Abstract
Clinically, SSRIs are widely prescribed in the treatment of several anxiety disorders, though very few pre-clinical studies have observed a beneficial effect of this class of drugs in animal models of anxiety. Furthermore, the biphasic pattern observed clinically, an exacerbation of anxiety followed by beneficial effects, is rarely observed in animal studies. In the present study we document this clinical phenomenon in several behavioral paradigms. While a single injection of citalopram induced anxiogenic effects, three administrations of citalopram were sufficient to elicit anxiolytic effects. Congruent with these data, we observed that short-term repeated administration with citalopram was accompanied by an increase activation of CREB in hippocampus and a desensitization of 5-HT1A receptors, two phenomenon well associated with chronic (2-3 week) rather than acute effects of antidepressants. Moreover, anxiolytic effects of citalopram were abolished in CREB αΔ mutant animals in elevated zero maze and tail suspension test, but not in novelty-induced hypophagia or 5-HT1A receptors desensitization. The significance of the elevated-zero maze and TST paradigms in predicting therapeutic efficacy is well known while effects in NIH and 5-HT1A sensitization are less well-established. These data demonstrate that behavioral response to citalopram is dependent on frequency of acministration which is associated with differences in CREB requirements.
Keywords: citalopram, anxiety, depression, cAMP response element binding protein, mouse models
Introduction
Depression and anxiety are both highly co-morbid, with a lifetime prevalence of 10-20% (Cryan and Holmes, 2005). In addition to co-morbidity, these two psychiatric disorders share common pharmacotherapeutic interventions (Vaswani et al, 2003). Antidepressants, such as selective serotonin reuptake inhibitors (SSRI), have been shown to be effective in treating major depression, but also several anxiety disorders. Traditional anxiolytics however, such as benzodiazepines, are only effective in treating general anxiety and panic disorders (Rickels, 1978). In contrast SSRIs appear to be the best strategy to treat a wide range of anxiety disorders such as panic (Gorman, 1997), social phobia (Fairbanks et al, 1997), obsessive-compulsive (Goodman, 1999) or post-traumatic stress disorders (PTSD) (Davidson, 1997) as well as general anxiety (Rocca et al, 1997). SSRIs exert their beneficial properties after chronic administration, as opposed to acute administration for benzodiazepines. However, exacerbation of anxiety during the initial phase of treatment with SSRIs has been reported in some patients (Gorman et al, 1987). In healthy volunteers, acute administration of citalopram precipitates anxiety using fear potentiated startle as the measure of the anxious state while chronic administration of citalopram decrease aversive states (Grillon et al, 2008; Grillon et al, 2007).
The biphasic response of SSRIs has been well characterized clinically, however there is less consistency regarding the effects of SSRIs in preclinical models of anxiety (see(Borsini et al, 2002) for review). Repeated administration of SSRIs seem to exert anxiolytic-like effects in tests such as marble-burying (Ichimaru et al, 1995), novelty-induced hypophagia (Dulawa et al, 2004) and mouse defensive test battery (Griebel et al, 1995) but not in paradigms typically used for screening anxiolytic compounds such as the open field, plus, or zero maze (Borsini et al, 2002). Only a few studies have demonstrated an anxiogenic effect of a single administration of SSRIs (Belzung et al, 2001; Griebel et al, 1994), however to date the biphasic anxiogenic and anxiolytic effects of SSRIs are not well documented in rodents (Griebel et al, 1995; Griebel et al, 1994).
Preclinical studies confirm that SSRIs increase extracellular levels of 5-HT, however the maximal effects caused by the acute systemic administration of SSR Is are only a 2-4 fold increase (Fuller, 1994) due to a restraining effect exerted by the activation of 5-HT autoreceptors that inhibit neurotransmitter synthesis and release. Pharmacological blockade or genetic disruption of inhibitory somatodendritic 5-HT1A and terminal 5-HT1B autoreceptors augments the magnitude of 5-HT increase produced by acute administration of SSRIs (Hjorth et al, 2000). In contrast, chronic administration of SSRIs cause diminished responsiveness of 5-HT1A receptors (Blier and de Montigny, 1994; Le Poul et al, 1995; Le Poul et al, 1997). These distinctions in 5-HT1A receptor regulation may contribute to the differential behavioral response seen after acute or chronic SSRI administration.
Depending on the specific SSRI, 5-HT receptors are activated directly or indirectly. While cAMP stimulation is commonly observed, not all 5-HT receptors increase cAMP levels. Most notably, the 5-HT1A receptor is coupled to Gi. Multiple mechanisms of 5-HT1A signal transduction have been proposed some of which may lead to extracellular-regulated kinase (ERK) microtubule-associated protein (MAP) kinases mobilization(Cowen et al, 1996). Among the many substrates of ERK in the brain, is the transcription factor CREB (cAMP response element binding protein). CREB appears to be a downstream molecule common to a number of signal transduction pathways activated by antidepressant drugs (Nestler et al, 2002). Most studies have demonstrated that chronic, but not acute antidepressants modulate CREB activity, however there are a few reports of acute effects following administration of SSRIs (Nibuya et al, 1996; Rantamaki et al, 2007; Thome et al, 2000; Tiraboschi et al, 2004). Thus, acute SSRI administration may have differential effects on CREB activity than chronic administration and the state of 5-HT1A receptor levels or activity may impact this as well.
To understand both behavioral and neuronal adaptations elicited by SSRIs and to investigate the transition between anxiety producing and anxiety reducing/antidepressant states we compared a single acute administration of citalopram with repeated short term exposure. We demonstrate that as few as three injections of citalopram, over 24 hours elicit antidepressant-like and anxiolytic effects on behavioral, cellular and molecular measures.
Materials and Methods
Animals
CREBαΔ mutant mice were backcrossed to the inbred mouse strains of either 129SvEv and C57Bl/6 for more than 20 generations. For these experiments, WT and CREBαΔ mutants were F1 hybrids obtained from crossing mice heterozygous for the CREB mutation from each strain. In order to minimize the influence of strain effects, pharmacological studies were carried out in 129SvEv and C57Bl/6 F1 hybrids. Age- and sex-matched mice (2-4 months old, 20-40g) were housed in groups of two (NIH paradigm) or groups of three to five (Elevated Zero Maze) and maintained on a 12 hr light/dark cycle with food and water available ad libitum in accordance with the University of Pennsylvania Animal Care and Use Committee. All experimental testing sessions were conducted between 9:00 AM and 3:00 PM, with animals randomly assigned to treatment conditions and tested in counterbalanced order.
Elevated Zero Maze (EZM)
The apparatus used for evaluating behavior in this paradigm was a circular track constructed in grey plexiglas elevated 24 inches from the ground. It consisted of two open quadrants with a raised, 0.5 cm edge and two closed quadrants with walls 12 cm high. Mice were placed in one of the closed quadrants designated as the starting quadrant and were allowed to investigate the zero-maze for a period of 5 min. Video tracking system (Viewpoint; Champagne au Mont d’or, France) was used to record both time spent in open-arm and locomotor activity.
Novelty Induced Hypophagia (NIH paradigm)
This paradigm was performed as described previously (Gur et al., 2007). For 1 week before the training period, and for the duration of the experiment, mice were housed in groups of two. Training consisted of daily sessions in which mice were exposed to a highly palatable food (peanut butter chips; Nestle, Glendale, CA) in a clear plastic dish. Plastic dividers were placed inside each cage to separate the mice during the training and home cage testing periods. Mice were acclimated for 1 h before placement of food. Food was placed in the cage for 15 min and latency to consume was measured. By the 12th day, a baseline latency to approach the food dish was reached such that there was <20% variability between mice.. For testing in the novel environment, mice were removed from the home cage and placed in an empty standard cage with no bedding, which was sprayed with a novel scent (Pine Sol) and was placed in a white box with bright light illumination. Latency to consume was recorded.
Tail suspension test (TST)
Mice were suspended by the tail with tape to a vertical aluminum bar. A 6 min test session is videotaped and scored by a trained observer who was blind to the experimental conditions. Mice demonstrated several escape-oriented behaviors interspersed with bouts of immobility as the session progressed The behavioral measure scored is the duration of “immobility,” defined as the time when mice were judged to cease escape-motivated behaviors.
8-OHDPAT-induced hypothermia
Body temperature was measured with a digital thermometer; TH5 Thermalert Monitoring Thermometer (Physitemp Instruments, Clifton, NJ) with a probe for mice (RET-3) inserted 1.0 cm into the rectum. All temperatures were measured at ambient temperature (21 ± 1° C). Basal temperature was measured 30 min and immediately prior to the subcutaneous injection of (±)-8-hydroxy-2-(di-n-propylamino) tetralin hydrobromide (8-OH-DPAT; 1 mg/kg, dissolved in 0.1 ml of saline). Body temperature was recorded every 15 min for 1 h after injection.
Western Blot
Mice were sacrificed 30 minutes after the last injection of either 10mg/kg citalopram or saline. Following cervical dislocation, brains were dissected on ice and stored at − 80 °C. Hippocampi were homogenized in 0.2 ml of ice-cold extraction buffer (50 mM Tris pH 7.5, 1% SDS, 1 mM PMSF, 1 mM EDTA, 1mM EGTA), sonicated briefly, and centrifuged at 13000 rpm at 4 °C for 10 min. The supernatant was collected and protein concentration was determined by Bradford assay. Samples were diluted in 50 mM Tris (pH 6.8), 1% SDS, 0.025% bromphenol blue, 10% glycerol, and 20 mM DTT and boiled for 5 min. Aliquots containing 12ug total protein were separated on 10% sodium dodecyl sulfate–polyacrylamide gels (SDS–PAGE) and transferred to nitrocellulose membranes (Trans-Blot Transfer Medium Bio-Rad Laboratories, Hercules, CA). Protein visualization and densitometry was performed using the Odyssey Infrared Imaging System and software (Li-Cor Biosciences) according to the manufacturer’s instructions. Monoclonal antibodies against P-CREB (1:1000) and CREB (1:2000) were obtained from Cell Signaling Technology (Beverly, MA) and secondary antibodies IR700 anti-rabbit and IR800 anti-mouse for visualization from Rockland Immunochemicals.
Drugs
Citalopram (Tocris, Ellisville MO), and chlordiazepoxide (CDP, Sigma, Saint-Louis MO) were prepared immediately before use and injected intraperitoneally using a volume of 10 ml/kg. The dose was calculated as mg/kg of the salt form and was dissolved in 0.9% saline. (+/−)-8-hydroxy-dipropylaminotetralin (8-OHDPAT, Sigma–Aldrich, St. Louis, MO) was dissolved in 0.9% saline and administrated sub-cutanously. For acute studies the last drug injection occurred 30-min to 1-hour prior to behavioral test. For repeated administration studies, mice were injected with drugs or 0.9% saline 23.5, 5 and 1 hour prior to behavioral testing for a total of three doses before exposure to behavioral paradigms. For Western analyses, animals were killed 30 min following the last saline or drug administration.
Statistics
All data were analyzed using the appropriate within-subject and mixed design ANOVAS or student’s t-test (in the case of comparisons between two groups of animals)) followed by, where appropriate, Bonferonni’s post-hoc tests. The level of significance was set at p≤ 0.05.
Results
Repeated, but not acute, administration of citalopram induced anxiolytic effects in mice
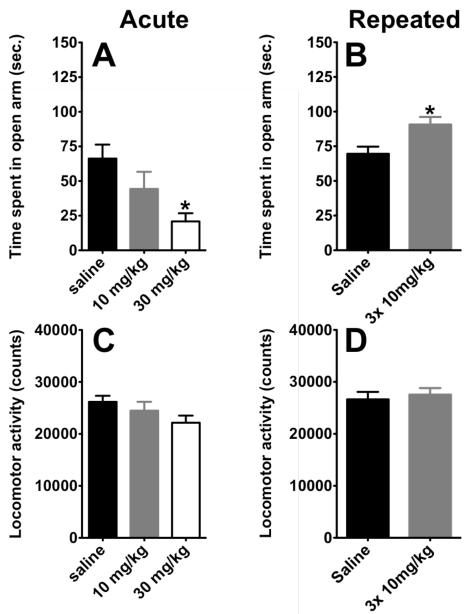
Mice receiving a single administration of citalopram ( 30 mg/kg ) spent significantly less time in the open-arm of the maze (p<0.05) compared to saline treated animals (Figure 1A; One way ANOVA, F(2,25)=4.414; p=0.029). Moreover, this anxiogenic effect was not due to any confounding effect of this treatment on locomotor activity (Fig 1C., F(2,25)=1.672; p=0.2099). In contrast, repeated administration of citalopram (3 injections of citalopram; 10mg/kg, i.p. over 24 hours) produces an increase in the time spent in open arm compared to saline-treated animals, indicating that sub-chronic administration of citalopram elicits an anxiolytic effect in this paradigm (t=2.798,df=17, p= 0.0124; Fig 1B.). This increase in time spent in the open arm was not due to a change in locomotion (t=0.4561, df=17, p= 0.6541; Fig 1D.). In both cohorts of mice, saline treated animals display comparable time spent in open arm confirming the consistency of this measure between groups.
Figure 1. A–D, Sub-chronic, but not acute, administration of citalopram elicits anxiolysis in elevated zero maze.
A) Acute administration of citalopram, at 30 mg/kg but not 10 mg/kg, induced anxiogenic effect in elevated plus maze (n=8-10, per group). Values are means +/−SEM. * groups that differed significantly from vehicle-treated animals (p<0.05). B) Sub-chronically, citalopram increased the time spent in open-arm (n=9-10, per group). Values are means +/−SEM. * groups that differed significantly from vehicle-treated animals (p<0.05). C-D) Absence of effect of acute and sub-chronic, respectively, injections of citalopram on locomotor activity in the elevated zero maze. Values are means +/−SEM.
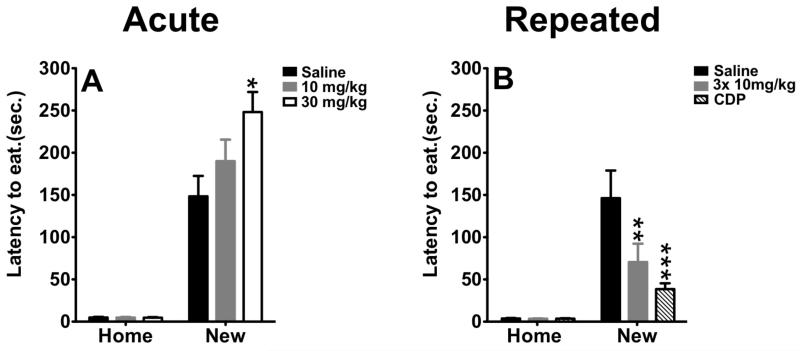
A second paradigm sensitive to anti-anxiety treatment is the novelty induced hypophagia (NIH) test. In this paradigm, latency to consume a highly palatable food in a novel environment is measured. Acute benzodiazepine treatment reduces latency to feed in a novel environment as does chronic, but not acute antidepressant treatment (Merali et al, 2003). Mice were injected with saline, 10mg/kg, or 30mg/kg citalopram one hour prior to exposure to the novel environment. One-way ANOVA repeated measures revealed a significant effect of treatment (F(2,49)=4.239; p=0.0201), a significant effect of condition (F(1,49)=184.5; p≤0.001) and a significant treatment × condition interaction (F(1,49)=4.259; p=0.0197). As expected, we observed that the majority of animals exhibit an increase in the latency to consume food in the novel environment compared to their home cage. Interestingly, this increase was exaggerated in mice treated with a single administration of citalopram at 30 mg/kg compared to saline-treated animals (p=0.05) recapitulating the anxiogenic properties of acute administration of a high dose of citalopram in the zero maze.
While acute citalopram was ineffective, repeated injection of citalopram in the NIH test significantly reduced the latency to consume food in the novel environment. One-way repeated measure revealed a significant effect of condition (F(1,37) = 31.76; p < 0.0001) on latency as a dependent variable, a significant effect of treatment (F(2,37) = 5.17; p =0.0104), and a significant treatment × condition interaction (F(2,37) = 5.11; p = .011). Of note, administration of citalopram in the home cage had no impact on the latency to feed in this environment. In the novel environment, subsequent post-hoc analysis indicates a decrease in the latency to consume the food in citalopram and chlordiazepoxide treated animals compared to saline controls (p=0.01 and p=0.001, respectively). Although the magnitude of the effects of repeated citalopram in this test are less robust compared to those induced by a benzodiazepine, the present data corroborate the potential anxiolytic properties of sub-chronic administration of citalopram. Previous studies using the same repeated dosing paradigm of the norepinephrine reuptake inhibitor desipramine had no effect in the NIH test (Gur et al, 2007).
Repeated administration of citalopram blocks 8-OHDPAT-induced hypothermia
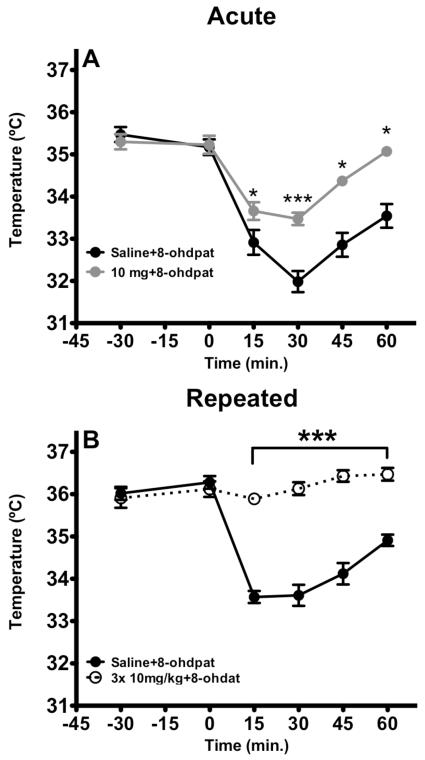
Following 5HT1A agonist stimulation, core body temperature is significantly reduced. This response is often used as a bioassay for 5HT1A function. Desensitization of 5-HT1A is thought to mediate at least some of the therapeutic efficacy of SSRIs. Therefore, to further explore the significance of acute vs repeated citalopram administration we assessed the effect on hypothermic response elicited by 5HT1A agonist 8-OHDPAT. 8-OHDPAT (1 mg/kg, s.c.) elicited a decrease in body temperature that was attenuated with an acute administration of citalopram (Fig 3A). One-way ANOVA repeated revealed significant a significant effect of treatment (F(1,72)= 50.74, p=0.001) of time (F(5,72)=52.06, p=0.001) and time × treatment interactions (F(5,72)= 6.842, p=0.001). Post-hoc analysis demonstrated that a single administration of citalopram (10 mg/kg, i.p.) attenuated, but did not block the hypothermic response to 8-OHDPAT. In contrast, repeated injection of citalopram was sufficient to totally block the hypothermic response to 8-OHDPAT (Fig. 3B). Two-way repeated measures of ANOVA revealed treatment (F1,108=188.9, p<0.001), Time (F5,108= 22,53 p<0.001), , time × treatment interaction (F5,108= 24.40, p<0.001).
Figure 3. A-B. Sub-chronic administration of citalopram blocked hypothermic response induced by 8-OHDPAT.
A) A single administration of citalopram 1h prior to 8-OHDPAT injection attenuated hypothermic response elicited by the prototypical agonist (n=5-7). Moreover, citalopram failed to affect basal temperature. Values are means +/− SEM.*, ***, group that differed from saline-8-OHDPAT treated animals. (p<0.05 and p<0.001, respectively). B) Three injections with citalopram totally blocked hypothermic response elicit by 8-OHDPAT (n=10). Values are means +/− SEM. *** groups that differed from saline treated animals. (p<0.001).
Sub-chronic administration of citalopram is sufficient to activate CREB
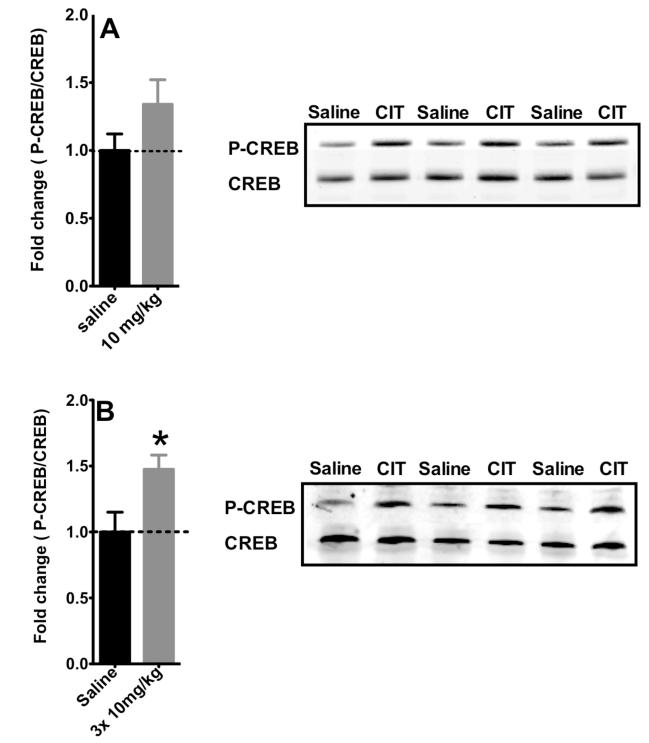
Previous studies have shown that chronic, but not acute antidepressant treatment induces phosphorylation of CREB in the hippocampus. However, we found that only 3 injections of citalopram over 24 hours increase CREB phosphorylation in the hippocampus (Fig. 4B, t=2.478, df=20, p=0.022) compared to saline-treated controls. A single administration of citalopram does not significantly increase CREB phosphorylation, although there is a trend toweard higher levels (t=1.557, df=16, p=0.139).
Figure 4. Effects of acute and sub-chronic administration of citalopram on CREB activation in the hippocampus.
A) A single administration of Citalopram (10mg/kg, 30 min.) failed to increase CREB activation in the hippocampus (n=9-10). Values are means +/−SEM. B) Three administrations of citalopram over 24 hours of citalopram increased P-CREB/CREB ratio in the hippocampus (n=7-9). Values are means +/−SEM. * groups that differed significantly from respective saline treated animals (p<0.05).
The role of CREB in Citalopram mediated behaviors
Since repeated administration of citalopram causes an increase in CREB phosphorylation as well as a reduction in anxiety in both the NIH and EZM paradigms along with an augmented effect of 5HT1A desensitization, we utilized CREB deficient mice to determine whether CREB was required for these behavioral and pharmacological effects.
Anxiolytic and antidepressant effects of citalopram are CREB dependent
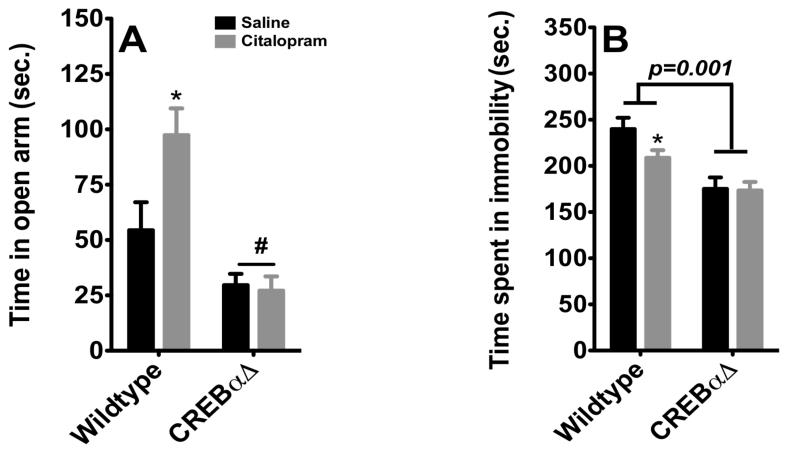
As demonstrated previously (Fig 1B) citalopram significantly increased the amount of time spent in the open arm of the EZM (Fig. 5). CREBαΔ mutant mice exhibit a decrease in the time spent in the open arm (p≤0.05), corroborating the exacerbated anxious phenotype previously observed in other behavioral paradigms (Graves et al, 2002; Gur et al, 2007). The two-way ANOVA revealed a main effect of genotype (F1,24= 24.85, p < 0.0001), and treatment (F1,24= 4.37, p =.0437) on time spent in the open arm. There was a significant genotype × treatment interaction (F1,24= 5.56, p = 0.0268) (n=7-8 per group). However, citalopram was ineffective in altering time spent in the open arm in CREBαΔ mutant, suggesting that CREB may be a required for anxiolytic properties of citalopram. In the tail suspension test, a behavioral paradigm associated with antidepressant–like efficacy, the response to citalopram is also completely abolished in CREBαΔ mutant. Two-way ANOVA revealed a effect of genotype on the time spent in immobility (F1,29=39.98, p<0.0001) but no effect of drugs (F1,29=4.31, p<0.13) and no interaction (F1,29=3.43, p<0.1749). Indeed, we recapitulate, here, the antidepressant-like phenotype of CREBαΔ mutant mice reflected by a significant decrease in the time spent immobile in these animals (Fig 4 and (Conti et al, 2002)). Further, post-hoc analysis revealed that citalopram decreased the time spent in immobility in wild-type animals but not in CREBαΔ mutant (p<0.05), suggesting CREB may also contribute to the antidepressant-like properties of citalopram.
Figure 5. Anxiolytic and antidepressant response of citalopram is abolished in CREBαΔ mice.
A) Citalopram induced an anxiolytic-like effect in wild-type animals in the elevated zero maze, while there was no effect in CREBαΔ mice (n=7-8). Values are means +/−SEM. * groups that differed significantly from respective saline treated animals (p<0.05). # groups that differed from wild-type animals(p<0.05).B) Sub-Chronic treatment with citalopram induced antidepressant-like effect in tail suspension in wild-type mice (n=6-10). Although CREB deletion elicited a decrease in time spent in immobility, this effect was not potentiated by citalopram. Values are means +/−SEM. *groups that differed significantly from respective saline treated animals (p<0.05).
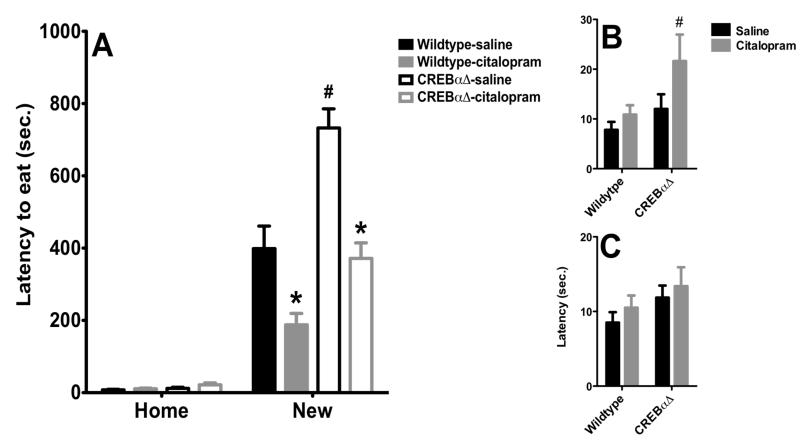
Citalopram effects in NIH are not dependent on CREB
While the NIH paradigm is acutely responsive to anti-anxiety compounds like CDP, acute antidepressant treatments are typically not effective. However, as shown in figure 2, three administrations of citalopram over 24 hours can significantly reduce latency, but this effect is not altered in CREBαΔ mutant mice (figure 6). A two-way repeated measures ANOVA revealed a significant effect of citalopram (F1,30 =29.31, p<0.001), a significant effect of genotype (F1,30= 27.37, p<0.001) and no citalopram × genotype interaction (F1,30= 1.932, p<0.17). Moreover, we also found a significant effect of time (F2,60=266.001, p<0.001), indicating animals exhibit an increase latency to feed in the new environment. In the first home test two-way ANOVA revealed a significant effect of genotype (F1,30=6.265, p=0.01080), but this appears to be due to an increased latency to feed only in the CREBαΔ mutant mice treated with citalopram. Post-hoc analysis revealed a significant difference in the latency to feed between CREBαΔ mutant treated with citalopram and wilt-type animals treated with the same regimen of drugs. On test day in the novel environment, two-way ANOVA revealed a significant effect of citalopram (F1,30=26.129, p<0.001), a effect of genotype (F1,30=31.858, p<0.001) but no genotype × drugs interaction (F1,30=1.313, p=0.14). Post-hoc analysis revealed sub-chronic administration of citalopram induced a large decrease in the latency to feed in both wildtype and CREBαΔ mutant mice. Moreover, we also observed that targeted deletion of CREB elicited a dramatic increase in the latency to feed in the new environment corroborating the anxious phenotype of CREBαΔ mutant observed previously. Thus, as observed above, three injections of citalopram is sufficient to elicit anxiolytic-like effects in this test and this effect appears to be independent of CREB. Finally, inspection of the second home-home cage test revealed no effect of drugs or genotype.
Figure 2. A–B, Sub-chronic, but not acute, administration of citalopram induced anxiolytic-like effects in the novelty-induced hypophagia paradigm.
A) Acute administration of citalopram, at 30 mg/kg but not 10 mg/kg, induced anxiogenic effect in novelty-induced hypopagia (n=9-10, per group). Values are means +/−SEM. * groups that differed significantly from saline treated animals (p<0.05). B) Sub-chronically, citalopram decreased the latency to feed. This effect was comparable to the effects of chlordiazepoxide (n=6-9, per group). Values are means +/−SEM. ** and *** group that differed significantly from vehicle-treated animals (p<0.01, p<0.001, respectively).
Figure 6. Anxiolytic and antidepressant response of citalopram is abolished in CREBαΔ mice.
A) Sub-chronic administration of citalopram treatment decreased the latency to consume peanut butter chips in the novel environment in both WT and CREBαΔ mutant mice (n=7-10). Moreover, saline-treated CREBαΔ mutant mice exhibited an increase in latency to feed in the novel environment. Values are means +/−SEM. * groups that differed significantly from respective saline treated animals (p<0.05). # groups that differed significantly from respective Wild-type animals (p<0.05). B) In the first home cage test, a single administration of citalopram increased the latency to feed in CREBαΔ mutant mice. Values are means +/−SEM. * groups that differed significantly from respective saline treated animals (p<0.05). C) No specific effect of citalopram or CREB deficiency was observed in the second home cage test. Values are means +/−SEM.
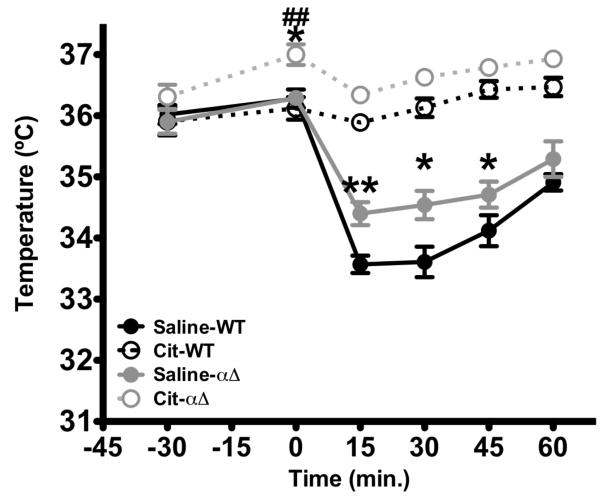
CREB deficiency decreases 5HT1A sensitivity at baseline and but is not required for citalopram effects in 8-OHDPAT hypothermia response
5HT1A receptors appear to be desensitized at baseline in CREBαΔ mutant mice. Two-way repeated measures of ANOVA revealed a significant effect of genotype (F1,180= 12.579, p<0.001), treatment (F1,180=117.597, p<0.001), Time (F5,180= 12.579, p<0.001), time × genotype interaction (F5,180=2.464, p=0.0346), time × treatment interaction (F5,180= 59.355, p<0.001) and triple interaction, time × genotype × treatment (F5,180= 4.245, p=0.0011). Subsequent post-hoc analysis revealed that CREB deficient mice show reduced 5-HT1A agonist-induced hypothermia following a single administration of 8-OHDPAT compared to wild-type animals. (Fig 7.). However, this effect is significantly greater when mice are pre-treated with a sub-chronic administration of citalopram, similar to the hypothermic response observed in wildtype mice. These data suggest that CREB is not required for citalopram-mediated augmentation of 5HT1A-induced hypothermia.
Figure 7. CREBαΔ mice exhibit a blunted hypothermic response to 8-OHDPAT.
CREBαΔ mice exhibited an attenuated response to 8-OHDPAT compared to wild-type animals. Moreover, three administration of citalopram blocked 8-OHDPAT-induced hypothermia in both wild-type animals and CREBαΔ mice (n=10). Values are means +/−SEM. *,**, groups that differed from saline-wild-type animals. (p< 0.05, p<0.01) respectively). ## groups that differed from citalopram-wild-type animals. (p< 0.05, respectively)
Discussion
In the present paper, we demonstrate that acute citalopram induces anxiety while only three injections of citalopram over 24 hours elicit anxiolytic effects. Furthermore, short-term repeated administration is sufficient to decrease the latency to feed in the NIH paradigm, and elicit desensitization of 5-HT1A receptors; effects typically seen with chronic, not acute antidepressant exposure.
The major clinical limitations of SSRI’s are that a period of 2-6 weeks of treatments is usually necessary before therapeutic effects develop and that the emergence of these effects is usually preceded by an episode of anxiety. In this study, we observed this characteristic biphasic phenomenon using citalopram in two well-characterized anxiety paradigms, namely the EZM and the NIH paradigm. Indeed, the fact that a single administration of citalopram at 30 mg/kg precipitate anxiety in these models (Fig 1. and Fig 2.) extend a broader literature showing that a single administration of SSRI consistently produce a anxiogenic effect in animal models of anxiety (Bagdy et al, 2001; Dekeyne et al, 2000; Griebel et al, 1994; Silva et al, 1999; To et al, 1999 ). For example, a single administration of fluoxetine and paroxetine decreased the time spent in open-arm of elevated plus maze (Drapier et al, 2007; Holmes and Rodgers, 2003). Regarding the NIH paradigm, we cannot exclude that anorectic properties of SSRI might participate in decreased latency to feed observed in the present study. Indeed, acute administration of SSRI, such as citalopram, has been shown to induce a reduction in food intake in rat (Grignaschi et al, 1998; Lucki et al, 1988). While we cannot discount the possibility that these anorectic properties might factor into the present observations, anxiogenic properties of acute administration of citalopram are also observed at the same dose (30 mg/kg) in another anxiety measure that is not dependent on food consumption (EPM). Together, the present results corroborate the fact that acute SSRI treatment is followed by a period of exacerbated anxiety.
Three injections of citalopram over 24 hours effectively reduced anxiety in two behavioral paradigms. Although the anxiolytic properties of SSRI’s have been characterized only after long-term administration, e.g., 21 days (Dulawa et al, 2004; Surget et al, 2008), this study demonstrates that a shorter exposure to citalopram is sufficient to produce anxiolysis in these two paradigms. Indeed, the few studies investigating the effects of sub-chronic treatment of SSRI’s on anxiety behavior failed to observe any effect of fluoxetine in both open-field and NIH paradigm, after 4-5 or 7 days of oral administration (Dulawa and Hen, 2005; Dulawa et al, 2004). However, the differences in the route of administration (intraperitoneal vs oral) and in the SSRI used (citalopram vs fluoxetine) limit the comparison between these studies and the present findings. Moreover, inter-strain variability might also accentuate these discrepancies. A recent study observed a remarkable difference in the sensitivity to chronic administration of fluoxetine between MRL/MpJ and C57Bl/6J mice in the NIH test (Balu et al, 2009). In this study, 21 days of treatment with fluoxetine failed to affect behavioral response in C57Bl/6J mice where it significantly altered behavior in MRL/MpJ mice.
The effect of repeated citalopram injections over 24 hours is not likely due to accumulation of drugs in plasma given the short plasma half-life of citalopram (1.5 h) (Fredricson Overo, 1982). Furthermore, the fact that a single administration of citalopram at 30 mg/kg induced an opposite effect on anxiety as that observed after three injections of 10 mg/kg suggests this repeated dosing paradigm is not due to a cumulative dose effect but may be sufficient to induce long lasting neuroadaptations usually produced by chronic administration of antidepressants.
Antidepressants, such as SSRIs, have been shown to exert their long lasting beneficial properties through the desensitization of somatodendritic 5-HT1A receptors (Blier et al, 1994). In effort to investigate the effect of acute and sub-chronic citalopram on this endpoint, we measured the hypothermic response induced by the prototypical 5-HT1A agonist, 8-OHDPAT. Indeed, 5-HT1A agonists have been report to elicit a profound hypothermia in several species, such as mouse, rat or human (Evrard et al, 2002; Hjorth, 1985; Seletti et al, 1995). Interestingly, chronic, but not acute, administration of antidepressants completely blunted this response, reflecting a potential desensitization of 5-HT1A receptors (Troelsen et al, 2005). This hypothermic response has been shown to reflect specifically, the sensitivity of somatodendritic 5-HT1A receptors in mice (Goodwin et al, 1985). In the present study, we observed that a single administration of citalopram attenuates the hypothermic response to 8-OHDPAT, while 3 administrations over 24 hours blocked this response (Fig 3.) an effect comparable to that observed after chronic administration (21 days) of SSRI (Troelsen et al, 2005). These data suggest involvement of a desensititization of 5-HT1A autoreceptors in anxiolytic effects of sub-chronic treatment with citalopram. Although it has been shown that some putative antidepressants, such as the 5-HT4 agonist (RS 67333), desensitize 5-HT1A autoreceptors in 3 days, the standard onset of SSRIs in this parameter is 2 or 3 weeks (Lucas et al, 2007). Moreover, it is plausible that the blockade of 8-OHDPAT-induced hypothermia might reflect other adaptations, such as desensitization of other 5-HT receptors. Indeed, it has been proposed that 8-OHDPAT-induced hypothermia might be mediated also through activation of 5-HT7 receptors (Hedlund et al, 2004). In addition, chronic antidepressant treatment has been show to induce a down-regulation of these receptors in hypothalamus (Sleight et al, 1995). Further studies are necessary to determine if modulation of 5-HT7 receptors contribute to the effects of citalopram after short-term exposure.
CREB phosphorylation is a common downstream target of several 5-HT receptors. Gi-coupled receptors, such as 5-HT1A receptors, might regulate CREB phosphorylation via activation of MAPK cascades (Cowen et al, 1996). In contrast, Gs-coupled receptors, such as 5-HT7 or 5-HT4, activate CREB through the recruitment of adenylyl cyclase or PKA (Johnson-Farley et al, 2005). We demonstrate here that CREB phosphorylation is significantly increased in the hippocampus following sub-chronic citalopram treatment (Fig 4B) and this correlated with a decreased in anxiety behavior and a blockade of 8-OHDPAT induced hypothermia. Thus, we can speculate that the rapid desensitization induced by 3 administrations of citalopram might increase 5-HT tone in the hippocampus and subsequently activate CREB through 5-HT1A, 5-HT4, or 5-HT7 This hypothesis is supported by the fact that a single administration, which results in less robust desensitization of 5-HT1A, tends to increase P-CREB/CREB ratio, but with a lower magnitude than short term repeated treatment (Fig. 4A). However, previous reports have rarely observed an increase in CREB activation in the hippocampus before 1.5-2 weeks of fluoxetine treatment (Thome et al, 2000; Tiraboschi et al, 2004). Further, a recent study failed to observe any elevation of P-CREB/CREB ratio in hippocampal tissue of rats treated during 3 days of citalopram (Lucas et al, 2007). Nevertheless, this study failed to report any activity of 3 days of citalopram in the forced swim test, an animal model assessing antidepressant activity, limiting their conclusion on the role of CREB in behavioral effects of citalopram. In contrast, we observed a n increase in CREB phosphorylation with behaviorally active treatments (3 administrations over 24 hours), suggesting that CREB may play a role in the anxiolytic activity of citalopram.
While not significant, we also see a trend towards increase CREB phosphorylation following a single administration of citalopram. It may appear surprising that both single and sub-chronic administration of citalopram result in increase in CREB activation and a desensitization of 5-HT1A receptors, when they induced opposite effects behaviorally. However, it is therefore possible that two distinct pathways might mediate anxiolytic/antidepressant and anxiogenic properties of citalopram. Indeed, elevation of CREB function, using viral mediated expression of constitutively active form of CREB, has been reported to elicit antidepressant-like effects in several paradigms (Chen et al, 2001) but didn’t elevated anxiety (Restivo et al, 2008), suggesting that CREB activation in the hippocampus might participate to antidepressant-like properties of citalopram treatment but not in anxiogenic properties. Thus, different neural mechanisms may underlie the anxiogenic properties of a single administration of citalopram. A growing body of evidence points to 5-HT2C receptors, but not 5-HT1A as primary mediator of anxiogenic response of acute administration of SSRIs (Bagdy et al, 2001; Dekeyne et al, 2000). Indeed, 5-HT2C receptors blockade have been shown to attenuate anxiogenic properties of fluoxetine, where the 5-HT1A antagonists, WAY 100635, was ineffective (Salchner and Singewald, 2006). According to Millan, this initial anxiogenic phase would be gradually attenuated through a progressive desensitization of these receptors (Millan, 2005). It may be reasonable to speculate that anxiogenic effect of citalopram mediated by 5-HT2C receptor activation masks early anxiolytic effects of citalopram elicited by CREB activation in the hippocampus. Nevertheless, it remains to be established whether these pathways contribute or not to the anxiolytic action elicited by short-term repeated administration of citalopram.
Lastly, to determine if CREB is required for the behavioral effects of sub-chronic citalopram, we treated WT and CREBαΔ mutant mice with 3 doses of CIT (10 mg/kg) or saline, and tested them in the EZM, NIH and tail suspension test.. CREBαΔ mutant mice did not demonstrated a behavioral response to citalopram in either the EZM or tail-suspension test (Fig 5.), demonstrating that CREB is required for both anxiolytic and antidepressant response of citalopram. Intriguingly, we did observed an effect of citalopram in the NIH paradigm in CREBαΔ mutant mice (Fig. 6). Although the EZM and the NIH are both an animal models of anxiety, it is becoming clear that both tests are probably different from each other in terms of the biological substrates that underlie their observed behaviors. Furthermore, the NIH test is reported to be sensitive to chronic, but not acute antidepressant treatment. Of interest, we demonstrated previously that three administrations of desipramine induced anxiolytic response in CREBαΔ mutant mice in NIH but not EZM (Gur et al, 2007). These results differ from the findings that CREBαΔ mutant mice are responsive to both DMI and fluoxetine in the FST and TST (Conti et al, 2002). The distinction between this study and our present results might be due to the fact that fluoxetine may act on both noradrenergic and serotoninergic systems, where citalopram act only on serotoninergic system. Indeed, a recent study demonstrates that antidepressant-like effect of citalopram persists in norepinephrine-deficient mice in the tail suspension test (Cryan et al, 2004). Conversely, antidepressant of fluoxetine was totally abolished in these mice. These data confirm the fact that CREB might play a key role in beneficial properties of the serotoninergic component of antidepressant.
In addition, we also observed a blunted hypothermic response to 8-OHDPAT in CREBαΔ mutant mice , suggesting a native 5-HT1A desensitization (Fig. 7). This desensitization might play a role in the antidepressant phenotype of CREBαΔ observed in the tail suspension test (Fig. 5). Alternatively, we can also postulate that this desensitization might contribute to the lack of efficacy of citalopram in the tail suspension test observed given the fact that genetic ablation of 5-HT1A receptors attenuates antidepressant effect in Forced swim test (Mayorga et al, 2001). The nature of the interactions between CREB and 5-HT remains to be established. Indeed, although the role of CREB in the excitability of dopaminergic system has been well characterized (Dong et al, 2006), its role on 5-HT neurons is still elusive.
In conclusion, the present results show that 3 administrations of citalopram are sufficient to induce both behavioral and neurochemical adaptations observed classically after three weeks of treatment. Moreover, we also demonstrate a putative involvement of CREB in anxiolytic and antidepressant properties of citalopram, through the serotoninergic system. Thus, uncovering factors both upstream and downstream of CREB that differentially modulate these phenomena will increase the understanding of the pathogenesis underlying affective disorders, and potentially generate new therapeutic targets.
Acknowledgements
This work was supported by National Institutes of Mental Health Grants U01MH0723832 and T32-MH14654. The authors would like to thank Dr Loic Lhuillier for critically reading the manuscript.
Footnotes
Statement of Interest
None.
References
- Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4(4):399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced Sensitivity of the MRL/MpJ Mouse to the Neuroplastic and Behavioral Effects of Chronic Antidepressant Treatments. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Le Guisquet AM, Barreau S, Calatayud F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12(3):151–162. doi: 10.1097/00008877-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15(7):220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Borsini F, Podhorna J, Marazziti D. Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl) 2002;163(2):121–141. doi: 10.1007/s00213-002-1155-6. [DOI] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001;49(9):753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22(8):3262–3268. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen DS, Sowers RS, Manning DR. Activation of a mitogen-activated protein kinase (ERK2) by the 5-hydroxytryptamine1A receptor is sensitive not only to inhibitors of phosphatidylinositol 3-kinase, but to an inhibitor of phosphatidylcholine hydrolysis. J Biol Chem. 1996;271(37):22297–22300. doi: 10.1074/jbc.271.37.22297. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4(9):775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A. 2004;101(21):8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JR. Biological therapies for posttraumatic stress disorder: an overview. J Clin Psychiatry. 1997;58(Suppl 9):29–32. [PubMed] [Google Scholar]
- Dekeyne A, Denorme B, Monneyron S, Millan MJ. Citalopram reduces social interaction in rats by activation of serotonin (5-HT)(2C) receptors. Neuropharmacology. 2000;39(6):1114–1117. doi: 10.1016/s0028-3908(99)00268-3. [DOI] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, et al. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9(4):475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Drapier D, Bentue-Ferrer D, Laviolle B, Millet B, Allain H, Bourin M, et al. Effects of acute fluoxetine, paroxetine and desipramine on rats tested on the elevated plus-maze. Behav Brain Res. 2007;176(2):202–209. doi: 10.1016/j.bbr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29(4-5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Evrard A, Malagie I, Laporte AM, Boni C, Hanoun N, Trillat AC, et al. Altered regulation of the 5-HT system in the brain of MAO-A knock-out mice. Eur J Neurosci. 2002;15(5):841–851. doi: 10.1046/j.1460-9568.2002.01917.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks JM, Pine DS, Tancer NK, Dummit ES, 3rd, Kentgen LM, Martin J, et al. Open fluoxetine treatment of mixed anxiety disorders in children and adolescents. J Child Adolesc Psychopharmacol. 1997;7(1):17–29. doi: 10.1089/cap.1997.7.17. [DOI] [PubMed] [Google Scholar]
- Fredricson Overo K. Kinetics of citalopram in test animals; drug exposure in safety studies. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6(3):297–309. doi: 10.1016/s0278-5846(82)80180-2. [DOI] [PubMed] [Google Scholar]
- Fuller RW. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 1994;55(3):163–167. doi: 10.1016/0024-3205(94)00876-0. [DOI] [PubMed] [Google Scholar]
- Goodman WK. Obsessive-compulsive disorder: diagnosis and treatment. J Clin Psychiatry. 1999;60(Suppl 18):27–32. [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR. Presynaptic serotonin receptor-mediated response in mice attenuated by antidepressant drugs and electroconvulsive shock. Nature. 1985;317(6037):531–533. doi: 10.1038/317531a0. [DOI] [PubMed] [Google Scholar]
- Gorman JM. The use of newer antidepressants for panic disorder. J Clin Psychiatry. 1997;58(Suppl 14):54–58. discussion 59. [PubMed] [Google Scholar]
- Gorman JM, Liebowitz MR, Fyer AJ, Goetz D, Campeas RB, Fyer MR, et al. An open trial of fluoxetine in the treatment of panic attacks. J Clin Psychopharmacol. 1987;7(5):329–332. [PubMed] [Google Scholar]
- Graves L, Dalvi A, Lucki I, Blendy JA, Abel T. Behavioral analysis of CREB alphadelta mutation on a B6/129 F1 hybrid background. Hippocampus. 2002;12(1):18–26. doi: 10.1002/hipo.10003. [DOI] [PubMed] [Google Scholar]
- Griebel G, Blanchard DC, Agnes RS, Blanchard RJ. Differential modulation of antipredator defensive behavior in Swiss-Webster mice following acute or chronic administration of imipramine and fluoxetine. Psychopharmacology (Berl) 1995;120(1):57–66. doi: 10.1007/BF02246145. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology (Berl) 1994;113(3-4):463–470. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Grignaschi G, Invernizzi RW, Fanelli E, Fracasso C, Caccia S, Samanin R. Citalopram-induced hypophagia is enhanced by blockade of 5-HT(1A) receptors: role of 5-HT(2C) receptors. Br J Pharmacol. 1998;124(8):1781–1787. doi: 10.1038/sj.bjp.0702028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Chavis C, Covington MF, Pine DS. Two-Week Treatment With the Selective Serotonin Reuptake Inhibitor Citalopram Reduces Contextual Anxiety but Not Cued Fear in Healthy Volunteers: A Fear-Potentiated Startle Study. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32(1):225–231. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, et al. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. J Neurosci. 2007;27(29):7860–7868. doi: 10.1523/JNEUROSCI.2051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487(1-3):125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm. 1985;61(1-2):131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Bengtsson HJ, Kullberg A, Carlzon D, Peilot H, Auerbach SB. Serotonin autoreceptor function and antidepressant drug action. J Psychopharmacol. 2000;14(2):177–185. doi: 10.1177/026988110001400208. [DOI] [PubMed] [Google Scholar]
- Holmes A, Rodgers RJ. Prior exposure to the elevated plus-maze sensitizes mice to the acute behavioral effects of fluoxetine and phenelzine. Eur J Pharmacol. 2003;459(2-3):221–230. doi: 10.1016/s0014-2999(02)02874-1. [DOI] [PubMed] [Google Scholar]
- Ichimaru Y, Egawa T, Sawa A. 5-HT1A-receptor subtype mediates the effect of fluvoxamine, a selective serotonin reuptake inhibitor, on marble-burying behavior in mice. Jpn J Pharmacol. 1995;68(1) doi: 10.1254/jjp.68.65. [DOI] [PubMed] [Google Scholar]
- Johnson-Farley NN, Kertesy SB, Dubyak GR, Cowen DS. Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J Neurochem. 2005;92(1):72–82. doi: 10.1111/j.1471-4159.2004.02832.x. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol. 1995;352(2):141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Hamon M, Lanfumey L. Fluoxetine-induced desensitization of somatodendritic 5-HT1A autoreceptors is independent of glucocorticoid(s) Synapse. 1997;27(4):303–312. doi: 10.1002/(SICI)1098-2396(199712)27:4<303::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Lucas G, Rymar VV, Du J, Mnie-Filali O, Bisgaard C, Manta S, et al. Serotonin(4) (5-HT(4)) receptor agonists are putative antidepressants with a rapid onset of action. Neuron. 2007;55(5):712–725. doi: 10.1016/j.neuron.2007.07.041. [DOI] [PubMed] [Google Scholar]
- Lucki I, Kreider MS, Simansky KJ. Reduction of feeding behavior by the serotonin uptake inhibitor sertraline. Psychopharmacology (Berl) 1988;96(3):289–295. doi: 10.1007/BF00216052. [DOI] [PubMed] [Google Scholar]
- Mayorga AJ, Dalvi A, Page ME, Zimov-Levinson S, Hen R, Lucki I. Antidepressant-like behavioral effects in 5-hydroxytryptamine(1A) and 5-hydroxytryptamine(1B) receptor mutant mice. J Pharmacol Exp Ther. 2001;298(3):1101–1107. [PubMed] [Google Scholar]
- Merali Z, Levac C, Anisman H. Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol Psychiatry. 2003;54(5):552–565. doi: 10.1016/s0006-3223(02)01827-9. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60(5):441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16(7):2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32(10):2152–2162. doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- Restivo L, Tafi E, Ammassari-Teule M, Marie H. Hippocampus. 2008. Viral-mediated expression of a constitutively active form of CREB in hippocampal neurons increases memory. [DOI] [PubMed] [Google Scholar]
- Rickels K. Use of antianxiety agents in anxious outpatients. Psychopharmacology (Berl) 1978;58(1):1–17. doi: 10.1007/BF00426784. [DOI] [PubMed] [Google Scholar]
- Rocca P, Fonzo V, Scotta M, Zanalda E, Ravizza L. Paroxetine efficacy in the treatment of generalized anxiety disorder. Acta Psychiatr Scand. 1997;95(5):444–450. doi: 10.1111/j.1600-0447.1997.tb09660.x. [DOI] [PubMed] [Google Scholar]
- Salchner P, Singewald N. 5-HT receptor subtypes involved in the anxiogenic-like action and associated Fos response of acute fluoxetine treatment in rats. Psychopharmacology (Berl) 2006;185(3):282–288. doi: 10.1007/s00213-005-0247-5. [DOI] [PubMed] [Google Scholar]
- Seletti B, Benkelfat C, Blier P, Annable L, Gilbert F, de Montigny C. Serotonin1A receptor activation by flesinoxan in humans. Body temperature and neuroendocrine responses. Neuropsychopharmacology. 1995;13(2):93–104. doi: 10.1016/0893-133X(95)00025-9. [DOI] [PubMed] [Google Scholar]
- Silva MT, Alves CR, Santarem EM. Anxiogenic-like effect of acute and chronic fluoxetine on rats tested on the elevated plus-maze. Braz J Med Biol Res. 1999;32(3):333–339. doi: 10.1590/s0100-879x1999000300014. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Carolo C, Petit N, Zwingelstein C, Bourson A. Identification of 5-hydroxytryptamine7 receptor binding sites in rat hypothalamus: sensitivity to chronic antidepressant treatment. Mol Pharmacol. 1995;47(1):99–103. [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64(4):293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, et al. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20(11):4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, et al. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29(10):1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- To CT, Anheuer ZE, Bagdy G. Effects of acute and chronic fluoxetine treatment of CRH-induced anxiety. Neuroreport. 1999;10(3):553–555. doi: 10.1097/00001756-199902250-00020. [DOI] [PubMed] [Google Scholar]
- Troelsen KB, Nielsen EO, Mirza NR. Chronic treatment with duloxetine is necessary for an anxiolytic-like response in the mouse zero maze: the role of the serotonin transporter. Psychopharmacology (Berl) 2005;181(4):741–750. doi: 10.1007/s00213-005-0032-5. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]