ± 2.1 | 0.95 |
| LDL cholesterol, mmol/l | 2.7 ± 1.0 | 3.6 ± 0.9 | 0.003 | 3.2 ± 1.8 | 0.39 | 3.2 ± 0.9 | 3.6 ± 0.9 | 0.08 | 3.2 ± 1.8 | 0.34 |
| HDL cholesterol, mmol/l | 0.8 ± 0.3 | 1.3 ± 0.3 | <0.001 | 0.8 ± 0.2 | <0.001 | 0.8 ± 0.3 | 1.3 ± 0.4 | <0.001 | 0.8 ± 0.2 | <0.001 |
| Triglycerides, mmol/l | — | — | — | 1.5 | — | — | — | — | — | — |
| Median | 1.0 | 1.2 | 0.65b | 1.3-2.1 | 0.02b | 1.25 | 1.25 | 0.22b | 1.5 | 0.23b |
| Interquartile range | 0.7–1.5 | 0.8–1.9 | — | — | — | 1.0–2.0 | 0.8–1.8 | — | 1.3–2.1 | — |
| Urinary free cortisol, nmol/24 h | 60.10 ± 31.66 | 59.27 ± 39.96 | 0.62b | n.a. | — | 50.33 ± 36.34 | 57.81 ± 35.19 | 0.31b | n.a. | — |
Blood pressure measurements were available in 21 ABCA1 mutation carriers and 17 controls and in 34 LCAT mutation carriers and 26 controls. Plasma ACTH levels were available in 18 ABCA1 mutation carriers and 18 controls and in 20 LCAT mutation carriers and 31 controls. Urinary cortisol levels were available in 22 ABCA1 mutation carriers and 22 controls and in 35 LCAT mutation carriers and 36 controls. Values are means ± SD unless otherwise indicated. No t-test was performed for history of coronary artery disease because referral bias was present. Partial overlap exists between the two control cohorts. P is for Student's t-test. n.a., not available.
P is for χ2 test.
Triglycerides, plasma ACTH, and urinary cortisol were log-transformed prior to t-test.
TABLE 2.
Free cholesterol and cholesteryl ester values in a subset of LCAT mutation carriers and controls
| Controls (n = 29) | Heterozygous carriers (n = 29) | P | Homozygous carriers (n = 3) | P | |
| Total cholesterol, mmol/l | 5.3 ± 1.2 | 4.5 ± 1.2 | 0.002 | 4.1 ± 0.6 | 0.09 |
| FC | 1.56 ± 0.35 | 1.4 ± 0.4 | 0.04 | 2.1 ± 0.3 | 0.02 |
| CE | 3.65 ± 1.04 | 3.0 ± 0.9 | 0.01 | 2.0 ± 0.6 | 0.01 |
| FC/CE ratio | 0.48 ± 0.30 | 0.5 ± 0.1 | 0.73 | 1.1 ± 0.4 | 0.001 |
| Cholesterol after apoB precipitation | 1.3 ± 0.4 | 0.7 ± 0.3 | 0.03 | 0.04 ± 0.01 | <0.001 |
| FC | 1.78 ± 0.71 | 0.1 ± 0.1 | 0.13 | 0.0 ± 0.0 | <0.001 |
| CE | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.02 | 0.04 ± 0.01 | <0.001 |
| FC/CE ratio | 0.24 ± 0.06 | 0 ± 0.12 | 0.50 | 1.1 ± 0.4 | <0.001 |
P is for Student's t-test compared with controls. FC, free cholesterol; CE, cholesteryl ester.
Compared with controls, hypertension was more prevalent among LCAT mutation carriers (P = 0.01). Statin use was more prevalent in the ABCA1 and LCAT mutation carriers compared with the control group. The low HDL-C group had similar baseline characteristics compared with the ABCA1 and LCAT groups.
Basal adrenal steroidogenesis
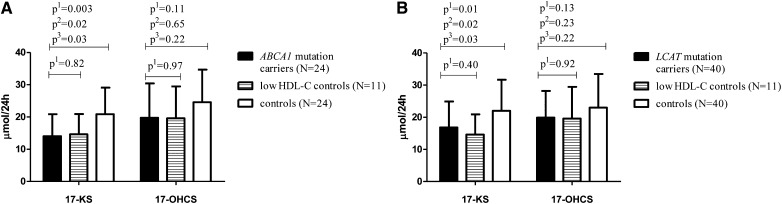
Compared with the control group, we identified lower 24 h urinary excretion of 17-KS in carriers of mutations in ABCA1 (33%, P = 0.003; Fig. 1A), in LCAT (27%, P = 0.01; Fig. 1B), and in the low HDL-C group (30%, P = 0.04 and 34%, P = 0.02; Fig. 1). These differences remained statistically significant after correcting for differences in plasma LDL-C and statin use using a linear regression model (P = 0.01 and P = 0.02 for carriers of ABCA1 mutations or LCAT mutations vs. controls, respectively). The mean 17-KS steroid excretion of 14.1 μmol/24 h in the ABCA1 mutation carriers was below the reference values for 17-KS in the appropriate age group (23).
Fig. 1.
Twenty-four hour urinary steroid excretion in male ABCA1 (A) and LCAT (B) mutation carriers compared with age-matched male controls. Data are presented as mean ± SD. p1, the uncorrected P value for Student's t-test; p2, the P value corrected for LDL-C; p3, the P value corrected for statin use.
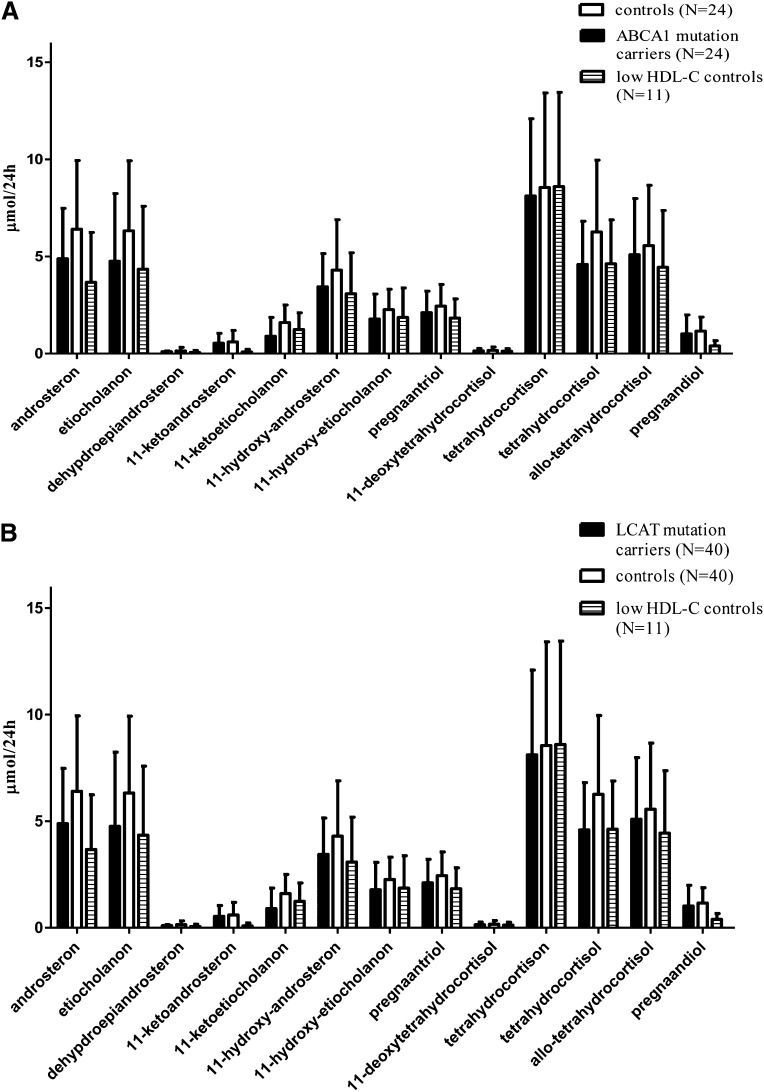
The lower levels of urinary 17-OHCS in both ABCA1 and LCAT mutation carriers, as well as the low HDL-C group, did not reach statistical significance (P = 0.11 and P = 0.15, respectively; Fig. 1A, B). The full panel of steroid metabolites is presented in Fig. 2A, B. No gene-dose effect was observed when comparing the two compound heterozygous ABCA1 mutation carriers or the three homozygous LCAT mutation carriers to heterozygous carriers.
Fig. 2.
Urinary steroid metabolites in male ABCA1 (A) and LCAT (B) mutation carriers compared with age-matched male controls. Data are presented as mean ± SD.
Free urinary cortisol was not significantly different between ABCA1 mutation carriers and controls (60.10 ± 31.66 vs. 59.27 ± 39.96 nmol/24 h, P = 0.62) or LCAT mutation carriers and controls (50.33 ± 36.34 vs. 57.81 ± 35.19, P = 0.31).
Plasma levels of ACTH did not differ significantly between ABCA1 mutation carriers and controls (23.11 ± 12.62 vs. 21.64 ± 15.10, P = 0.25) or LCAT mutation carriers and controls (22.00 ± 14.25 vs. 24.57 ± 23.08, P = 0.81).
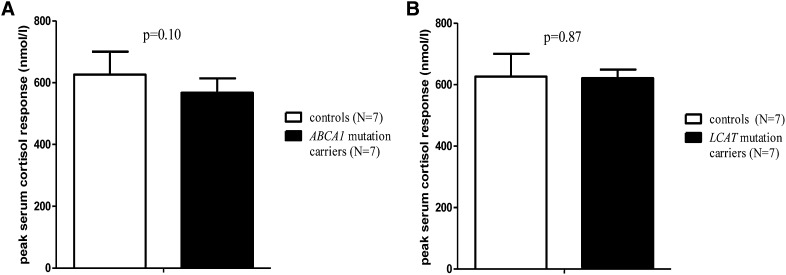
Adrenal response to cosyntropin
In a cosyntropin stimulation test, the cortisol response to physiological levels of ACTH was measured as an approach to assess adrenal cortical function (25, 26). The peak serum cortisol response to ACTH was comparable in ABCA1 and LCAT mutation carriers and did not differ from controls (P = 0.10 and P = 0.87 respectively, Fig. 3A, B). Also peak plasma levels of free cortisol, taking into account possible differences in CBG levels (16), were not different. Plasma lipid levels did not differ significantly before and after cosyntropin testing (data not shown).
Fig. 3.
Peak serum cortisol increase after cosyntropin administration in male ABCA1 (A) and LCAT (B) mutation carriers versus male controls. Data are presented as mean ± SD. P values for Student's t-test.
DISCUSSION
The present study demonstrates that under normal conditions male individuals with low levels of HDL-C, irrespective of molecular origin, have significantly lower urinary excretion of total 17-KS compared with normolipidemic controls, indicative of disturbed basal corticosteroid synthesis. A parallel reduction in hydroxycorticosteroid excretion in both groups did not reach statistical significance which may suggest a preferential adrenal pathway for HDL driven steroidogenesis. Mutations in either ABCA1 or LCAT did not, however, affect the response to ACTH, while physical examination and a standardized questionnaire for signs of adrenal insufficiency (16) did not reveal symptoms of clinically relevant adrenal dysfunction. These findings are in line with those in mice, and tissue culture showing that the supply of plasma lipoprotein-derived cholesterol is used for basal adrenal steroidogenesis, but that this pathway is not able to respond to acute stress (27–29). For a proper quick response, endogenous adrenal cortisol reserves are secreted upon stimulation. Because steroidogenic tissues are rapidly depleted of cortisol following stimulation, replenishing is thought to occur via uptake of cholesterol from lipoproteins.
We previously reported that individuals with reduced SRB1 function displayed mild adrenal insufficiency in addition to a reduced urinary excretion of steroid hormones (16). Thus reduced SRB1 function on adrenal cells has a larger impact on adrenal steroidogenesis when compared with low levels of plasma HDL-C caused by reduced ABCA1 or LCAT function.
There were no differences in urinary excretion of free cortisol. However, particularly in the lower ranges the quantification of free cortisol has been shown to be less reliable as compared with the quantification of full urinary steroid metabolites in diagnosing the presence of hypocortisolism (30, 31).
In accordance with LCAT's mechanism of action, cholesteryl ester concentration is lower in LCAT mutation carriers, with the most pronounced effect in homozygous carriers. Because adrenal steroidogenesis was not further decreased in homozygous carriers compared with heterozygous carriers, there is no indication that the adrenal gland has a preference for cholesteryl esters as a substrate for steroidogenesis. This is supported by the fact that a similar reduction in steroid metabolites was observed in subjects with low HDL-C not related to defects in LCAT.
Plasma levels of ACTH did not differ between the low HDL-C group and controls. The latter most likely reflects the fact that the decrease in urinary steroid metabolites is only mild, thereby precluding a compensatory ACTH increase. In line, none of the study participants reported signs of hypocortisolism.
We would like to discuss three limitations of our study. First, adrenal cells express ABCA1 (32). Thus reduced adrenal ABCA1 expression could conceptually lead to accumulation of cholesterol in adrenal cells, compromising intra-adrenal signaling and steroidogenesis due to cholesterol toxicity as proposed for pancreatic β-cell dysfunction in individuals carrying ABCA1 mutations (33, 34). Although we cannot exclude this possibility, mutations in LCAT as well as low HDL-C in noncarrier individuals are associated with similar reductions in HDL-C and urinary steroid metabolite excretion, making an ABCA1-specific effect implausible.
A second limitation is that carriers of ABCA1 mutations showed significantly lower levels of plasma LDL-C levels in addition to low levels of HDL-C. This could mean that combined low levels of HDL-C and LDL-C account for the effects observed, but as already discussed, a role for LDL-C in human adrenal corticoid production is unlikely (4–6). In line, statistical corrections for the observed reductions in LDL-C did not affect outcome.
Third, in the urinary steroid metabolites, the 17-KS were particularly decreased in carriers compared with controls. Because 17-KS are metabolites derived largely from the androgenic pathway, this finding hints toward the preferential use of HDL-derived cholesterol in adrenal steroidogenesis. Further studies are needed to dissect whether, and to what extent, HDL-derived cholesterol contributes to steroidogenesis in either adrenal or testicular steroid production.
In conclusion, we demonstrate that basal adrenal steroidogenesis is compromised in males with low levels of plasma HDL-C, establishing a role for HDL-derived cholesterol in adrenal steroidogenesis in humans.
Acknowledgments
The authors would like to thank C. A. M. Koch and J. F. Los for their assistance in expanding the pedigrees and J. Peter for his help in the identification of ABCA1 and LCAT mutations.
Footnotes
Abbreviations:
- A
- androsteron
- ABCA1
- ATP binding cassette transporter A1
- ACTH
- adrenocorticotropic hormone
- ALLO
- allo-tetrahydrocortisol
- CBG
- cortisol binding globulin
- D
- dehydroepiandrosteron
- E
- etiocholanolon
- HA
- 11-hydroxy-androsteron
- HDL-C
- HDL cholesterol
- HE
- 11-hydroxy-etiocholanolon
- KA
- 11-ketoandrosteron
- KE
- 11-ketoetiocholanolon, 17-KS, 17 ketogenic steroid metabolites
- LDL-C
- LDL cholesterol
- 17-OHCS
- 17 hydroxycorticosteroids
- P2
- pregnaandiol
- P3
- pregnaantriol
- SRB1
- scavenger receptor type B1
- THE
- tetrahydrocortison
- THF
- tetrahydrocortisol
- THS
- 11-deoxytetrahydrocortisol
This study was supported by three grants from the Dutch Heart Foundation (numbers 2009B027, 2008B070, and 2008T070). G.K.H. and A.G.H. are supported by Veni Grants (project numbers 91612122 and 91613031, respectively) from the Netherlands Organisation for Scientific Research (NWO).
REFERENCES
- 1.Hu J., Zhang Z., Shen W. J., Azhar S. 2010. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. (Lond.). 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borkowski A., Delcroix C., Levin S. 1972. Metabolism of adrenal cholesterol in man. I. In vivo studies. J. Clin. Invest. 51: 1664–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borkowski A. J., Levin S., Delcroix C., Mahler A., Verhas V. 1967. Blood cholesterol and hydrocortisone production in man: quantitative aspects of the utilization of circulating cholesterol by the adrenals at rest and under adrenocorticotropin stimulation. J. Clin. Invest. 46: 797–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Illingworth D. R., Lees A. M., Lees R. S. 1983. Adrenal cortical function in homozygous familial hypercholesterolemia. Metabolism. 32: 1045–1052 [DOI] [PubMed] [Google Scholar]
- 5.Illingworth D. R., Alam N. A., Lindsey S. 1984. Adrenocortical response to adrenocorticotropin in heterozygous familial hypercholesterolemia. J. Clin. Endocrinol. Metab. 58: 206–211 [DOI] [PubMed] [Google Scholar]
- 6.Illingworth D. R., Kenny T. A., Orwoll E. S. 1982. Adrenal function in heterozygous and homozygous hypobetalipoproteinemia. J. Clin. Endocrinol. Metab. 54: 27–33 [DOI] [PubMed] [Google Scholar]
- 7.van der Voort P. H., Gerritsen R. T., Bakker A. J., Boerma E. C., Kuiper M. A., de Heide L. 2003. HDL-cholesterol level and cortisol response to synacthen in critically ill patients. Intensive Care Med. 29: 2199–2203 [DOI] [PubMed] [Google Scholar]
- 8.Marik P. E., Gayowski T., Starzl T. E. 2005. The hepatoadrenal syndrome: a common yet unrecognized clinical condition. Crit. Care Med. 33: 1254–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaguchi H., Tsutsumi K., Shimono K., Omura M., Sasano H., Nishikawa T. 1998. Involvement of high density lipoprotein as substrate cholesterol for steroidogenesis by bovine adrenal fasciculo-reticularis cells. Life Sci. 62: 1387–1395 [DOI] [PubMed] [Google Scholar]
- 10.Azhar S., Reaven E. 2002. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation of steroidogenesis. Mol. Cell. Endocrinol. 195: 1–26 [DOI] [PubMed] [Google Scholar]
- 11.Azhar S., Leers-Sucheta S., Reaven E. 2003. Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and ‘selective’ pathway connection. Front. Biosci. 8: s998–s1029 [DOI] [PubMed] [Google Scholar]
- 12.Reaven E., Chen Y. D., Spicher M., Azhar S. 1984. Morphological evidence that high density lipoproteins are not internalized by steroid-producing cells during in situ organ perfusion. J. Clin. Invest. 74: 1384–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azhar S., Stewart D., Reaven E. 1989. Utilization of cholesterol-rich lipoproteins by perfused rat adrenals. J. Lipid Res. 30: 1799–1810 [PubMed] [Google Scholar]
- 14.Hoekstra M., Meurs I., Koenders M., Out R., Hildebrand R. B., Kruijt J. K., van Eck M., Van Berkel T. J. 2008. Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J. Lipid Res. 49: 738–745 [DOI] [PubMed] [Google Scholar]
- 15.Hoekstra M., Ye D., Hildebrand R. B., Zhao Y., Lammers B., Stitzinger M., Kuiper J., Van Berkel T. J., van Eck M. 2009. Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J. Lipid Res. 50: 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergeer M., Korporaal S. J., Franssen R., Meurs I., Out R., Hovingh G. K., Hoekstra M., Sierts J. A., Dallinga-Thie G. M., Motazacker M. M., et al. 2011. Genetic variant of the scavenger receptor BI in humans. N. Engl. J. Med. 364: 136–145 [DOI] [PubMed] [Google Scholar]
- 17.Candini C., Schimmel A. W., Peter J., Bochem A. E., Holleboom A. G., Vergeer M., Dullaart R. P., Dallinga-Thie G. M., Hovingh G. K., Khoo K. L., et al. 2010. Identification and characterization of novel loss of function mutations in ATP-binding cassette transporter A1 in patients with low plasma high-density lipoprotein cholesterol. Atherosclerosis. 213: 492–498 [DOI] [PubMed] [Google Scholar]
- 18.Duivenvoorden R., de Groot E., Elsen B. M., Lameris J. S., van der Geest R. J., Stroes E. S., Kastelein J. J., Nederveen A. J. 2009. In vivo quantification of carotid artery wall dimensions: 3.0-Tesla MRI versus B-mode ultrasound imaging. Circ. Cardiovasc. Imaging. 2: 235–242 [DOI] [PubMed] [Google Scholar]
- 19.Duivenvoorden R., Holleboom A. G., van den Bogaard B., Nederveen A. J., de Groot E., Hutten B. A., Schimmel A. W., Hovingh G. K., Kastelein J. J., Kuivenhoven J. A., et al. 2011. Cholesterol acyltransferase gene mutations have accelerated atherogenesis as assessed by carotid 3.0-T magnetic resonance imaging. J. Am. Coll. Cardiol. 58: 2481–2487 [DOI] [PubMed] [Google Scholar]
- 20.van Dam M. J., de Groot E., Clee S. M., Hovingh G. K., Roelants R., Brooks-Wilson A., Zwinderman A. H., Smit A. J., Smelt A. H., Groen A. K., et al. 2002. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: an observational study. Lancet. 359: 37–42 [DOI] [PubMed] [Google Scholar]
- 21.Holleboom A. G., Kuivenhoven J. A., Peelman F., Schimmel A. W., Peter J., Defesche J. C., Kastelein J. J., Hovingh G. K., Stroes E. S., Motazacker M. M. 2011. High prevalence of mutations in LCAT in patients with low HDL cholesterol levels in The Netherlands: identification and characterization of eight novel mutations. Hum. Mutat. 32: 1290–1298 [DOI] [PubMed] [Google Scholar]
- 22.van de Calseyde J. F., Scholtis R. J., Schmidt N. A., Leijten C. J. 1972. Profiling urinary steroids. A reliable procedure. Clin. Chim. Acta. 38: 103–111 [DOI] [PubMed] [Google Scholar]
- 23.Weykamp C. W., Penders T. J., Schmidt N. A., Borburgh A. J., van de Calseyde J. F., Wolthers B. J. 1989. Steroid profile for urine: reference values. Clin. Chem. 35: 2281–2284 [PubMed] [Google Scholar]
- 24.Coolens J. L., van Baelen H., Heyns W. 1987. Clinical use of unbound plasma cortisol as calculated from total cortisol and corticosteroid-binding globulin. J. Steroid Biochem. 26: 197–202 [DOI] [PubMed] [Google Scholar]
- 25.Doi S. A., Lasheen I., Al-Humood K., Al-Shoumer K. A. 2006. Relationship between cortisol increment and basal cortisol: implications for the low-dose short adrenocorticotropic hormone stimulation test. Clin. Chem. 52: 746–749 [DOI] [PubMed] [Google Scholar]
- 26.Thaler L. M., Blevins L. S., Jr 1998. The low dose (1-microg) adrenocorticotropin stimulation test in the evaluation of patients with suspected central adrenal insufficiency. J. Clin. Endocrinol. Metab. 83: 2726–2729 [DOI] [PubMed] [Google Scholar]
- 27.Vahouny G. V., Chanderbhan R., Hinds R., Hodges V. A., Treadwell C. R. 1978. ACTH-induced hydrolysis of cholesteryl esters in rat adrenal cells. J. Lipid Res. 19: 570–577 [PubMed] [Google Scholar]
- 28.Azhar S., Chen Y. D., Reaven G. M. 1983. Stimulation of lipoprotein receptors and role of lipoprotein and cellular cholesterol during gonadotropin-induced desensitization of steroidogenic response in luteinized rat ovary. J. Biol. Chem. 258: 3735–3740 [PubMed] [Google Scholar]
- 29.Schumacher M., Schwarz M., Leidenberger F. 1985. Desensitization of mouse Leydig cells in vivo: evidence for the depletion of cellular cholesterol. Biol. Reprod. 33: 335–345 [DOI] [PubMed] [Google Scholar]
- 30.Snow K., Jiang N. S., Kao P. C., Scheithauer B. W. 1992. Biochemical evaluation of adrenal dysfunction: the laboratory perspective. Mayo Clin. Proc. 67: 1055–1065 [DOI] [PubMed] [Google Scholar]
- 31.Streeten D. H., Anderson G. H., Jr, Dalakos T. G., Seeley D., Mallov J. S., Eusebio R., Sunderlin F. S., Badawy S. Z., King R. B. 1984. Normal and abnormal function of the hypothalamic-pituitary-adrenocortical system in man. Endocr. Rev. 5: 371–394 [DOI] [PubMed] [Google Scholar]
- 32.Wellington C. L., Walker E. K., Suarez A., Kwok A., Bissada N., Singaraja R., Yang Y. Z., Zhang L. H., James E., Wilson J. E., et al. 2002. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab. Invest. 82: 273–283 [DOI] [PubMed] [Google Scholar]
- 33.Brunham L. R., Kruit J. K., Pape T. D., Timmins J. M., Reuwer A. Q., Vasanji Z., Marsh B. J., Rodrigues B., Johnson J. D., Parks J. S., et al. 2007. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13: 340–347 [DOI] [PubMed] [Google Scholar]
- 34.Vergeer M., Brunham L. R., Koetsveld J., Kruit J. K., Verchere C. B., Kastelein J. J., Hayden M. R., Stroes E. S. 2010. Carriers of loss-of-function mutations in ABCA1 display pancreatic beta-cell dysfunction. Diabetes Care. 33: 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]