Osteosarcoma is the most common type of primary bone malignancy in children and young adults [1-3]. This malignancy is rare, with an annual incidence of approximately 1 case per 100,000 children. In the 1970s, the 2-year survival was low (15–20%). Since then, the introduction of effective multiagent chemotherapy regimens has helped improve outcomes considerably, with the current overall 5-year survival being 50–75% [2,4,5].
Despite the improvement in survival rates over the past 30 years, patients with osteosarcoma who present with metastatic lung disease do not fare well. At best, they have a 5-year survival of 30%, provided complete resection of lung metastases is achieved and there are no metastases in other sites [4,5].
The current recommended surgical approach for patients with osteosarcoma having lung metastases is meticulous resection of all metastatic disease. This includes metastatic lesions that are detected by imaging and those identified by palpation at thoracotomy [4–11]. Reports from various centers agree that complete resection of all lung metastases is the best prognostic predictor of survival in these patients[8-12]. In 1971, before the introduction of adjuvant chemotherapy, Martini et al. [13] reported 22 young patients with metastatic osteosarcoma who underwent a total of 59 thoracotomies. The 3-year survival for patients who underwent metastasectomy was 40%. The overall 5-year survival in this series was 32%, compared with 17% in patients who did not undergo metastasectomy.
Currently, surgical management of the contralateral hemithorax in patients presenting with unilateral pulmonary nodules on computed tomography (CT) scans within 24 months of diagnosis is controversial. Some authors advocate a contralateral thoracotomy in all patients with early metastases [10]. At the St. Jude Children’s Research Hospital, ipsilateral thoracotomy is performed if CT scan identifies unilateral metastases. Performing a bilateral thoracotomy in patients presenting with unilateral metastatic disease may be associated with added morbidity therefore demands careful analysis.
The aim of this study was to conduct a retrospective review of osteosarcoma patients presenting with unilateral pulmonary metastases within the first 24 months of diagnosis at St. Jude from 1980 to 2005 to determine whether contralateral exploratory thoracotomy is beneficial.
Materials and methods
Study Population
The study was approved by the St. Jude Institutional Review Board. We retrospectively reviewed the clinical charts and institutional databases of patients presenting with or developing early pulmonary metastases (within 24 months of diagnosis) at St. Jude from June 1980 to September 2005. Patients who were not considered surgical candidates were not included in this review.
According to our protocol, patients who presented with unilateral lung metastases had unilateral thoracotomy and those with bilateral disease had staged bilateral thoracotomies. Patients who had radiographically suspected recurrence were operated upon and suspected nodules were removed. For the purpose of this study, “pulmonary metastases” refer to the presence of pathologically proven osteosarcoma lung metastases and “recurrence” refers to new pulmonary metastases that appeared after resection of all detectable pulmonary nodules.
Factors assessed for prognostic significance included unilateral versus bilateral pulmonary metastases at presentation, number of pulmonary metastatic nodules at initial resection, and laterality of pulmonary recurrence after the initial resection. Because we reviewed records from over 25 years, we also analyzed survival along a timeline to determine the effect of evolving imaging techniques and treatment protocols on the prognosis.
Statistical Methods
Descriptive statistics were reported for continuous and categorical variables. The Shapiro-Wilk test was used to test the normality of each continuous variable. Group medians were compared using the Wilcoxon-Mann-Whitney exact test. Associations from singly ordered contingency tables were tested using the Kruskal-Wallis exact test. Exact simultaneous confidence intervals (CI) for the incidence of each type of recurrence were calculated using the methods of Blyth-Still-Casella and Goodman at the 95% level. The Kaplan-Meier method was used to estimate survival curves for the whole cohort and by analysis group. The log rank test was used to test for significant differences in the survival of analysis groups.
Results
Demographics
Table 1 lists the demographics and site of primary tumor for the patients. From June 1980 and September 2005, 109 osteosarcoma patients (70 males and 39 females) with resectable early pulmonary metastases were admitted to St. Jude. Median age of the patients was 14 years (range: 2.4–24.3 years). The follow-up period ranged from 4 months to 24 years with a median of 1.5 years (table 1)
Table 1.
Patient demographics and site of primary tumor
| N (%) | |
|---|---|
| Age (years) | |
| 0-5 | 5 (4.6) |
| 6-10 | 15(13.8) |
| 11-15 | 44 (40.4) |
| >15 | 45 (41.3) |
| Gender | |
| Male | 70 (64.2) |
| Female | 39 (35.8) |
| Site of primary tumor | |
| Femur | 66 (60.6) |
| Tibia | 21 (19.3) |
| Humerus | 12 (12.8) |
| Fibula | 3 (2.8) |
| Radius | 1 (0.9) |
| Pelvis | 3 (2.8) |
| Rib | 1 (0.9) |
Site of primary tumor
Of the 109 patients, 105 (96.4%) had primary tumors in the extremities: 66 (60.6%) in the femur, 21(19.3%) in the tibia, 14 (12.8%) in the humerus, 3(2.8%) in the fibula, and 1 (0.9%) in the radius. Four (3.7%) patients presented with primary axial disease: 3 (2.8%) with pelvic disease and 1(0.9%) with a primary tumor in the rib (Table 1).
Pulmonary metastases
Of the patients, 81 (74.3%) presented with early unilateral pulmonary metastatic disease: 35 (43.2%) had left pulmonary involvement only and 46 (56.8%) had right pulmonary involvement only. Twenty-eight (25.7%) patients had bilateral involvement.
The median time from presentation to detection of pulmonary metastases was 12 months (SD = 14.46 months) for bilateral and 15 months (SD = 20.26 months) for unilateral disease. This difference was not statistically significant (p = 0.43).
The number of metastatic nodules resected at surgery was recorded in 108 (99%) patients. Of the 27 patients with bilateral disease, 11 (41%) had 4 or fewer nodules and 16 (59%) had 5 or more nodules. Of the 81 patients with unilateral pulmonary involvement, 73 (90%) had 4 or fewer nodules and 8 (10%) had 5 or more nodules.
Recurrence patterns
Of the 81 patients presenting with unilateral metastatic disease, 39 (48%) had subsequent pulmonary recurrence, which for most occurred within 6 months. Within the first 6 months, 9 (11%) patients had a recurrence on the same side and 10 (12%) on the contralateral side. One year following thoracotomy, 12 (15%) patients had recurrence on the same side, 12 (15%) had recurrence on the contralateral side, and 1(1.2%) had bilateral recurrence. Two years later, 13 (16%) had recurrence on the same side, 19 (23%) had recurrence on the contralateral side, and 2 (2.5%) had bilateral recurrence.
Overall recurrence after initial resection of unilateral pulmonary metastases was as follows: 17.3% (95% CI:9.8%,26.6%) on the same side, 27.1%(95%CI:17.9%,38.1%) on the contralateral side, and 3.7%(95% CI:1%,9.8%) bilateral. In patients presenting with unilateral disease the overlapping confidence intervals indicate that we have no evidence of a statistically significant difference between the number of patients with ipsilateral and contralateral recurrence. (Table 2)
Table 2.
Time after the first thoracotomy to recurrence in patients with unilateral disease
| Months |
||||
| Recurrence site | 6 | 12 | 24 | Overall |
|
| ||||
| Ipsilateral | 9 | 12 | 13 | 14(17.3%) |
| Contralateral | 10 | 12 | 19 | 22(27.2%) |
|
| ||||
| Bilateral | 0 | 1 | 2 | 3(3.7%) |
Of the 28 patients who presented with bilateral disease, 11(39.3%) had unilateral recurrence; no patient had bilateral recurrence.
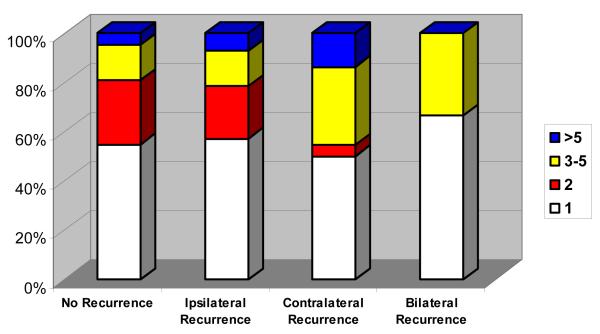
The number of metastatic pulmonary nodules was recorded in 81 patients with unilateral disease: 44 (54.32%) had 1 nodule, 15 (18.52%) had 2 nodules, 16 (19.75%) had 3–5 nodules, and 6 (7.41%) had more than 5 nodules. There was no association between the number of metastatic nodules in patients with early unilateral disease and the risk or side of recurrence (p = 0.69; Fig. 1).
Fig. 1.
Number of pulmonary metastases at presentation in patients with osteosarcoma and unilateral lung metastases as a factor in different recurrence patterns
Survival analysis
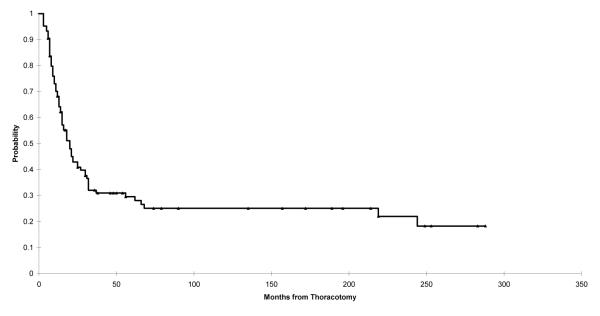
The overall survival of patients diagnosed with osteosarcoma from June 1980 to September 2005 and presenting with early pulmonary metastases was 43% at 2 years and 30% at 5 years (Fig. 2).
Fig. 2.
The Kaplan–Meier plot showing overall survival of patients diagnosed with at St. Jude with osteosarcoma from June 1980 to September 2005 and presenting with pulmonary metastases. Point estimates and 95% CIs for survival after 2 years are 0.43 (0.33, 0.53) and after 5 years are 0.30 (0.19, 0.40).
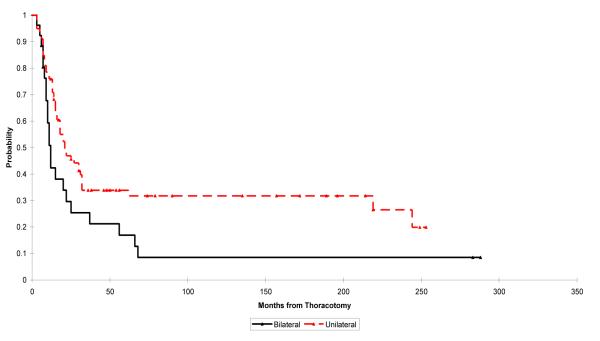
Patients presenting with unilateral disease had 2- and 5-year survivals of 47% and 34%, respectively, and those presenting with bilateral disease had 2- and 5-year survivals of 30% and 17%, respectively. There was no statistically significant difference in survival between the patient with unilateral or bilateral disease, but a larger cohort might have supported this finding (p = 0.051; Fig. 3).
Fig. 3.
Kaplan–Meier plot showing overall survival of patients presenting with unilateral pulmonary metastases vs. that for patients presenting with bilateral pulmonary metastasis. The log rank test p-value for the difference between the survival rates in these groups is 0.051. Point estimates and 95% CIs for survival for unilateral and bilateral pulmonary metastases, respectively, after 2 years are 0.47 (0.36, 0.58) and 0.30 (0.12, 0.47) and after 5 years are 0.34 (0.21, 0.47) and 0.17 (0.03, 0.30).
Patients with ipsilateral recurrence had both 2- and 5-year survivals of 35% and those with contralateral recurrence had 2- and 5-year survivals of 62% and 28%, respectively. There was no significant difference in survival between patients with ipsilateral or contralateral recurrences (p = 0.34; Fig. 4).
Fig. 4.
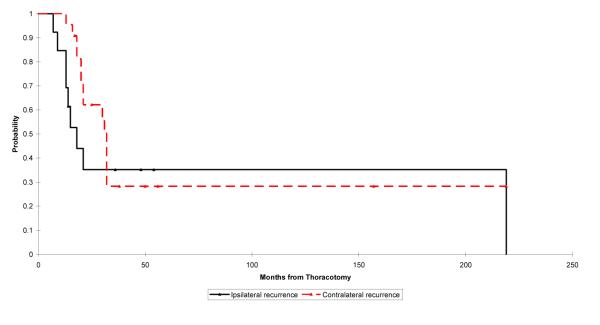
Kaplan–Meier plot showing survival of patients diagnosed with osteosarcoma with pulmonary metastases where reccurrence is ipsilateral vs. contralateral. The log rank test p-value for the difference between the survival rates in these groups is 0.34. Point estimates and 95% CIs for survival for ipsilateral and contralateral recurrence, respectively, after 2 years are 0.35 (0.10, 0.60) and 0.62 (0.43, 0.82) and after 5 years are 0.35 (0.00, 0.74) and 0.28 (0.01, 0.55).
Timeline analysis
Improvements in imaging techniques
Because imaging techniques improved significantly during the study period (from 1980 to 2005), data were subdivided into 3 time periods. For 1980–1987, 1988–2000, and 2001–2005, the number of patients who had unilateral disease or bilateral disease, respectively, were 28 (68.3%) and 13 (31.7%), 35 (74.5%) and 12 (25.5%), and 18 (85.7%) and 3 (14.3%).
Changes in treatment
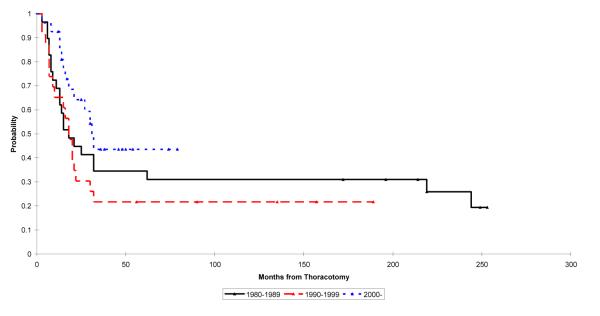
To study the effect of advances in treatment protocols on survival of patients with early unilateral disease, we evaluated survival rates in 3 periods. For 1980–1989, 1990–1999, and 2000–2005, the 2- and 5-year survivals for patients, respectively, were 45% and 35%, 30% and 22%, and 64% and 44%. These differences were not statistically significant (p = 0.14; Fig. 5).
Fig. 5.
Kaplan–Meier plot showing survival of patients diagnosed with osteosarcoma with unilateral pulmonary metastases. The log rank test p-value for the difference between the survival rates in these groups is 0.14. Point estimates and 95% CIs for survival are as follows: 1980-1989 2 year: 0.45 (0.27, 0.62); 5 year: 0.35 (0.19, 0.51) 1990-1999: 2 year: 0.30 (0.13, 0.48); 5 year: 0.22 (0.05, 0.39) 2000-2005: 2 year: 0.64 (0.45, 0.83); 5 year: 0.44 (0.07, 0.80)
Discussion
We reviewed the recurrence patterns and survival data of 109 children with osteosarcoma and early resectable pulmonary metastases admitted to St. Jude over a period of 25 years (1980–2005). All 81 patients with early unilateral metastatic disease underwent unilateral thoracotomy; none of them had an exploratory thoracotomy of the contralateral side. Our analysis suggests that osteosarcoma patients with early unilateral pulmonary metastases may not benefit from exploration of the contralateral hemithorax.
In 2003, Su and coworkers [10] from the Memorial Sloan-Kettering Cancer Center reported on 9 osteosarcoma patients having CT findings of early unilateral pulmonary metastases. Despite negative CT findings, all patients underwent contralateral exploratory thoracotomy, and 6 (86%) had metastases in the contralateral lung. In the same study, in another group of patients presenting with late metastases (more than 2 years from initial diagnosis), only 1 of 5 patients (20%) relapsed in the contralateral lung. On the basis of these results, the authors advocated contralateral exploratory thoracotomy in patients with osteosarcoma and early unilateral pulmonary metastases.
Because we did not perform an exploration of the contralateral lung, it was reasonable to expect that our patients would have an increased recurrence in the contralateral lung. However, Twelve months after resection 12 patient had ipsilateral recurrence and 12 had contralateral recurrence representing a total of 30% one year recurrence rate. Two years into the study 13 (16%) had unilateral recurrence and 19(23%) patients had contralateral recurrence While small foci of tumor cells may lie dormant for quite some time, it is unlikely that an early contralateral thoracotomy would identify these foci of tumor. Overall on long term follow-up 22 (27%) patients had a recurrence on the contralateral side versus 14 (17%) with recurrence on the same side. There was no statistically significant difference between the recurrences in the contralateral and ipsilateral lung.
The difference between our findings and those from the study by Su et al. [10] should be interpreted with care. It is possible that if we had performed routine contralateral explorations as suggested, we might have found a higher incidence of patients with bilateral metastases. However, these “occult” metastases, even if present, may not have developed into recognizable metastases and detected by imaging and their clinical significance is uncertain.
The prognostic significance of unilateral versus bilateral metastatic lung disease has been discussed by several authors. In a Pediatric Oncology Group study, Harris et al. [14] reported that patients presenting with unilateral disease had a 5-year event-free survival (EFS) of 75% after resection and treatment and those presenting with bilateral disease had a 5-year EFS of 35%. However, this excellent survival rate for patients with unilateral disease could not be reproduced in studies by other groups [7,9,10,11]. Kempf-Bielack et al. [15] analyzed 576 eligible patients of all ages with relapsed osteosarcoma from the Cooperative Osteosarcoma Study Group (COSS). There was a statistically significant 5-year survival advantage for patients with unilateral disease (34%) over those with bilateral disease (14%) [15]. In contrast, Harting et al. [11] in their retrospective study of 137 patients with osteosarcoma and pulmonary metastases found no difference in survival of patients with bilateral and unilateral metastatic disease.
In our series no patients were lost to follow-up but 57% of patients presenting with unilateral disease died in the first 2 years. This mortality rate is high but not different from survival data of other studies. Patients presenting with unilateral metastatic disease had a 5-year survival of 34% and those presenting with bilateral disease had a 5-year survival of 17%. While there was a trend indicating an improved survival patients with unilateral disease, the difference in survival did not reach statistical significance (p = 0.051). A larger cohort might have provided more conclusive data. Our results correlate with the survival data published in the COSS study [15].
Younes et al. [16] investigated the need for bilateral thoracotomies in 179 patients with various types of solid tumors presenting with unilateral pulmonary metastases. All the patients in their study had only ipsilateral thoracotomies. On follow up, the overall contralateral recurrence rate was 22%. The predictors of a contralateral metastatic lesion were the type of the tumor (osteosarcoma) and more than 2 metastatic nodules in the ipsilateral lung. Younes et al. do not advocate routine bilateral thoracotomy in patients with unilateral disease. Bacci et al. [17] also found a striking correlation between the number of pulmonary nodules and relapse: patients with 1 or 2 nodules had a recurrence rate of 10.8% and those with more than 2 nodules had a recurrence rate of 53.1% [17]. In contrast, Harting et al. [11] did not find a significant correlation between the number of nodules and recurrence rate. Although it seems logical to assume that a higher tumor burden on one side might indicate bilateral disease, in our study there was no correlation between the number of metastatic nodules and contralateral recurrence.
There are several studies on the correlation between bilateral or unilateral lung involvement and survival. Younes et al. [16] found the survival of patients with contralateral recurrence to be comparable with that of patients presenting with bilateral disease. Harting et al. [11] also found no correlation between laterality of recurrence and survival. In our study, there was no statistically significant difference (p = 0.34) between the 5-year survival rates for patients with ipsilateral recurrence (35%) and for those with contralateral recurrence (28%).
With advancements in imaging techniques, such as introduction of the spiral CT and protocols involving smaller slices (5 mm vs. 10 mm), we expected an increase in patients diagnosed with bilateral metastatic disease. However, our data indicated a reverse trend. During the first period of our study (1980–1989), 13 (31.7%) patients had bilateral metastatic disease. Recent data (2001–2005) showed that only 3 (14.3%) patients had bilateral disease. This might be because of earlier detection of early small pulmonary lesions, better local control, and better chemotherapy treatment protocols.
Since methotrexate was introduced in the 1960s, treatment protocols have evolved significantly. In 1976, Rosen introduced the T5 protocol, which included multidrug therapy with vincristine, doxorubicin, and weekly high-dose methotrexate. Since then, treatment has continued to evolve and improve. The current standard therapy includes high-dose methotrexate, doxorubicin, and cisplatin, and some protocols add cyclophosphamide, ifosfamide, etoposide, or carboplatin [18]. To show the impact of evolving imaging techniques, surgical approach and treatment protocols we looked at patient survival over three decades. In our series, patients with early unilateral pulmonary metastases had a 5-year survival rate of 35% and 22% for 1980–1989 and 1990–1999, respectively. The survival for 2000–2005 rose to 44%, but the differences for the 3 periods are not statistically significant and are comparable to reports from other studies. These results also show that the survival rates of these patients have not improved despite the improvement in imaging techniques and more efficient treatment protocols. Prospective studies should focus on finding ways to improve the survival of patients with osteosarcoma and pulmonary metastases.
Conclusions
This retrospective study of 109 osteosarcoma patients with early resectable pulmonary metastases admitted to our institution over a period of 25 years suggests that osteosarcoma patients with early unilateral pulmonary metastases may not benefit from exploration of the contralateral hemithorax. Based on evidence presented in this study a prospective randomized long term study would be needed to establish the best approach to these children before routine bilateral thoracotomy can be recommended.
Acknowledgments
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bacci G, Longhi A, Bertoni F, et al. Primary high-grade osteosarcoma: comparison between preadolescent and older patients. J Pediatr Hematol Oncol. 2005;27(3):129–134. doi: 10.1097/01.mph.0000155860.38641.83. [DOI] [PubMed] [Google Scholar]
- 2.Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity. Updated results of the Multi-Institutional Osteosarcoma Study. Clin Orthop. 1991;(270):8–14. [PubMed] [Google Scholar]
- 3.Kellie SJ, Pratt CB, Parham DM, et al. Sarcomas (other than Ewing’s) of flat bones in children and adolescents. A clinicopathologic study. Cancer. 1990;65(4):1011–1016. doi: 10.1002/1097-0142(19900215)65:4<1011::aid-cncr2820650428>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Goorin AM, Shuster JJ, Baker A, et al. Changing pattern of pulmonary metastases with adjuvant chemotherapy in patients with osteosarcoma: results from the Multi-Institutional Osteosarcoma Study. J Clin Oncol. 1991;9(4):600–605. doi: 10.1200/JCO.1991.9.4.600. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson WS, Harris MB, Goorin AM, et al. Presurgical window of carboplatin and surgery and multidrug chemotherapy for the treatment of newly diagnosed metastatic or unresectable osteosarcoma: Pediatric Oncology Group Trial. J Pediatr Hematol Oncol. 2001;23(6):340–348. doi: 10.1097/00043426-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 6.McCarville MB, Kaste SC, Cain AM, et al. Prognostic factors and imaging patterns of recurrent pulmonary nodules after thoracotomy in children with osteosarcoma. Cancer. 2001;91(6):1170–1176. doi: 10.1002/1097-0142(20010315)91:6<1170::aid-cncr1114>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Kaste SC, Pratt CB, Cain AM, et al. Metastases detected at the time of diagnosis of primary pediatric extremity osteosarcoma at diagnosis: imaging features. Cancer. 1999;86(8):1602–1608. doi: 10.1002/(sici)1097-0142(19991015)86:8<1602::aid-cncr31>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Strauss SJ, McTiernan A, Whelan JS. Late relapse of osteosarcoma: implications for follow-up and screening. Pediatr Blood Cancer. 2004;43(6):692–697. doi: 10.1002/pbc.20154. [DOI] [PubMed] [Google Scholar]
- 9.Chou AJ, Merola PR, Wexler LH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104(10):2214–2221. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 10.Su WT, Chewning J, Abramson S, et al. Surgical management and outcome of osteosarcoma patients with unilateral pulmonary metastases. J Pediatr Surg. 2004;39(3):418–423. doi: 10.1016/j.jpedsurg.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Harting MT, Blakely ML, Jaffe N, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg. 2006;41(1):194–199. doi: 10.1016/j.jpedsurg.2005.10.089. [DOI] [PubMed] [Google Scholar]
- 12.Divis G. Ein Beitrag zur operativen Behandlung der Lung-engeschwulste. Acta Chir Scand. 1927;62:329–341. [Google Scholar]
- 13.Martini N, Bains MS, Huvos AG, et al. Surgical treatment of metastatic sarcoma to the lung. Surg Clin North Am. 1974;54(4):841–848. doi: 10.1016/s0039-6109(16)40387-7. [DOI] [PubMed] [Google Scholar]
- 14.Harris MB, Gieser P, Goorin AM, et al. Treatment of metastatic osteosarcoma at diagnosis: a Pediatric Oncology Group Study. J Clin Oncol. 1998;16(11):3641–3648. doi: 10.1200/JCO.1998.16.11.3641. [DOI] [PubMed] [Google Scholar]
- 15.Kempf-Bielack B, Bielack SS, Jürgens H, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23(3):559–568. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 16.Younes RN, Gross JL, Deheinzelin D. Surgical resection of unilateral lung metastases: is bilateral thoracotomy necessary? World J Surg. 2002;26(9):1112–1116. doi: 10.1007/s00268-002-6209-8. [DOI] [PubMed] [Google Scholar]
- 17.Bacci G, Briccoli A, Longhi A, et al. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. 2005;44(7):748–755. doi: 10.1080/02841860500327503. [DOI] [PubMed] [Google Scholar]
- 18.Marina NM, Pratt CB, Rao BN, et al. Improved prognosis of children with osteosarcoma metastatic to the lung(s) at the time of diagnosis. Cancer. 1992;70(11):2722–2727. doi: 10.1002/1097-0142(19921201)70:11<2722::aid-cncr2820701125>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]