Abstract
Background
Limited data describing the perceived strength of evidence behind practices to prevent common healthcare-associated infections (HAIs) exist. We conducted a national survey of infection prevention personnel to assess perception of the evidence for various preventive practices. We were also curious if lead infection preventionist certification in infection prevention and control (CIC) correlated with perceptions of the evidence.
Methods
In 2009, we mailed surveys to 703 infection prevention personnel using a national random sample of U.S. hospitals and all VA hospitals; the response rate was 68%. The survey asked the respondent to grade the strength of evidence behind prevention practices. We considered “strong” evidence as being 4 and 5 on a Likert scale. Multivariable logistic regression models assessed associations between CIC status and the perceived strength of the evidence.
Results
The following practices were perceived by 90% or more of respondents as having strong evidence: alcohol-based hand rub, aseptic urinary catheter insertion, chlorhexidine for antisepsis prior to central venous catheter insertion, maximum sterile barriers during central venous catheter insertion, avoiding the femoral site for central venous catheter insertion, and semi-recumbent positioning of the ventilated patient. CIC status was significantly associated with the perception of the evidence for several practices.
Conclusions
Successful implementation of evidence-based practices should consider how key individuals in the translational process assess the strength of that evidence.
Background
Preventing healthcare-associated infection (HAI) enhances patient safety. There has been a recent proliferation of guidelines, systematic reviews, meta-analyses, and other evidence-based recommendations that clinicians and policy-makers can use to decide which HAI preventive practices to implement in their hospitals.1-10 While the availability of such information is helpful, it is important to understand how those who might champion HAI prevention activities in the hospital view the recommendations that are being provided. For instance, if a practice is perceived as being supported by weak evidence by those in a position to affect change among front-line healthcare personnel, uptake and implementation of this practice is unlikely – irrespective of the underlying strength of the evidence.11
Infection preventionists (IPs) play a key role in preventing HAI within U.S. hospitals. Every U.S. hospital is required to comply with a condition of participation issued by the Centers for Medicare and Medicaid Services (CMS) stating that each hospital must designate a person(s) who serves as the IP to develop and implement policies and practices aimed at prevention and control of infections and communicable diseases.12 IPs are not only expected to keep up with the prevention literature and make recommendations as to what practices to use to prevent infection, they may lead hospital efforts to implement the practice for routine use by front-line healthcare personnel. In this manner, IPs are a key link in the diffusion of innovation process by taking recommendations from the scientific literature and implementing certain recommendations in their facility.13 For example, Furuya and colleagues recently found that a central venous catheter bundle was associated with a lower infection rate only when compliance with bundle elements was high.14 IPs can become certified in infection prevention and control (CIC), a designation that requires that an IP pass a comprehensive examination that demonstrates their mastery of the knowledge necessary to be a highly capable IP (Certification Board of Infection Control & Epidemiology (CBIC) http://www.cbic.org/). Given the emphasis on credentialing healthcare personnel by all stakeholders – coupled with the recent intense focus on HAI prevention – understanding the impact of board certification on the effectiveness of an infection prevention program is imperative.15
Despite the importance of IPs in helping ensure the safety of hospitalized patients, little is known about how infection prevention personnel responsible for implementing infection prevention practices perceive the strength of evidence behind these practices. By appreciating which preventive practices are believed to have strong, moderate, or weak evidence, we can better tailor implementation strategies in actual clinical settings to address such perceptions. As a secondary goal, we sought to determine whether CIC status influences the perceived strength of evidence for various infection prevention practices.
Methods
We conducted a national survey study to compare the use of specific infection prevention practices by U.S. hospitals. In March 2009, using a national sample of non-federal and all VA hospitals, we surveyed infection prevention personnel to understand how they rated the evidence for general infection prevention practices and specific practices to prevent catheter-associated urinary tract infection (CAUTI), central line-associated bloodstream infection (CLABSI), and ventilator-associated pneumonia (VAP). The study sample had been originally derived for a similar survey study conducted in 2005.11,16,17 Specifically, we identified all non-federal, general medical and surgical hospitals with an intensive care unit (ICU) and at least 50 hospital beds using the 2005 American Hospital Association (AHA) Database™ (fiscal year 2003 data). We then stratified hospitals into 2 bed size groups (50-250 beds and ≥ 251 beds), and selected a random sample of 300 hospitals from each group. The 2009 survey was sent to the same hospitals sampled in 2005 with a few exceptions due to closure or merger between the longitudinal survey time points. We sent the survey out to a total of 586 non-federal hospitals. The VA sample consisted of all VA medical centers with primarily general medical and surgical acute care operating beds in 2005 (n=119). Our 2009 survey was sent to 117 VA hospitals. Following a modified Dillman approach,18 we sent an initial mailing, a reminder letter, and a second mailing of the survey after 4 weeks to those who had not yet responded.
All mailings were addressed to the “Infection Control Coordinator” with the following instructions for survey completion: if there were more than 1 infection preventionist (IP) at that particular facility, then the IP who supervises and/or coordinates the other IPs should complete the survey. If an IP was unavailable, the survey should be completed by someone involved in infection prevention such as a hospital epidemiologist, the chair of the infection control committee or the chief of nursing.
The University of Michigan and VA Ann Arbor Healthcare System provided institutional review board approval.
Study Measures
The survey asked about the perception of the evidence for use of general infection prevention practices and practices specific to the prevention of CAUTI, CLABSI, and VAP in adult acute care patients, with attention directed to practices identified in published guidelines or recommendations from the Centers for Disease Control and Prevention (CDC) or professional associations.2,4,6,9,19,20 Using a Likert scale from 1 to 5 (1 being no evidence and 5 extremely strong evidence), respondents were asked to rate how strong they felt the evidence was for specific infection prevention practices. For our descriptive analysis, we categorized responses of 1 or 2 to correspond to “weak” evidence, responses of 3 to “moderate” evidence, and responses of 4 or 5 to “strong” evidence. For our regression analysis, strong evidence was defined as receiving a rating of 4 or 5. The perceptions of the evidence for all practices examined were dichotomized into binary dependent variables, with strong evidence (as defined above) coded as 1 and 0 otherwise. Information about general hospital characteristics and the infection control program were also collected, including whether the facility was participating in an infection prevention-related collaborative. Participation in a collaborative is measured as a “yes” response to the question “Is your facility involved in a collaborative effort to reduce healthcare-associated infections?” We also collected data on the number of full-time equivalent IPs, whether the lead IP is CIC, and the number of years the lead IP has been in current infection prevention position. The number of hospital beds was obtained from the AHA Database™ for fiscal year 2007, and was dichotomized as ≥ 250 beds or < 250 beds for each hospital.
Statistical Analysis
Means and standard deviations for continuous variables, and frequencies and percentages for categorical variables, were used to summarize selected hospital and IP characteristics. Descriptive statistics were used to generate frequency distributions of the perception of evidence for the infection prevention practices investigated. We used covariate adjusted logistic regression to examine multivariable associations between the CIC status of the lead IP and the perceived strength of the evidence for use of the various infection prevention practices. The following covariates were included in our final adjustment model: the number of years the respondent has been in their current position, the number of full-time equivalent IPs, hospital bed-size, and hospital participation in a collaborative focused on reducing HAI. All analyses were conducted using Stata version 11.0 (Stata Corp, College Station, TX).
Results
A total of 478 hospitals responded for an overall response rate of 68%. Demographic characteristics of the respondent hospitals are outlined in Table 1. While our respondents had a number of different job titles, the most frequently identified titles were infection preventionist (60%), various infection prevention-related leadership titles such as director of infection prevention (23%), and infection control and/or employee health nurse (11%). The remainder listed a number of miscellaneous titles (e.g., nurse manager, risk manager, hospital epidemiologist, and director of quality).
Table 1.
Select Characteristics of Respondent Hospitals; N= 478
| Characteristic | Percent Yes or Mean |
|---|---|
| Lead infection preventionist certified in infection prevention and control | 60% |
| Participate in collaborative effort focused on reducing healthcare-associated infection | 72% |
| Number of years the respondent has held current position | 9.25 |
| Number of full-time equivalent infection preventionists | 1.74 |
| Number of hospital beds | 223 |
We first provide descriptive results for perception of evidence divided into the following sections: general infection prevention practices, CAUTI, CLABSI, and VAP. We then provide multivariable associations between perception of evidence and respondent characteristics focusing on the IP’s CIC status.
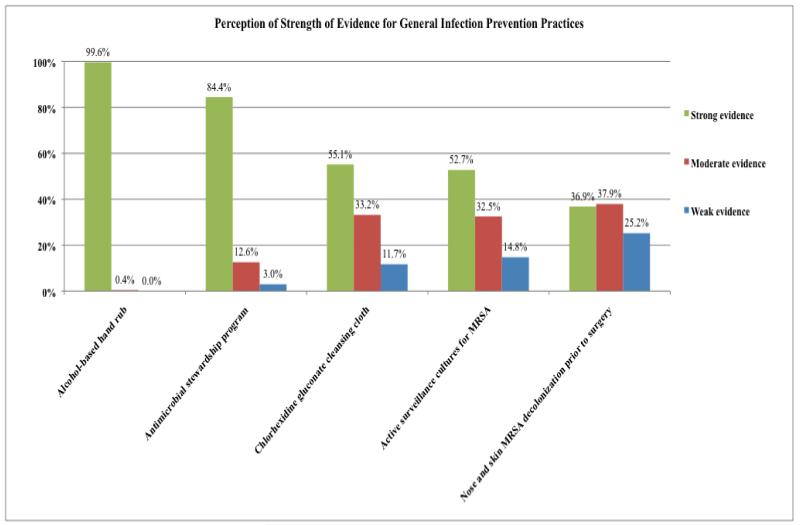
General Infection Prevention Practices (Figure 1)
Figure 1.
Perception of Strength of Evidence for General Infection Prevention Practices
Nearly all respondents believe that hand hygiene with alcohol-based hand wash has strong evidence to support its use. Antimicrobial stewardship programs also were perceived as having strong or moderate evidence supporting their use by 97% of respondents. Support for infection prevention practices focusing on preventing MRSA had more modest support.
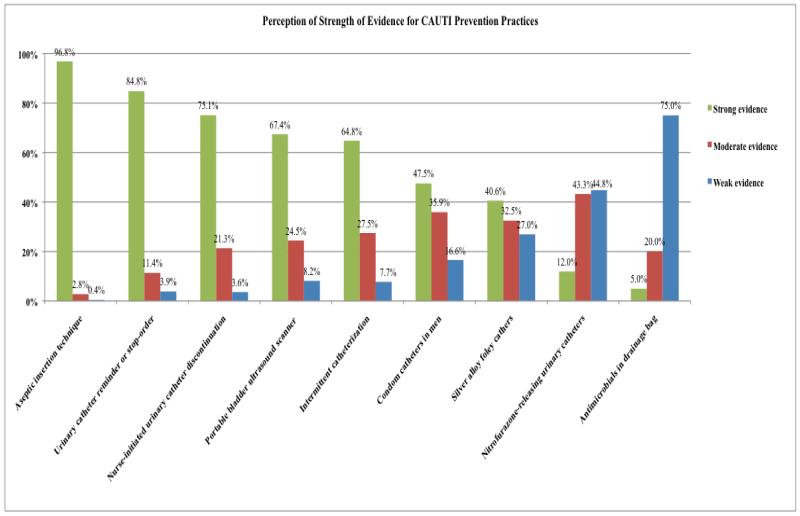
CAUTI Prevention Practices (Figure 2)
Figure 2.
Perception of Strength of Evidence for CAUTI Prevention Practices
Among practices to prevent CAUTI, respondents believed the evidence supporting aseptic insertion technique was the strongest, followed by timely removal of the urinary catheter (utilizing either a urinary catheter reminder or stop-order) or a nurse-initiated removal protocol. Bladder ultrasound scanning also was perceived as having reasonably strong supporting evidence, as was the use of intermittent catheterization. The perceived strength of evidence supporting condom catheters in men and antimicrobial urinary catheters, on the other hand, was much lower.
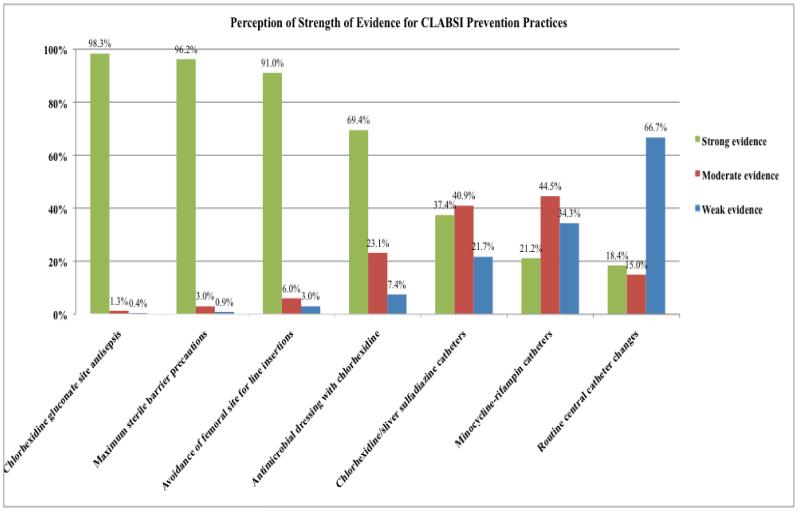
CLABSI Prevention Practices (Figure 3)
Figure 3.
Perception of Strength of Evidence for CLABSI Prevention Practices
Among respondents, 90% or more IPs perceived the strength of evidence supporting the following practices as strong: chlorhexidine gluconate for insertion site skin antisepsis, maximum sterile barriers during central venous catheter (CVC) insertion, and avoiding the femoral site for central venous catheterization. The perceived strength of evidence supporting the use of chlorhexidine sponge dressings at site of CVC insertion was less strong. The evidence supporting the use of antimicrobial catheters was perceived as moderate. Routine CVC changes were perceived as having weak evidence.
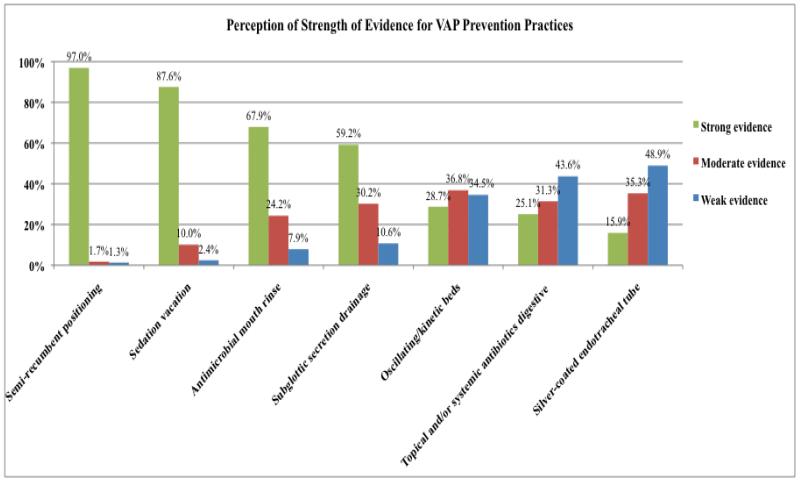
VAP Prevention Practices (Figure 4)
Figure 4.
Perception of Strength of Evidence for VAP Prevention Practices
Semi-recumbent positioning was perceived as having strong evidence for preventing VAP by 97% of respondents, followed by “sedation vacation” (88%), antimicrobial mouth rinse (68%), and subglottic secretion drainage (59%). Other practices – oscillating/kinetic beds, topical and/or systemic antibiotics for selective digestive tract decontamination, and silver coated endotracheal tubes – were perceived as having less strength of evidence.
CIC Status (Table 2)
Table 2.
Significant Multivariable Associations between Certification in Infection Prevention and Control and Strong Perception of Evidence for Infection Control Practices
| Strong Perception of evidence for: | OR (95%CI) |
|---|---|
| GENERAL INFECTION CONTROL: | |
| Antimicrobial stewardship program | 2.13 (1.16 – 3.92) |
|
| |
| CAUTI: | |
| Nurse initiated urinary catheter discontinuation | 2.03 (1.20 – 3.41) |
|
| |
| CLABSI: | |
| Routine central venous catheter changes | 0.38 (0.21 – 0.69) |
|
| |
| VAP: | |
| Oscillating/kinetic beds | 0.46 (0.27 – 0.76) |
| Antimicrobial mouth rinse | 0.56 (0.34 – 0.94) |
| “Sedation vacation” (e.g., regular interruption of sedation) | 2.72 (1.35 – 5.48) |
OR: odds ratio; CI: confidence interval; IP: infection preventionist; CAUTI: catheter-associated urinary tract infection; CLABSI: central line-associated bloodstream infection; VAP: ventilator-associated pneumonia. Multivariable logistic regression results presented are also adjusted for the following covariates: the number of years the respondent has held current position, the number of full-time equivalent infection preventionists, hospital bed-size, and hospital participation in a collaborative focused on reducing healthcare-associated infection.
Having an IP with CIC was associated with a 2-fold increase in the odds of reporting strong perception of the evidence for antimicrobial stewardship programs (p = 0.02). Furthermore, several significant associations were found between CIC status and perceived strength of evidence for device-specific HAIs. Within CAUTI, CIC status was associated with a 2-fold increase in the odds of reporting strong perception of evidence for nurse-initiated urinary catheter discontinuation protocols (p = 0.01). Among CLABSI prevention activities, having an IP CIC was associated with approximately a 40% decreased odds of reporting strong perception of evidence for routine CVC changes (p = 0.001). Finally, within VAP prevention, having an IP CIC was associated with approximately a 45% and 55% decreased odds of reporting strong perception of evidence for oscillating/kinetic beds (p = 0.002) and for antimicrobial mouth-rinse (p = 0.03), respectively. CIC status was also associated with nearly a 3-fold increase in the odds of reporting strong perception of evidence for “sedation vacation” (p = 0.01).
Discussion
Given the clinical and economic consequences of HAI, several organizations and government agencies have issued guidelines for preventing infectious episodes in hospitalized patients.1,2,5,8-10,21 The U.S. government has further incentivized hospitals to enhance the safety of their patients through infection prevention since CMS no longer reimburses hospitals for the additional costs of caring for patients who develop certain infections during hospitalization.22 In order to reach their fullest potential for impact, however, guidelines should be implemented into everyday practice by taking into account the contextual factors at each hospital, including such factors as perceived strength of evidence supporting the use of a particular practice.23 Through our national survey of hospitals, we provide data that can be used by clinicians and policy-makers to guide infection prevention strategies.
Our primary findings are two-fold. First, our respondents, who are primarily IPs, appear to have general agreement about which practices have strong or weak evidence supporting their use in order to prevent HAI. We found that 90% or more of respondents believed that the following practices had strong evidence supporting their use: alcohol-based hand rub, aseptic urinary catheter insertion technique, chlorhexidine gluconate for skin antisepsis, maximum sterile barriers during catheter insertion, avoiding the femoral site for catheter insertion, and semi-recumbent positioning of the ventilated patient. Second, CIC status was associated with strong perceived strength of evidence for several practices. This finding corresponds with our previously reported results that CIC status is associated with a higher likelihood of implementing certain infection prevention practices.11,16,17 Others have found that the incidence of bloodstream infections caused by multidrug-resistant organisms such as methicillin-resistant S. aureus are lower at facilities where at least one of the IPs is CIC.24 CIC status may thus accelerate the implementation of preventive strategies but additional studies are needed before making this conclusion firm.
In general, perceived strength of evidence tracked with the actual strength of evidence that has been reported in evidence-based guidelines. We will discuss these below for each HAI after first discussing the perceived importance of hand hygiene. Respondents believed that hand hygiene using alcohol-based hand rub had the strongest strength of evidence among all the practices queried. This is not surprising given that both the World Health Organization and the U.S. Centers for Disease Control and Prevention (CDC) emphasize improving healthcare worker hand hygiene, as do several noted infection prevention authorities.25-28 For the CAUTI prevention practices surveyed, perceived strength of the evidence is generally in line with the strength of recommendation for the practices as assigned by the CDC HICPAC guideline,2 which is based on an algorithm incorporating the quality of evidence and whether it is already an accepted practice without harms noted. For example, the practices of aseptic insertion and catheter removal reminders or protocols are both a “IB” category recommendation in the HICPAC guideline (IB defined as “strong recommendation supported by low quality evidence suggesting net clinical benefits or is an accepted or established practice (e.g., aseptic technique) supported by low to very low or no quality evidence”). However, even though more respondents assessed the evidence for aseptic insertion as strongest (and as having stronger evidence than for removal using reminders or stop orders), there is actually little evidence available regarding the benefit of aseptic insertion of urinary catheters29– or the strength of association between increased use of aseptic technique with reduced CAUTI rates – though it is a standard practice and consistent with general principles of preventing device-associated infection. In contrast, several studies have demonstrated reduced catheter use and/or CAUTI rates with use of catheter reminders or stop orders.7
Regarding CLABSI, the 2011 updated HICPAC Guidelines for the Prevention of Intravascular Catheter-Related Infections highlight evidence-based practices proven to decrease the risk of this adverse outcome.1 Importantly, the guidelines endorse the performance of specific practices in tandem (or “bundling”) as a vehicle for reducing the burden of this HAI.30 In our survey, we found that most respondents correctly recognized elements comprising the CLABSI bundle (e.g., hand hygiene by the provider prior to insertion, use of maximal sterile barrier precautions, chlorhexidine for skin antisepsis, and avoidance of the femoral vein for site of insertion) as being supported by strong evidence. However, we found some important variations. For example, about one-third of respondents indicated that routine CVC changes had moderate or strong evidence supporting its use. On the contrary, several randomized trials have found this practice not to be beneficial.31,32 In fact, routine changes over guide wires have been associated with a trend towards increased risk of CLABSI.33
For VAP, the 2008 compendium “Strategies to Prevent Ventilator-Associated Pneumonia in Acute Care Hospitals” is the most recent summary of evidence-based practices relevant to prevention of VAP.34 In our survey, we found that some but not all of the practices that respondents felt were associated with the strongest evidence base – such as semi-recumbent positioning, sedation vacation, antimicrobial mouth rinse, and subglottic secretion drainage – were concordant with the guidelines recommendations. Semi-recumbent positioning had a “B” recommendation indicating moderate strength of evidence, as did subglottic secretion drainage and sedation vacation, while antiseptic mouth rinse had an “A” recommendation. Practices not recommended in the guideline – such as routine use of oscillating/kinetic beds – were generally perceived by respondents to have less than strong evidence supporting its use. The finding that the silver-coated endotracheal tubes were perceived to have the weakest strength of evidence supporting their use may also stem from the fact that the study results assessing the efficacy of the silver coated tube35were reported after the publication of the guideline. Importantly, the evidence base supporting VAP preventive practices appears to be substantially less robust than that for CLABSI as highlighted in a recent article.21
CIC has been adopted as the centerpiece of a new competency model developed by the Association for Professionals in Infection Control & Epidemiology (APIC).36 The rationale for this direction is derived from the identification and ongoing updates of core competencies addressed by CIC and emerging appreciation of the value this brings to patient safety. In general, we found that CIC appeared to correlate with strength of evidence for several of the HAI-specific practices.
Our study has a number of important limitations. First, since the response rate was less than 100%, our results have some susceptibility to non-response bias. If the non-respondents were systematically different from those responding, generalizing our results to the full population of U.S. hospitals may not be possible. Second, we relied on self-reported data from one respondent, generally the lead IP, at each hospital to determine the perceived strength of evidence for the various infection prevention practices used and it is possible that the perceptions of this individual may not reflect those of other infection prevention personnel working in the same facility. Third, in a small proportion of hospitals, someone other than an IP filled out the survey, such as a nurse manager or a hospital epidemiologist. Their perceptions may differ from that of the IP at that particular hospital. Fourth, decisions regarding the implementation of infection control practices are often made by multidisciplinary groups. Thus, factors beyond the perception of the strength of evidence may be relevant in choosing whether or not to implement a certain practice. Finally, participating in multistate or national collaboratives with designated (or even mandated) interventions37,38 may influence perception of the strength of evidence.
Despite these limitations, our findings may help inform hospital epidemiologists, clinicians, policy-makers and decision-makers about how best to implement evidence-based recommendations within infection prevention. Specifically, implementation has both technical aspects as well as socio-adaptive components.13 While the actual strength of the evidence is a technical issue, the perception of the strength of that evidence falls within the socio-adaptive realm. Front-line clinicians and IPs primarily react to their perceived strength of the evidence, as that is most immediate. Ideally, implementation scientists will focus not just on the strength of the evidence but how that evidence is perceived by the end-user. Those that develop guidelines should consider how to incorporate new evidence in a timely manner and modify recommendations accordingly.
Acknowledgments
Funding/Support: This project was supported by the National Institute of Nursing Research (5 R01 NR010700), the Blue Cross Blue Shield of Michigan Foundation, and the Ann Arbor VAMC/University of Michigan Patient Safety Enhancement Program.
Acknowledgements: We thank Christine Kowalski, MPH, for her help in data collection.
Footnotes
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011;39(4 Suppl 1):S1–34. doi: 10.1016/j.ajic.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319–326. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 3.Casey AL, Mermel LA, Nightingale P, Elliott TS. Antimicrobial central venous catheters in adults: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(12):763–776. doi: 10.1016/S1473-3099(08)70280-9. [DOI] [PubMed] [Google Scholar]
- 4.Lo E, Nicolle L, Classen D, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S41–50. doi: 10.1086/591066. [DOI] [PubMed] [Google Scholar]
- 5.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 6.Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S22–30. doi: 10.1086/591059. [DOI] [PubMed] [Google Scholar]
- 7.Meddings J, Rogers MA, Macy M, Saint S. Systematic review and meta-analysis: reminder systems to reduce catheter-associated urinary tract infections and urinary catheter use in hospitalized patients. Clin Infect Dis. 2010;51(5):550–560. doi: 10.1086/655133. [DOI] [PubMed] [Google Scholar]
- 8.Rebmann T, Aureden K. Preventing methicillin-resistant Staphylococcus aureus transmission in hospitals: an Executive Summary of the Association for Professionals in Infection Control and Epidemiology, Inc, Elimination Guide. Am J Infect Control. 2011;39(7):595–598. doi: 10.1016/j.ajic.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 9.Tablan OC, Anderson LJ, Besser R, et al. Guidelines for preventing health-care--associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(RR-3):1–36. [PubMed] [Google Scholar]
- 10.Yokoe DS, Mermel LA, Anderson DJ, et al. A compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S12–21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 11.Krein SL, Kowalski CP, Damschroder L, Forman J, Kaufman SR, Saint S. Preventing ventilator-associated pneumonia in the United States: a multicenter mixed-methods study. Infect Control Hosp Epidemiol. 2008;29(10):933–940. doi: 10.1086/591455. [DOI] [PubMed] [Google Scholar]
- 12.Department of Health and Human Services . Centers for Medicare and Medicaid Services Condition of participation: Infection control. 2002. 42 CFR 482.42. [Google Scholar]
- 13.Saint S, Howell JD, Krein SL. Implementation science: how to jump-start infection prevention. Infect Control Hosp Epidemiol. 2010;31(Suppl 1):S14–7. doi: 10.1086/655991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One. 2011;6(1):e15452. doi: 10.1371/journal.pone.0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crist KS, Russell BS, Farber MR. The value of certification and the CIC credential. Am J Infect Control. 2012;40(4):294–295. doi: 10.1016/j.ajic.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Krein SL, Hofer TP, Kowalski CP, et al. Use of central venous catheter-related bloodstream infection prevention practices by US hospitals. Mayo Clin Proc. 2007;82(6):672–678. doi: 10.4065/82.6.672. [DOI] [PubMed] [Google Scholar]
- 17.Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: a national study. Clin Infect Dis. 2008;46(2):243–250. doi: 10.1086/524662. [DOI] [PubMed] [Google Scholar]
- 18.Dillman D. Mail and internet surveys: the tailored design method. J. Wiley; New York: 2000. [Google Scholar]
- 19.O’Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. Infect Control Hosp Epidemiol. 2002;23(12):759–769. doi: 10.1086/502007. [DOI] [PubMed] [Google Scholar]
- 20.Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S31–40. doi: 10.1086/591062. [DOI] [PubMed] [Google Scholar]
- 21.O’Grady NP, Murray PR, Ames N. Preventing ventilator-associated pneumonia: does the evidence support the practice? JAMA. 2012;307(23):2534–2539. doi: 10.1001/jama.2012.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicare and Medicaid Services (CMS), HHS Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Fed Regist. 2007;72(162):47129–48175. [PubMed] [Google Scholar]
- 23.Shekelle PG, Pronovost PJ, Wachter RM, et al. Advancing the science of patient safety. Ann Intern Med. 2011;154(10):693–6. doi: 10.7326/0003-4819-154-10-201105170-00011. [DOI] [PubMed] [Google Scholar]
- 24.Pogorzelska M, Stone PW, Larson EL. Certification in infection control matters: Impact of infection control department characteristics and policies on rates of multidrug-resistant infections. Am J Infect Control. 2012;40(2):96–101. doi: 10.1016/j.ajic.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251–69. doi: 10.1016/0196-6553(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 26.Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51(RR-16):1–45. quiz CE1-4. [PubMed] [Google Scholar]
- 27.WHO guidelines on hand hygiene in health care: a summary. World Health Organization; 2005. [Google Scholar]
- 28.Pittet D, Allegranzi B, Storr J, Donaldson L. ‘Clean Care is Safer Care’: the Global Patient Safety Challenge 2005-2006. Int J Infect Dis. 2006;10(6):419–24. doi: 10.1016/j.ijid.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Carapeti EA, Andrews SM, Bentley PG. Randomised study of sterile versus non-sterile urethral catheterisation. Ann R Coll Surg Engl. 1996;78(1):59–60. [PMC free article] [PubMed] [Google Scholar]
- 30.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 31.Eyer S, Brummitt C, Crossley K, Siegel R, Cerra F. Catheter-related sepsis: prospective, randomized study of three methods of long-term catheter maintenance. Crit Care Med. 1990;18(10):1073–1079. [PubMed] [Google Scholar]
- 32.Uldall PR, Merchant N, Woods F, Yarworski U, Vas S. Changing subclavian haemodialysis cannulas to reduce infection. Lancet. 1981;1(8234):1373. doi: 10.1016/s0140-6736(81)92553-8. [DOI] [PubMed] [Google Scholar]
- 33.Cook D, Randolph A, Kernerman P, et al. Central venous catheter replacement strategies: a systematic review of the literature. Crit Care Med. 1997;25(8):1417–1424. doi: 10.1097/00003246-199708000-00033. [DOI] [PubMed] [Google Scholar]
- 34.Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S31–40. doi: 10.1086/591062. [DOI] [PubMed] [Google Scholar]
- 35.Kollef MH, Afessa B, Anzueto A, et al. Silver-coated endotracheal tubes and incidence of ventilator-associated pneumonia: the NASCENT randomized trial. JAMA. 2008;300(7):805–13. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 36.Murphy DM, Hanchett M, Olmsted RN, et al. Competency in infection prevention: a conceptual approach to guide current and future practice. Am J Infect Control. 2012;40(4):296–303. doi: 10.1016/j.ajic.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419–30. doi: 10.1056/NEJMoa1007474. [DOI] [PubMed] [Google Scholar]
- 38.Saint S, Olmsted RN, Fakih MG, et al. Translating health care-associated urinary tract infection prevention research into practice via the bladder bundle. Jt Comm J Qual Patient Saf. 2009;35(9):449–55. doi: 10.1016/s1553-7250(09)35062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]