Abstract
Escherichia coli mutants lacking multiple penicillin-binding proteins (PBPs) produce aberrantly shaped cells. However, most of these experiments have been performed in E. coli K12 strains, which do not attach a complete O-antigen to their outer membrane lipopolysaccharide. We constructed mutants in different genetic backgrounds and found that the frequency of morphological deformities was higher in strains lacking the O-antigen. Also, complementing O-negative mutants with a heterologous O-antigen from Klebsiella returned a substantial fraction of misshapen cells to a normal morphology. Thus, the O-antigen contributes to cell shape in E. coli, perhaps by reducing the number of ectopic poles, which may be the proximal cause of shape abnormalities.
Keywords: Escherichia coli, penicillin-binding proteins, O-antigen, cell shape
Introduction
Although it is clear that bacterial morphology is determined by the shape of the rigid peptidoglycan cell wall, the molecular details of how the wall adopts a specific shape are just beginning to become clear (Carballido-Lopez & Errington, 2003; Young, 2003; Cabeen & Jacobs-Wagner, 2005). The most favored current hypothesis is that peptidoglycan synthesis is directed by enzymes associated with one or more shape-directing, polymeric, cytoplasmic scaffolds composed of homologues of actin (e.g. MreB) or tubulin (e.g. FtsZ) (Carballido-Lopez & Errington, 2003; Figge et al., 2004). According to this view, the dimensions and overall shape of the peptidoglycan sacculus are determined by controlling the spatial insertion of monomeric precursors into the existing cell wall.
In Gram-negative cells, other factors may also contribute to bacterial shape, including a possible role for the outer membrane (OM) and its components, which might act collectively as a type of external scaffold (Young, 2003). For example, such a mechanism could, in theory, influence cell length (Cooper, 1991). More tangible experimental evidence for the idea includes the fact that some OM mutants are misshapen. An Escherichia coli strain lacking the OmpA and Lpp proteins is spherical instead of rod shaped when grown in the presence of magnesium ions (Sonntag et al., 1978; Hiemstra et al., 1987), as are certain E. coli mutants lacking the Lpp lipoprotein by itself (Hiemstra et al., 1987). Ellipsoidal-shaped cells are produced by overexpressing the NlpI OM lipoprotein (Ohara et al., 1999), and removal of the OmpA protein creates irregular and flattened cells (Belaaouaj et al., 2000). It appears, therefore, that at least some of the protein components of the OM affect the general shape of E. coli.
Previously, we reported that deleting certain low-molecular-weight penicillin-binding proteins (LMW PBPs) from E. coli, yields cells with aberrant morphologies (Nelson & Young, 2000, 2001). However, these experiments were performed in an E. coli K12 background. This is a potentially important consideration because K12 strains express a truncated form of lipopolysaccharide (LPS), a major constituent of the outer leaflet of the OM in Gram-negative bacteria (Liu & Reeves, 1994). Such strains are described as ‘rough’ – that is, they lack the O-antigen characteristic of wild-type lipopolysaccharide. The reason is that most K12 strains carry an IS5 insertion in the rfb gene cluster that encodes the proteins that synthesize the lipopolysaccharide component (Liu & Reeves, 1994).
To test whether the O-antigen might affect morphology in E. coli, we examined the shapes of PBP mutants constructed in a strain of E. coli that produces an intact O-antigen. The frequency of cell shape abnormalities in these strains was compared with isogenic mutants in which the rfb gene cluster was inactivated and with rfb mutants complemented with O-antigen from another genus. The results indicate that the presence of O-antigen reduces PBP-related deformities, indicating that the lipid composition of the OM also plays a role in the generation or maintenance of bacterial cell shape.
Materials and methods
Bacteria, plasmids and general techniques
The bacterial strains used in this study are listed in Table 1. Plasmid pBMM1 (Meberg et al., 2004) carries a res-npt-res kanamycin-resistant cassette from pCK155 (Kristensen et al., 1995). Plasmid pWQ5 [pBluescript KS(+) vector, AmpR] carries the rfb genes that encode the pathway for synthesizing the Klebsiella O1-antigen (Bronner et al., 1994), and was provided by C. Whitfield. Plasmid pSH55 [pBluescript KS(+) vector, AmpR] carries the E. coli slyD and fkpA genes cloned into the EcoRI site (S. Horne and K. Young, unpublished).
Table 1.
Escherichia coli strains
| Strain | Genotype | O-antigen | Source |
|---|---|---|---|
| 2443 |
thr-1 leuB6 Δ(gpt-proA)66 argE3 thi-1 rfbO8 lacY1 ara-14 galK2 xyl-5 mtl-1 mgl51 rpsL31 kdgK51 supE44 |
O8 | A.T. Maurelli (Rick et al., 1994; Sandlin et al., 1996) |
| 2443T | 2443 zba-3000::Tn10 (TetR) (by P1 transduction from KL743) | O8 | Ghosh & Young (2005) |
| CS109 | W1485 rpoS rph | None | C. Schnaitman |
| CS315-1 | CS109 ΔdacA ΔdacB ΔpbpG | None | Meberg et al. (2001) |
| CS446-1 | CS109 ΔdacA ΔdacB ΔdacC ΔpbpG | None | Meberg et al. (2001) |
| KL743 | MG1655 zba-3000::Tn10 LAM− rph− | None | M. Goldberg (CGSC 6213) |
| KM32 |
leuB6 proA2 thr-1 argE3 lacY1 galK2 ara-14 xyl-5 thi-1 rpsL31 mtl-1 tsx-33 supE44 Δ(recC ptr recB recD):: Plac-bet exo(CamR) |
None | K.C. Murphy (Murphy, 1998) |
| KM32I-9 | KM32 hisI::npt (KanR) (by λ Red recombination) | None | Ghosh & Young (2005) |
| AGTO2-1K | 2443T (TetR) hisI::npt (KanR) rfbK12 | None | Ghosh & Young (2005) |
| AG375-3 | 2443 zba-3000::Tn10 (TetR) ΔdacA ΔdacB ΔpbpG | O8 | This work |
| AG375-3* | AG375(TetR) hisI::npt (KanR) rfbK12 | None | This work |
| AG430-2K | 2443 hisI::npt (KanR) rfbK12 | None | Ghosh & Young (2005) |
| AG456-1 | 2443 zba-3000::Tn10 (TetR) ΔdacA ΔdacB ΔdacC ΔpbpG | O8 | Nilsen et al. (2004) |
| AG456-1* | AG456-1 hisI::npt (KanR) rfbK12 | None | This work |
| AG60B-1 | AG456-1ΔmrcB ΔdacD | O8 | Nilsen et al. (2004) |
| AG60B-1* | AG60B-1 hisI::npt (KanR) rfbK12 | None | This work |
| AG70A-6 | AG456-1ΔmrcAΔdacD ΔampC | O8 | Nilsen et al. (2004) |
| AG70A-6* | AG70A-6 hisI::npt (KanR) rfbK12 | None | This work |
| AG70C-12 | AG60B-1 ΔampC | O8 | Nilsen et al. (2004) |
| AG70C-12* | AG70C-12 hisI::npt (KanR) rfbK12 | None | This work |
Bacteria were grown in Luria–Bertani (LB) broth, on LB agar plates, or in M9 minimal glucose medium supplemented with 40 μg mL−1 each of L-threonine, L-leucine, L-arginine, L-lysine and L-histidine (Miller, 1992). Where appropriate, antibiotics were added to the following final concentrations: ampicillin, 100 μg mL−1; chloramphenicol, 20 μg mL−1; kanamycin, 50 μg mL−1; and tetracycline, 25 μg mL−1. Unless otherwise specified, chemicals and reagents were from Sigma Chemical Co. (St Louis, MO). Microscopy was performed as described previously (Ghosh & Young, 2005).
Strain constructions
PBPs were deleted from E. coli 2443 by introducing a kanamycin resistance gene cassette flanked with res sites into individually targeted PBP genes, as described previously (Denome et al., 1999; Nilsen et al., 2004). Gene deletions were confirmed by labeling the PBPs with 125I-penicillin X, followed by autoradiography (Henderson et al., 1994; Denome et al., 1999). For creating multiple mutants, the kanamycin resistance cassette was removed by expressing the RP4ParA resolvase (Kristensen et al., 1995; Denome et al., 1999; Nilsen et al., 2004), and the procedures were repeated to remove successive genes.
The rfbO8 genes in E. coli 2443 were replaced by the inactive rfbK12 gene segment from E. coli K12 by P1 cotransduction with a hisI::Kan marker, as described previously (Ghosh & Young, 2005). Cells that had lost the O8-antigen formed rough colonies, and the rfb gene replacement was confirmed by diagnostic PCR and the inability to bind concanavalin A-conjugated Alexa-Fluor 488 (Ghosh & Young, 2005). Lipopolysaccharide was extracted and the absence of the O-antigen was confirmed by gel electrophoresis and lipopolysaccharide detection (Ghosh & Young, 2005).
Results and discussion
PBP mutants expressing O8-antigen have fewer morphological deformities
Mutants lacking certain low molecular weight penicillin-binding proteins (LMW PBPs) have extensive morphological malformations (Denome et al., 1999; Nelson & Young, 2000, 2001). However, these strains were derived from E. coli K12, which does not express O-antigen (Liu & Reeves, 1994). To test the proposition that components of the OM might contribute to the control of cell shape, we deleted multiple PBP genes from E. coli 2443, which expresses the O8 antigen (Sandlin et al., 1996), and characterized the cells as normal, abnormal or branched (Fig. 1). In the E. coli K12 strain CS315-1, approximately half the population exhibited shape abnormalities. However, deleting the same PBPs from the O8-positive E. coli 2443 background yielded a population in which only 23% of the population was abnormal (a decrease of 54%) (Table 2, strain AG375-3). A similar tendency was observed in strains lacking four PBPs. Approximately half of the cells of the E. coli K12 strain CS446-1 displayed deformities, whereas similarly aberrant cell shapes were observed in 36% of the cells of the O8-positive strain, AG456-1 (a decrease of 27%) (Table 2). Thus, the presence of the O8-antigen appeared to reduce the number and extent of shape abnormalities in E. coli.
Fig. 1.
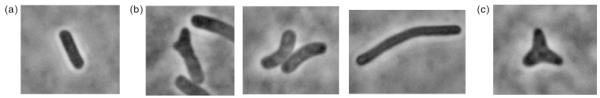
Morphological scoring for bacterial shape. (a) ‘Normal’ cells were those with a uniform rod shape. (b) ‘Abnormal’ cells were those exhibiting significant curvatures, bulges or other deformities. (c) ‘Branched’ bacteria were those having three or more arms, each of which comprised a significant portion of the cell volume.
Table 2.
Effect of O-antigen on cellular deformities
| Strains* | PBPs deleted |
O8-antigen | Deformities (% population) |
Total cells |
|---|---|---|---|---|
| CS315-1 | 4 7 5 | − | 50 | 529 |
| AG375-3 | 4 7 5 | + | 23 | 707 |
| AG375-3* | 4 7 5 | − | 29 | 750 |
| CS446-1 | 4 7 5 6 | − | 49 | 408 |
| AG456-1 | 4 7 5 6 | + | 36 | 414 |
| AG456-1* | 4 7 5 6 | − | 49 | 432 |
Strains designated with the ‘CS’ prefix were derived from Escherichia coli K-12, which lacks the O-antigen (Liu & Reeves, 1994). Strains designated with the prefix ‘AG’ was derived from E. coli 2443, which expresses the O8-antigen (Rick et al., 1994). AG names followed by an asterisk (*) designate strains from which the O8-antigen genetic locus was deleted and replaced by mutated genes from E. coli K12.
If the presence of the O-antigen minimizes the number of cellular deformities in PBP mutants, then its removal should increase the percentage of deformed cells. Therefore, we deleted the rfbO8 gene cluster from E. coli 2443 strains and replaced it with the corresponding cluster from E. coli KM32, a K12 strain. The O8-antigen polysaccharide ladder was confirmed to be absent from 2443-derived mutants in which the rfbO8 genes had been replaced (e.g. see strain AG456-1* in Fig. 2, lane 6).
Fig. 2.
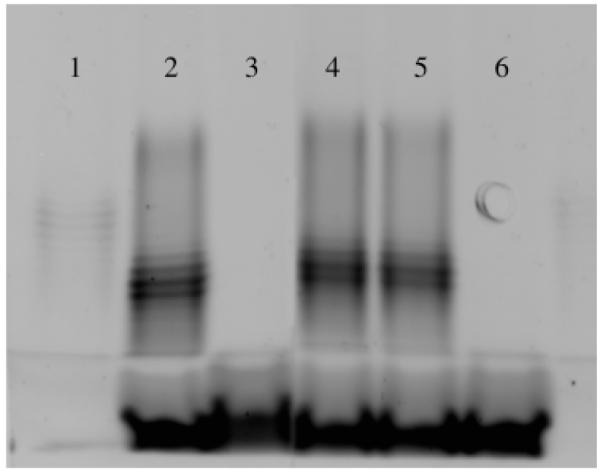
Lipopolysaccharide profiles of Escherichia coli strains with and without the O-antigen. Lipopolysaccharide from E. coli was separated by SDS-PAGE and visualized with Pro-Q Emerald LPS stain. Lipopolysaccharide was prepared from the following strains. Lane 1, E. coli serotype O55:B5; Lane 2, E. coli 2443T (O8-antigen); Lane 3, E. coli CS109 (O-negative K-12 strain); Lane 4, AG375-3; Lane 5, AG456-1; Lane 6, AG456-1*. The banding patterns represent O-antigen side chains present in E. coli 2443T and its derivatives (lanes 2, 4 and 5) but absent in the control K12 strain E. coli CS109 (lane 3) and in strain AG456* (lane 6), where the O8-antigen was replaced with that of CS109.
When the morphology of four strains was examined in four independent experiments, in 15 of 16 cases it was clear that removing the O8-antigen increased the proportion of cells that exhibited deformities (Table 3). The single exception (Table 3, AG70C-12* Exp #1) was a strain that displayed substantial increases in deformities in three other experiments. In the O-negative mutant AG456-1* (lacking four PBPs), the percentage of the population exhibiting deformities increased to a level similar to that of PBP mutants constructed in the naturally occurring O-negative E. coli K12 strain CS446-1 (Table 2). However, although removing the O-antigen from AG375-3 increased the proportion of abnormal cells to 29% (Table 2, AG375-3*), this was not quite as high as seen in the K12 strain CS315-1 (Table 2). It should be noted that the absolute values of deformed cells varied from day to day for unknown reasons. Nonetheless, O8-negative strains exhibited greater morphological variation than did their paired O8-positive parents in all but one case (Tables 2 and 3). In addition, three other PBP mutants lacking five, six or seven PBPs exhibited the same increase in deformities upon deletion of the rfbO8 genes (data not shown). Overall, therefore, the results support the idea that the O-antigen helps maintain normal cellular morphology in E. coli.
Table 3.
Effect of removing O8-antigen on shape deformities in Escherichia coli
| Exp #1 |
Exp #2 |
Exp #3 |
Exp #4 |
Cumulative |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | O8- antigen |
Total # cells |
Abnormal (%) |
Increase (%) |
Total # cells |
Abnormal (%) |
Increase (%) |
Total # cells |
Abnormal (%) |
Increase (%) |
Total # cells |
Abnormal (%) |
Increase (%) |
Total # cells |
Abnormal (%) |
Increase (%) |
| AG60B-1 | + | 358 | 24 | − | 386 | 33 | − | 65 | 23 | − | 57 | 40 | − | 866 | 29 | − |
| AG60B-1* | − | 240 | 51 | 113 | 741 | 38 | 15 | 90 | 52 | 126 | 71 | 52 | 30 | 1142 | 43 | 48 |
| AG70A-6 | + | 815 | 38 | − | 371 | 47 | − | 77 | 28 | − | 81 | 17 | − | 1344 | 39 | − |
| AG70A-6* | − | 521 | 50 | 32 | 346 | 62 | 32 | 286 | 30 | 7 | 329 | 30 | 76 | 1482 | 44.6 | 14 |
| AG70C-12 | + | 177 | 42 | − | 203 | 51 | − | 219 | 26 | − | 175 | 32 | - | 774 | 38 | − |
| AG70C-12* | − | 960 | 42 | 0 | 194 | 67 | 31 | 139 | 48 | 85 | 128 | 55 | 72 | 1421 | 47 | 24 |
| AG456-1 | + | 298 | 64 | − | 263 | 57 | − | 136 | 24 | − | 126 | 36 | − | 823 | 51 | − |
| AG456-1* | − | 287 | 77 | 20 | 407 | 66 | 16 | 424 | 48 | 100 | 519 | 49 | 36 | 1637 | 58 | 14 |
A heterologous O-antigen reduces the frequency of shape deformities
The heterologous rfb genes from Klebsiella (Vinés et al., 2005), cloned in a pBluescript vector, were introduced into two E. coli K12 mutants to determine whether a non-E. coli O-antigen would complement the misshapen phenotype. In both CS446-1 (which lacks four PBPs) and CS703-1 (which lacks seven PBPs), introduction of the pBluescript vector by itself increased the proportion of the population with abnormal shapes. The percentage of abnormal cells for CS446-1 increased from 60% of the population to 82% in cells carrying the vector, and for CS703-1 the fraction of abnormal cells increased from 73% of the population to 87% (Table 4). The reason for this effect is unknown. Nonetheless, using these vector-containing cells as a baseline, it was clear that expressing a heterologous O-antigen complemented the phenotype in the PBP mutants (Table 4). Adding the Klebsiella O1-antigen increased the fraction of normally shaped cells from 9% to 22% in CS446-1 and from 9% to 28% in CS703-1 (Table 4, pWQ5-containing cells). To eliminate the possibility that cloning just any gene into the pBluescript vector would reverse these morphological effects, plasmid pSH55, which contains unrelated E. coli genes, was introduced into the mutants. The presence of this plasmid did not reduce the number of abnormal cells in either mutant (data not shown), indicating that O-antigen complementation was not an artifact of cloning into the vector. Therefore, the frequency of misshapen cells in PBP mutants of E. coli was reduced when these strains expressed an O-antigen from a related genus.
Table 4.
Complementation of shape defects by heterologous O-antigen
| Strain | Plasmid | O-antigen | Cells (Total #) |
Normal (%) |
Abnormal (%) |
Branched (%) |
|---|---|---|---|---|---|---|
| CS446-1 | None | − | 1008 | 29 | 60 | 11 |
| pBluescript | − | 594 | 9 | 82 | 9 | |
| pWQ5 | Klebsiella O1 | 952 | 22 | 76 | 2 | |
| CS703-1 | None | − | 1313 | 23 | 73 | 4 |
| pBluescript | − | 498 | 9 | 87 | 4 | |
| pWQ5 | Klebsiella O1 | 359 | 28 | 69 | 3 |
CS446-1 and CS703-1 are Escherichia coli K12 strains lacking four and seven pencillin-binding proteins, respectively.
The O-antigen was introduced into these strains on plasmid pWQ5 (Vinés et al., 2005), and cells categorized as in Fig. 1.
Possible mechanisms for OM effects on cell shape
At first, it seems odd that the state of the OM helps shape bacteria since the peptidoglycan sacculus and the enzymes that construct it are located internal to this structure. However, altering the OM could affect bacterial shape either directly, by disrupting an external mechanical scaffold, or indirectly, by interfering with the activities of enzymes that synthesize the peptidoglycan sacculus. The idea of the OM as a physical scaffold has been discussed (Cooper, 1991), but little experimental evidence exists for the idea. The main support for such a view is that OM proteins (Braun’s lipoprotein, Pal or OmpA) bind peptidoglycan by covalent and noncovalent interactions (Braun & Rehn, 1969; Sonntag et al., 1978; Wang, 2002; Parsons et al., 2006). These intimate connections may impose physical stresses on peptidoglycan that affect how the wall is constructed.
The likelihood of an indirect relationship between the OM and cell wall has more support. Defects in OM synthesis or structure might affect the sacculus either by altering the supply of new peptidoglycan precursors or by changing the way they are incorporated into the pre-existing wall. With regard to precursor supply, it seems reasonable that synthesis of O-antigen and peptidoglycan should be coordinated so that the surface area of the OM and murein sacculus will increase in tandem, to avoid stretching or buckling one or the other (Ehlert & Höltje, 1996). Because the precursors for synthesizing O-antigen and murein are translocated to the periplasm by the same phospholipid carrier (Höltje, 1998), disrupting O-antigen synthesis may alter the sites or rate at which peptidoglycan precursors are delivered to the periplasm. Such an imbalance might, in turn, affect cell shape. This possibility is made more plausible because insertion of O-antigen into the OM has a spatial component (Ghosh & Young, 2005). As for incorporation into the pre-existing wall, previously created peptidoglycan bonds may be held in a particular conformation by virtue of numerous OM protein-to-peptidoglycan interconnections. Should these be disrupted, improper insertion of new material by the penicillin-binding proteins might lead to aberrant shapes in mutants lacking specific DD-carboxypeptidases or endopeptidases (Nelson & Young, 2000, 2001).
Regardless of whether the OM affects cell wall synthesis by mechanical or enzymatic means, it seems clear that neither mechanism affects cell shape by changing the general characteristics of the poles. Whether or not the O-antigen is present, pole-specific proteins localize to natural and ectopic poles in malformed cells (Nilsen et al., 2004) and a subset of OM proteins are retained for long periods at these same sites (Nilsen et al., 2004; Ghosh & Young, 2005). Instead, the present results show that in the absence of O-antigen, the number of ectopic poles increases, and it is these structures that provoke the increase in cellular deformities (de Pedro et al., 2003). Therefore, on the one hand, the OM may provide a mechanical stability that restricts peptidoglycan-synthesis so that the placement of new cell poles is constrained. Alterations in the OM might weaken this mechanical restraint and allow the production of poles at unusual sites. On the other hand, if the OM affects cell shape indirectly by regulating peptidoglycan-synthesizing enzymes, then altering the membrane composition may interfere with an inhibitory function, thus allowing the creation of multiple, misplaced polar zones.
Acknowledgements
This work was supported by Grant R01-GM061019 from the National Institutes of Health to K.D.Y. A.S.G. was supported by the Department of Biotechnology, Government of India, and by a UNESCO-ASM travel award.
References
- Belaaouaj A, Kim KS, Shapiro SD. Degradation of outer membrane protein A in Escherichia coli killing by neutrophil elastase. Science. 2000;289:1185–1188. doi: 10.1126/science.289.5482.1185. [DOI] [PubMed] [Google Scholar]
- Braun V, Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969;10:426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Bronner D, Clarke BR, Whitfield C. Identification of an ATP-binding cassette transport system required for translocation of lipopolysaccharide O-antigen side-chains across the cytoplasmic membrane of Klebsiella pneumoniae serotype O1. Mol Microbiol. 1994;14:505–519. doi: 10.1111/j.1365-2958.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Cabeen MT, Jacobs-Wagner C. Bacterial cell shape. Nat Rev Microbiol. 2005;3:601–610. doi: 10.1038/nrmicro1205. [DOI] [PubMed] [Google Scholar]
- Carballido-Lopez R, Errington J. A dynamic bacterial cytoskeleton. Trends Cell Biol. 2003;13:577–583. doi: 10.1016/j.tcb.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Cooper S. Bacterial Growth and Division: Biochemistry and Regulation of Prokaryotic and Eukaryotic Division Cycles. Academic Press Inc.; San Diego: 1991. pp. 228–230. [Google Scholar]
- de Pedro MA, Young KD, Höltje JV, Schwarz H. Branching of Escherichia coli cells arises from multiple sites of inert peptidoglycan. J Bacteriol. 2003;185:1147–1152. doi: 10.1128/JB.185.4.1147-1152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denome SA, Elf PK, Henderson TA, Nelson DE, Young KD. Escherichia coli mutants lacking all possible combinations of eight penicillin-binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J Bacteriol. 1999;181:3981–3993. doi: 10.1128/jb.181.13.3981-3993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert K, Höltje JV. Role of precursor translocation in coordination of murein and phospholipid synthesis in Escherichia coli. J Bacteriol. 1996;178:6766–6771. doi: 10.1128/jb.178.23.6766-6771.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figge RM, Divakaruni AV, Gober JW. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol Microbiol. 2004;51:1321–1332. doi: 10.1111/j.1365-2958.2003.03936.x. [DOI] [PubMed] [Google Scholar]
- Ghosh AS, Young KD. Helical disposition of proteins and lipopolysaccharide in the outer membrane of Escherichia coli. J Bacteriol. 2005;187:1913–1922. doi: 10.1128/JB.187.6.1913-1922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA, Dombrosky PM, Young KD. Artifactual processing of penicillin-binding proteins 7 and 1b by the OmpT protease of Escherichia coli. J Bacteriol. 1994;176:256–259. doi: 10.1128/jb.176.1.256-259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemstra H, Nanninga N, Woldringh CL, Inouye M, Witholt B. Distribution of newly synthesized lipoprotein over the outer membrane and the peptidoglycan sacculus of an Escherichia coli lac-lpp strain. J Bacteriol. 1987;169:5434–5444. doi: 10.1128/jb.169.12.5434-5444.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J-V. Growth of the stress-bearing and shape-maintaining murein sacculus of Escherichia coli. Microbiol Mol Biol Rev. 1998;62:181–203. doi: 10.1128/mmbr.62.1.181-203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen CS, Eberl L, Sanchez-Romero JM, Givskov M, Molin S, de Lorenzo V. Site-specific deletions of chromosomally located DNA segments with the multimer resolution system of broad-host-range plasmid RP4. J Bacteriol. 1995;177:52–58. doi: 10.1128/jb.177.1.52-58.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Reeves PR. Escherichia coli K12 regains its O-antigen. Microbiology. 1994;140(Part 1):49–57. doi: 10.1099/13500872-140-1-49. [DOI] [PubMed] [Google Scholar]
- Meberg BM, Sailer FC, Nelson DE, Young KD. Reconstruction of Escherichia coli mrcA (PBP 1a) mutants lacking multiple combinations of penicillin-binding proteins. J Bacteriol. 2001;183:6148–6149. doi: 10.1128/JB.183.20.6148-6149.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg BM, Paulson AL, Priyadarshini R, Young KD. Endopeptidase penicillin-binding proteins 4 and 7 play auxiliary roles in determining uniform morphology of Escherichia coli. J Bacteriol. 2004;186:8326–8336. doi: 10.1128/JB.186.24.8326-8336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. A Short Course in Bacterial Genetics: A Laboratory Manual and Handbook for Escherichia coli and Related Bacteria. Cold Spring Harbor Laboratory Press; Plainview, NY: 1992. [Google Scholar]
- Murphy KC. Use of bacteriophage λ recombination functions to promote gene replacement in Escherichia coli. J Bacteriol. 1998;180:2063–2071. doi: 10.1128/jb.180.8.2063-2071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Young KD. Penicillin-binding protein 5 affects cell diameter, contour, and morphology of Escherichia coli. J Bacteriol. 2000;182:1714–1721. doi: 10.1128/jb.182.6.1714-1721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Young KD. Contributions of PBP 5 and DD-carboxypeptidase penicillin-binding proteins to maintenance of cell shape in Escherichia coli. J Bacteriol. 2001;183:3055–3064. doi: 10.1128/JB.183.10.3055-3064.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T, Ghosh AS, Goldberg MB, Young KD. Branching sites and morphological abnormalities behave as ectopic poles in shape-defective Escherichia coli. Mol Microbiol. 2004;52:1045–1054. doi: 10.1111/j.1365-2958.2004.04050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara M, Wu HC, Sankaran K, Rick PD. Identification and characterization of a new lipoprotein, NlpI, in Escherichia coli K-12. J Bacteriol. 1999;181:4318–4325. doi: 10.1128/jb.181.14.4318-4325.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LM, Lin F, Orban J. Peptidoglycan recognition by Pal, an outer membrane lipoprotein. Biochemistry. 2006;45:2122–2128. doi: 10.1021/bi052227i. [DOI] [PubMed] [Google Scholar]
- Rick PD, Hubbard GL, Barr K. Role of the rfe gene in the synthesis of the O8 antigen in Escherichia coli K-12. J Bacteriol. 1994;176:2877–2884. doi: 10.1128/jb.176.10.2877-2884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandlin RC, Goldberg MB, Maurelli AT. Effect of O side-chain length and composition on the virulence of Shigella flexneri 2a. Mol Microbiol. 1996;22:63–73. doi: 10.1111/j.1365-2958.1996.tb02656.x. [DOI] [PubMed] [Google Scholar]
- Sonntag I, Schwarz H, Hirota Y, Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. J Bacteriol. 1978;136:280–285. doi: 10.1128/jb.136.1.280-285.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinés ED, Marolda CL, Balachandran A, Valvano MA. Defective O-antigen polymerization in tolA and pal mutants of Escherichia coli in response to extracytoplasmic stress. J Bacteriol. 2005;187:3359–3368. doi: 10.1128/JB.187.10.3359-3368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. The function of OmpA in Escherichia coli. Biochem Biophys Res Commun. 2002;292:396–401. doi: 10.1006/bbrc.2002.6657. [DOI] [PubMed] [Google Scholar]
- Young KD. Bacterial shape. Mol Microbiol. 2003;49:571–580. doi: 10.1046/j.1365-2958.2003.03607.x. [DOI] [PubMed] [Google Scholar]


