Abstract
Background
Heavy alcohol consumption is associated with severe bronchitis. This is likely related to increased inflammation in the airways of alcohol abusers. Toll-like receptor 2 (TLR2) is an important mediator of inflammation in the airway epithelium. TLR2 initiates an inflammatory cascade in response to gram-positive bacteria. We have previously shown that alcohol upregulates TLR2 in the airway epithelium. However, the mechanism of alcohol-mediated upregulation of TLR2 has not been identified.
Methods
A human airway epithelial cell line, 16HBE14o-, was exposed to biologically relevant concentrations of alcohol (100mM) in the presence and absence of L-NAME, a nitric oxide synthase (NOS) inhibitor; and Rp-8-Br-cGMP-S, an antagonist analogue of cGMP. TLR2 was measured using real-time PCR and Western blots. In addition, 16HBE14o- cells were incubated with sodium nitroprusside (SNP), an NO donor, and 8-Br-cGMP, a cGMP analogue. TLR2 was measured using real-time PCR.
Results
L-NAME blocked the alcohol-mediated upregulation of TLR2. This indicates that NO plays a key role in alcohol's upregulation of TLR2. SNP, a nitric oxide donor, up-regulated TLR2. Rp-8-Br-cGMPS attenuated alcohol's upregulation of TLR2, suggesting that NO was working through cGMP/PKG. 8-Br-cGMP upregulated TLR2, also demonstrating the importance of cGMP/PKG.
Conclusions
Alcohol upregulates TLR2 through a NO/cGMP/PKG dependent pathway in the airway epithelium. This is an important observation in the understanding how alcohol modulates airway inflammation. In addition, this is the first time that cyclic nucleotides have been shown to play a role in the regulation of TLR2.
Keywords: Lung, nitric oxide, cyclic nucleotide, ethanol, inflammation
Introduction
Heavy alcohol use is clearly associated with lung disease. Alcohol intake is an independent risk factor for developing acute respiratory distress syndrome (Moss and Burnham, 2003), pneumonia (de Roux et al., 2006; Fernandez-Sola et al., 1995; Saitz et al., 1997), and chronic bronchitis (Jalleh et al., 1993; Oleru, 1987; Suadicani et al., 2001). Although alcohol is associated with increased incidence and severity of lung disease, very little is known about how alcohol affects the innate immunity of the airway epithelium. Understanding how alcohol affects the airway epithelium is particularly important, because it is the body's first line of defense against inhaled microorganisms. Toll-like receptor 2 (TLR2) is an important part of the innate immunity of the airway epithelium. TLR2 recognizes peptidoglycan, a component of the gram-positive cell wall of common respiratory pathogens such as streptococcus pneumoniae. When peptidoglycan binds TLR2, it initiates a cascade of intracellular signaling which includes activation of MAPK and NF-κB (Akira and Takeda, 2004). This ultimately leads to release of pro-inflammatory cytokines such as IL-8.
We have previously shown that alcohol upregulates TLR2 in airway epithelial cells (Bailey et al., 2008). We demonstrated a concentration-dependent increase in TLR2 mRNA and protein when cells were exposed to 100mM alcohol. The increase in surface expression of TLR2 made the cells more responsive to peptidoglycan, a ligand for TLR2. The alcohol-primed cells secreted twice as much IL-8 in response to stimulation with a low concentration (40ng) of peptidoglycan. These findings are in stark contrast to how alcohol modulates immune effector cells. Others have shown that alcohol downregulates TLR2-mediated inflammatory signaling in macrophages (Frost et al., 2005; Goral and Kovacs, 2005; Pruett et al., 2004). The mechanism for alcohol's alternative upregulation of TLR2 in the airway epithelium is unknown.
We hypothesized that alcohol's novel upregulation of TLR2 in airway epithelial cells is through the NO/cGMP/PKG pathway. We have previously shown that airway epithelial cells produce nitric oxide in response to alcohol stimulation (Sisson, 1995). Nitric oxide in turn, binds to soluble guanylyl cyclase and increases intracellular concentrations of cGMP (Wyatt et al., 1998). Cyclic GMP then binds to and activates its major cellular receptor, the cGMP-dependent protein kinase (PKG). The catalytic activation of PKG then leads to substrate phosphorylation and a subsequent increase in TLR2 expression. We have tested this hypothesis by stimulating airway epithelial cells with alcohol in the presence of inhibitors of this pathway. In addition, we have reproduced the effects of alcohol using agonists to this pathway.
Methods
Cell culture
Dr. Dieter Gruenert provided the 16HBE14o- transformed airway epithelial cell line (Gruenert et al., 1988). We have previously published that 16HBE14o- respond to alcohol in the same way as normal human bronchial epithelial cells (Bailey et al., 2008). 16HBE14o- were grown in tissue culture plates coated with 27μg/ml Type I collagen (Sigma, St. Louis, MO) and 3μg/ml Type III collagen (Sigma, St. Louis, MO). Cells were grown in submerged culture in Dulbecco's Modified Eagle Medium (Media Tech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA). They were maintained in a 37°C incubator with 5% carbon dioxide. The cells used for these experiments were passage 35-50. Like primary smooth muscle cells, these cells down regulate PKG with extended passages (Browner et al., 2004). After passage 50 there was no active PKG.
Cell exposure to alcohol
16HBE14o- cells were exposed to alcohol by adding ethanol (Pharmco-AAPER, Shelbyville, KY) to their growth media for the times and concentrations indicated for individual experiments. Serum remained in the growth media when exposing the 16HBE14o- to alcohol for all experiments. We chose to use 100mM ethanol in these experiments, because we have previously published that this is the concentration that maximally up-regulates TLR2 (Bailey et al., 2008). This concentration is biologically relevant and is frequently seen in intoxicated individuals.
RNA extraction
16HBE14o- cells were exposed to experimental conditions for the times indicated. Cell monolayers were washed twice with PBS, then trypsin was used to remove the cells from the plate. The cells were stored in RNAlater (Applied Biosystems, Carlsbad CA) until RNA extraction could be performed. RNA extraction was performed using the MagMax 96 RNA extraction kit (Applied Biosystems, Foster City, CA) according to the manufacturer's directions. RNA quantity and quality was evaluated using the Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE). All RNA samples had an A260/A280 ratio of 1.8-2.0.
Synthesis of cDNA
cDNA was synthesized using the Taqman reverse transcription kit (Applied Biosystems). Reactions were prepared using 100 ng of template RNA, 1× Taqman RT buffer, 1.25 U/μl of Multiscribe reverse transcriptase, 500 μM of each dNTP, 5.5 mM magnesium chloride, 2.5 μM random hexamers, and 0.4 U/μl RNase inhibitor. The reactions were incubated in a PTC 200 thermocycler (MJ Research, Waltham, MA) for 10 minutes at 25°C, 30 minutes at 48°C, and 5 minutes at 95°C.
Real-time PCR
Real-time PCR was performed on the cDNA using the following reaction: 1× Gene master mix, and 1× human TLR2 primer and probe mix (Applied Biosystems Hs00152932_m1) in 25 μl reactions in a 96-well plate. Reactions were performed in duplicate. The plate was then placed in an ABI Prism 7500 Sequence detection system (Applied Biosystems). Reactions underwent 2 minutes at 50°C, 10 minutes at 95°C, then 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Ribosomal RNA (Applied Biosystems) was used as an endogenous control. For relative comparison of TLR2 to ribosomal RNA, we analyzed the cycle threshold value (Ct) using the ΔΔCt method (Livak and Schmittgen, 2001). Data are then reported as a fold-change from control.
Western blotting
16HBE14o- cells were grown to 30-40% confluency in 60mm tissue culture dishes as described above. Half the cells were pre-treated for 2 hours with the cGMP antagonist analogue Rp-8-Br-cGMPS (10μM) (Biomol, Plymouth Meeting, PA) in the normal growth media (DMEM with 10% FBS). The rest of the cells were treated with media alone. Ethanol was added to the appropriate wells for a final concentration of 100 mM or the same volume of media was added for 48 hours. The cell layers were washed twice with PBS, then lysed on ice in a lysis buffer containing 10 mM Tris, 150 mM NaCl, 3 mM EDTA, 20 μg/ml of soybean trypsin inhibitor, 5 mM Benzamidine, 1× protease inhibitor cocktail (Sigma). The cells were then mechanically scraped using a cell lifter. The concentration of protein in each sample was determined using the Bradford method (Bradford, 1976). Total protein (50 μg) was resolved in a 10% polyacrylamide gel. It was then electroblotted onto nitrocellulose using a semi-dry transfer at 10V for 45 minutes. The blot was blocked for at least 2 hours in 5% blotto w/v in PBS at room temperature. The blot was probed with rabbit anti-human TLR2 (H175) antibody (SantaCruz Biotechnology, Santa Cruz, CA) at a 1:400 dilution in 5% blotto overnight at 4°C. The blot was then washed 5 times in PBS with 0.5% Tween. The blot was then incubated in a 1:10,000 dilution of goat-anti-rabbit HRP-conjugated antibody (Jackson ImmunoResearch, West Grove, PA) in 5% blotto for 2 hours at room temperature. The blot was then washed 5 times in PBS-Tween, then developed using Pierce SuperSignal kit (ThermoScientific) The blots were then exposed to X-ray film and developed. Beta actin was used as a loading control. Densitometry was performed using ImageJ software (Downloaded from http://rsbweb.nih.gov/ij/).
Statistics
All data are reported as mean ± SEM. Statistics were performed using GraphPad/Prism 4 software (La Jolla, CA). Data were analyzed using the one-way analysis of variance (ANOVA) followed by Dunnett's multiple comparison test or Tukey's multiple comparison test as appropriate. p values < 0.05 were considered statistically significant.
Results
Alcohol upregulates TLR2 through a nitric oxide (NO) dependent mechanism
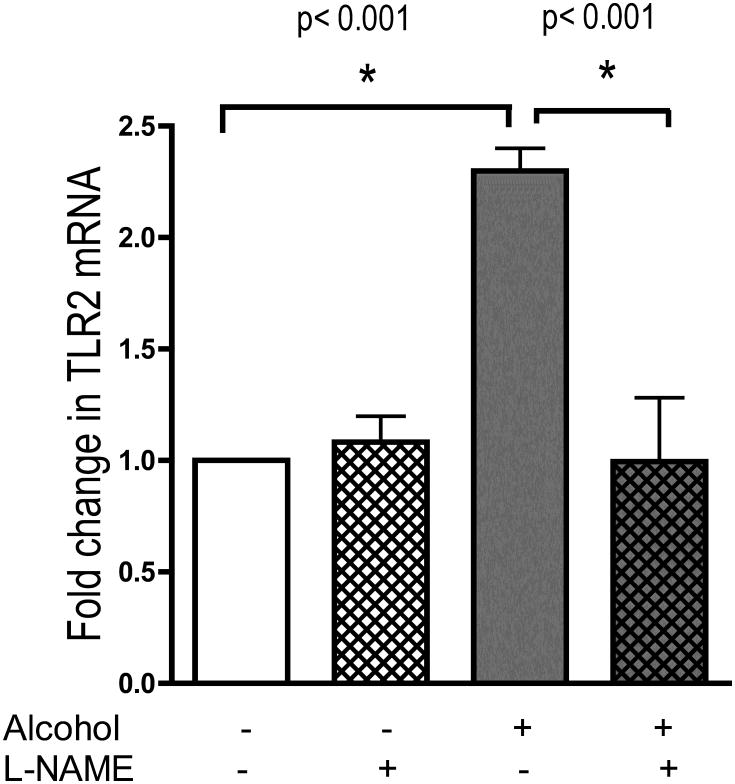
We first tested the hypothesis that nitric oxide was playing a role in the alcohol-triggered upregulation of TLR2. We inhibited nitric oxide synthase production of NO in the airway epithelial cells using the NOS inhibitor, Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME) as previously reported (Wyatt et al., 2003). Cells were preincubated for 1 hour with media alone or media containing L-NAME (1 mM). With L-NAME remaining in the media, the cells were exposed to 100 mM alcohol for 6 hours. Cell monolayers were harvested and real-time PCR was performed. Alcohol stimulated cells showed a 2-fold increase (p<0.001, n=4) in TLR2 mRNA at 6 hours over control (Figure 1). This increase was completely blocked by L-NAME (p<0.001). L-NAME did not decrease the baseline expression of TLR2 compared to control. This suggests that nitric oxide plays an integral role in alcohol's upregulation of TLR2.
Figure 1.
16HBE14o- cells were preincubated with and without L-NAME, an NOS inhibitor, for 1 hour. The indicated cells were then exposed to 100mM alcohol for 6 hours. TLR2 mRNA was measured using real-time PCR. L-NAME blocked alcohol's upregulation of TLR2 (n=4).
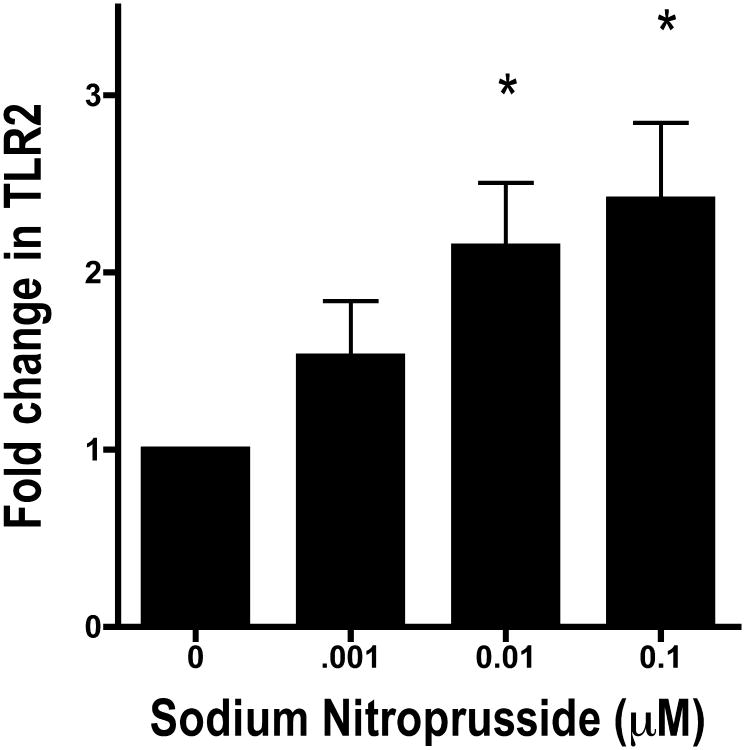
To further test the role NO plays in the regulation of TLR2, we stimulated cells with a NO donor, sodium nitroprusside (SNP). We found that SNP produces the same effect on TLR2 as alcohol. Cells were incubated with 1 μM, 10 μM and 100 μM SNP for 24 hours. Cell monolayers were harvested and TLR2 mRNA was measured using real-time PCR. SNP induced a concentration-dependent increase in TLR2 of a similar magnitude as alcohol (Figure 2). At the 10 μM and 100 μM concentrations, we observed a statistically significant difference in alcohol treatment (p <0.05, n=5) over control.
Figure 2.
16HBE14o- cells were exposed to 0.001-0.1 μM Sodium Nitroprusside (SNP), a nitric oxide donor, for 6 hours. TLR2 mRNA was measured using real-time PCR. SNP upregulated the amount of TLR2 mRNA as much as alcohol (n=5).
NO signals through cGMP
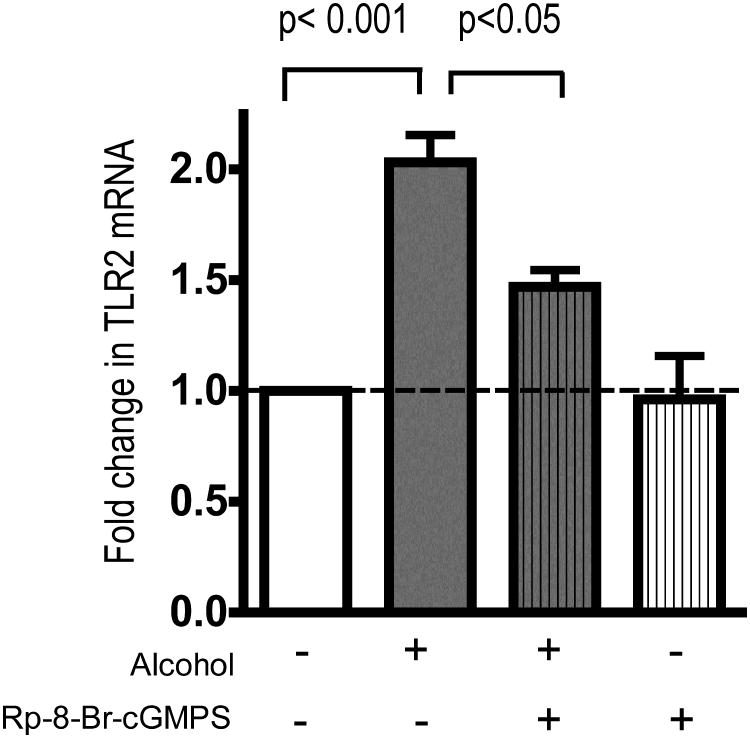
NO often signals through the activation of guanylyl cyclase and the production of cGMP. To determine if cGMP regulates alcohol-mediated upregulation of TLR2, cells were exposed to alcohol in the presence of a cGMP antagonist analogue, Rp-8-Br-cGMP-S. Cells were pre-incubated with 10 μM Rp-8-Br-cGMPS for one hour. Alcohol was then added to the wells for a final concentration of 100 mM for 24 hours. Cell monolayers were harvested and real-time PCR for TLR2 was performed. Again, alcohol produced a 2.1-fold increase in TLR2 mRNA (p< 0.001) while this increase was attenuated by Rp-8-Br-cGMPS (p<0.05, n=4) (Figure 3). Rp-8-Br-cGMP did not cause a decrease in baseline production of TLR2 mRNA (Figure 3).
Figure 3.
16HBE14o- cells were pre-incubated for 1 hour with Rp-8-Br-cGMPS, an antagonist analogue of cGMP. Cells were then stimulated with 100 mM alcohol for 24 hours in the presence of the antagonist. Alcohol caused a 2-fold increase in TLR2 mRNA (p< 0.001) Rp-8-Br-cGMPS inhibited the upregulation of TLR2 mRNA by alcohol (p<0.05). The alcohol + Rp-8-Br-cGMPSgroup is not statistically different from control (p>0.05;n=4).
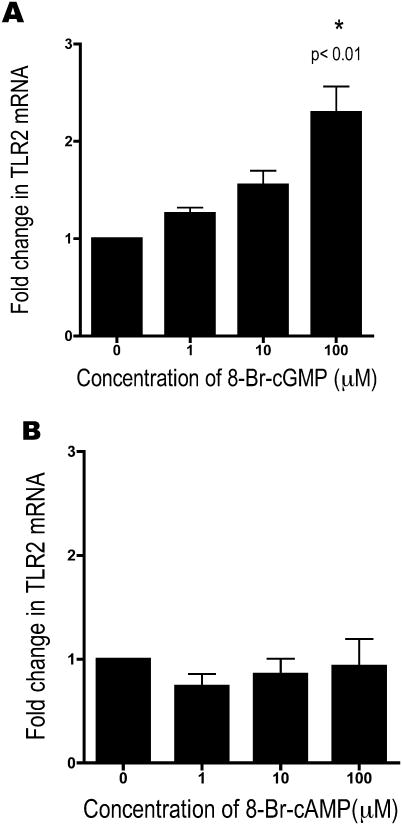
We then used a cGMP agonist, 8-Br-cGMP, to mimic the effect of alcohol on TLR2. Cells were exposed to 1, 10, and 100 μM 8-Br-cGMP for 6 hours. We observed a statistically significant increase in TLR2 mRNA of 2.2 fold (p<0.01, n=4) with 100 μM cGMP (Figure 4a). At high concentrations (>0.1 M), cGMP can cross-activate airway epithelial cell PKA (Wyatt et al., 2003). To ensure that the increase in TLR2 mRNA was due to the action of cGMP on PKG and not the cross-activation of PKA, we also stimulated cells with a cAMP analogue, 8-Br-cAMP. The cells were exposed to 1, 10, and 100 μM 8-Br-cAMP for 6 hours. Even at very high doses of cAMP, we did not see any increase in TLR2 mRNA (n=4) (Figure 4b). This indicates that the increase in TLR2 is related to the activation of PKG, and not due to a cGMP-mediated cross-activation of PKA.
Figure 4.
16HBE14o- were exposed to 0, 1, 10, and 100 μM 8-Br-cGMP (A) and 8-Br-cAMP (B) for 6 hours. TLR2 mRNA increased with 8-Br-cGMP, as with alcohol (n=4).
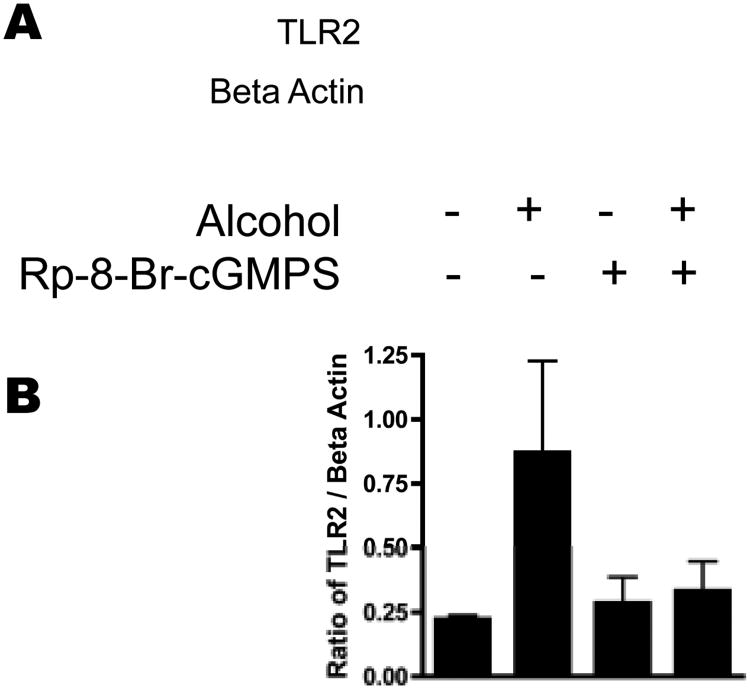
The alcohol-triggered upregulation of TLR2 mRNA appears to be dependent on the NO/cGMP/PKG pathway. We wanted to confirm that the changes in TLR2 mRNA correlated with the changes in TLR2 protein. 16HBE14o- were stimulated with 100 mM alcohol for 48 hours in the presence or absence of 10 μM Rp-8-Br-cGMP-S, the cGMP antagonist analog. Although we see an increase in TLR2 mRNA at 24 hours, it takes 48 hours for TLR2 protein expression to increase in response to alcohol. For this reason, the cells were exposed to alcohol for 48 hours. A representative Western blot for TLR2 (out of 5 blots showing the same results) is shown (Figure 5A) along with a summary graph of the data (Figure 5B). We observed that alcohol increases TLR2 protein 3.9 fold and that this increase is attenuated by Rp-8-Br-cGMP-S.
Figure 5.
16HBE14o- were stimulated with 100 mM alcohol in the presence or absence of Rp-8-Br-cGMPS for 48 hours. Cell monolayers were harvested and a Western blot for TLR2 was performed. A. Representative blot of 5 separate experiments. B. Densitometry of the ratio of TLR2: Beta Actin ratio of the 5 Western blots performed. TLR2 protein is upregulated by alcohol, and this effect is blocked by Rp-8-Br-cGMPS.
Discussion
We have previously shown that biologically relevant concentrations of alcohol functionally upregulate TLR2 (Bailey et al., 2008). In the current studies, we sought to define the mechanism of the alcohol-stimulated upregulation of TLR2. We found that alcohol upregulates TLR2 through the NO/cGMP/PKG pathway in the airway epithelium. We have shown that NO production is an essential part of this upregulation of TLR2 by inhibiting nitric oxide synthase using L-NAME. Likewise, we can mimic alcohol's upregulation of TLR2 using the NO donor SNP. We have also shown that cGMP/PKG are essential in the alcohol-triggered upregulation of TLR2. We blocked alcohol's upregulation of TLR2 by using the cGMP antagonist analogue, Rp-8-Br-cGMPS. Likewise, we can mimic alcohol's upregulation of TLR2 using the cGMP analogue 8-Br-cGMP.
Our findings demonstrate a novel mechanism of regulation of TLR2 in airway epithelium. Alcohol upregulates TLR2 through a NO/cGMP/PKG pathway. Alcohol has been shown to regulate other airway epithelial cell processes through the same pathway, such as ciliary beat frequency (Li et al., 2000; Wyatt et al., 1998), wound repair (Spurzem et al., 2005), and adenosine transport (Diane S.Allen-Gipson, 2008). However, the upregulation of TLR2 through this pathway has never been demonstrated in any cell type.
Most of the study of alcohol's effect on TLR's has been confined to monocytes and macrophages. Our study is unique in that it focuses on the airway epithelium. Understanding how alcohol influences the innate immune system in the airway epithelium is important, because the lining of the conducting airways represent the lung's first defense against infection by inhaled pathogens. Immune effector cells such as monocytes and macrophages behave very differently than airway epithelial cells in response to alcohol. Alcohol is known to decrease the downstream signaling of TLR2 in monocytes and macrophages (Goral and Kovacs, 2005; Oak et al., 2006). This is in sharp contrast to the upregulation of TLR2 that we see in airway epithelial cells
It is possible that a simple upregulation of TLR2 by alcohol does not necessarily translate into increased inflammation of the airway epithelium in the absence of an additional injury. We have previously published that cells ‘primed’ with alcohol produce twice as much IL-8 as unprimed cells in response to low concentrations of peptidoglycan, a ligand for TLR2 (Bailey et al., 2008). In this context, it appears that alcohol triggers a functional upregulation of TLR2. This alcohol-induced “priming” of the airway epithelium could then lead to the potentiation of a significantly enhanced inflammatory response to the inhalation of gram-positive bacteria or organic dusts, both of which are highly enriched in peptidoglycan.
In general, much more is known about the downstream signaling of TLRs than how TLRs themselves are modulated. The study of nitric oxide in relation to TLRs is a good example of this. Nitric oxide has long been known to be produced by macrophages in response to inflammatory stimuli (Stuehr and Marletta, 1985). In macrophages, NO is produced by iNOS (NOS2) as a consequence of a ligand binding a TLR. For example, Lipotechteic acid, a component of the gram positive cell wall, binds to TLR2 and stimulates macrophages to secrete NO (Ryu et al., 2009). Other organisms such as Peptostreptococcus (Marcato et al., 2008), Mycobacterium tuberculosis (Doz et al., 2007), and enterococcus faecalis (Baik et al., 2008) have similar effects in macrophages. Other cell types also produce NO in response to activation of TLR2. Astrocytes (Phulwani et al., 2008), microglial cells (Kinsner et al., 2006), pancreatic stellate cells (Masamune et al., 2008), epididymal epithelial cells (Zhao et al., 2008)and gastric epithelial cells (Uno et al., 2007) all secrete NO after TLR2 has been activated. Little is known about NO and TLR2 in the airway epithelium. An attempt was made to study the effect of NO on TLR2 mRNA expression in the whole lung homogenate of rats (Wu et al., 2005). In this study, acute hemorrhagic pancreatitis was induced and mice were treated with intravenous L-arginine. The mice with pancreatitis that received L-arginine had higher levels of NO and somewhat decreased levels of TLR2 mRNA. This is at odds with our findings, but it is impossible to determine which cells within the whole lung homogenate were responsible for this change. The upregulation of TLR2 may be specific to the airway epithelium. This may be because the airway epithelium is the first level of defense against inhaled pathogens.
How cyclic nucleotides modulate toll-like receptors and their signalling is largely unknown. The few studies that have looked at the relationship between TLRs and cyclic nucleotides have been focused on the downstream signaling of TLRs In one study, osteoblasts were stimulated with LPS to activate TLR4. The addition of a cAMP analog enhanced production of NO by the osteoblasts (Sosroseno et al., 2009). In contrast, two studies in monocytes (Kaufmann et al., 2005) and dendritic cells(Harzenetter et al., 2007) show that cAMP activation led to diminished inflammatory cytokine production in response to stimulation with TLR2 agonists. Our findings are the first to suggest that cyclic nucleotides play a role in the regulation of TLR2 expression.
Many questions remain to be answered in this area. We do not know how alcohol affects the down-stream signaling of TLR2 in the airway epithelium. It is also possible that the upregulation of TLR2 in the airway epithelium is only an acute reaction to alcohol, and that chronic exposures may have very different effects. In addition, because we see different trends in TLR2 regulation between the airway epithelium and immune effector cells, it is impossible to know what the net inflammatory outcome is by in vitro studies alone. In vivo studies will be essential to determine the overall effect of both acute and chronic alcohol exposure on TLR2-mediated airway inflammation.
Conclusion
Alcoholics have more severe airway disease than their non-alcoholic counterparts. This is likely due to increased inflammation in the airways of alcoholics. At least in part, this inflammation is likely due to alcohol's upregulation of TLR2. In this paper, we have shown that alcohol modulates its effect on TLR2 expression through the NO/cGMP/PKG pathway. Understanding the mechanism through which alcohol upregulates TLR2 brings us closer to developing better therapies for alcohol-related airway inflammation.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Omaha VA Medical Center, Omaha, NE (Department of Veterans Affairs [VA Merit Review] to TAW and DJR.) Funding from the National Institutes of Health supported this work: 5F32AA016433 to KLB, R37AA008769 to JHS, R01OH008539 to DJR, and R01AA017993 to TAW.
References
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Baik JE, Ryu YH, Han JY, Im J, Kum KY, Yun CH, Lee K, Han SH. Lipoteichoic acid partially contributes to the inflammatory responses to Enterococcus faecalis. J Endod. 2008;34(8):975–82. doi: 10.1016/j.joen.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Bailey KL, Wyatt TA, Romberger DJ, Sisson JH. Alcohol Functionally Upregulates Toll-Like Receptor 2 in Airway Epithelial Cells. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Browner NC, Dey NB, Bloch KD, Lincoln TM. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J Biol Chem. 2004;279(45):46631–6. doi: 10.1074/jbc.M408518200. [DOI] [PubMed] [Google Scholar]
- de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, Torres A. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129(5):1219–25. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- Allen-Gipson Diane S, PJCJ, BS, Kharbanda Kusum K, PhD, Bailey Kristina L, MD, Robinson James E, BS, Sisson Joseph H, MD, Wyatt Todd A., PhD Ethanol Blocks Adenosine Uptake via Inhibiting the Nucleoside Transport System In Bronchial Epithelial Cells. Alcoholism: Clinical and Experimental Research. 2008 doi: 10.1111/j.1530-0277.2009.00897.x. Accepted Dec. 2008, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doz E, Rose S, Nigou J, Gilleron M, Puzo G, Erard F, Ryffel B, Quesniaux VF. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J Biol Chem. 2007;282(36):26014–25. doi: 10.1074/jbc.M702690200. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155(15):1649–54. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- Frost RA, Nystrom G, Burrows PV, Lang CH. Temporal differences in the ability of ethanol to modulate endotoxin-induced increases in inflammatory cytokines in muscle under in vivo conditions. Alcohol Clin Exp Res. 2005;29(7):1247–56. doi: 10.1097/01.alc.0000171935.06914.5d. [DOI] [PubMed] [Google Scholar]
- Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174(1):456–63. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- Gruenert DC, Basbaum CB, Welsh MJ, Li M, Finkbeiner WE, Nadel JA. Characterization of human tracheal epithelial cells transformed by an origin-defective simian virus 40. Proc Natl Acad Sci U S A. 1988;85(16):5951–5. doi: 10.1073/pnas.85.16.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzenetter MD, Novotny AR, Gais P, Molina CA, Altmayr F, Holzmann B. Negative regulation of TLR responses by the neuropeptide CGRP is mediated by the transcriptional repressor ICER. J Immunol. 2007;179(1):607–15. doi: 10.4049/jimmunol.179.1.607. [DOI] [PubMed] [Google Scholar]
- Jalleh R, Fitzpatrick MF, Jan MA, MacNee W, Douglas NJ. Alcohol and cor pulmonale in chronic bronchitis and emphysema. Bmj. 1993;306(6874):374. doi: 10.1136/bmj.306.6874.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann A, Musset B, Limberg SH, Renigunta V, Sus R, Dalpke AH, Heeg KM, Robaye B, Hanley PJ. “Host tissue damage” signal ATP promotes non-directional migration and negatively regulates toll-like receptor signaling in human monocytes. J Biol Chem. 2005;280(37):32459–67. doi: 10.1074/jbc.M505301200. [DOI] [PubMed] [Google Scholar]
- Kinsner A, Boveri M, Hareng L, Brown GC, Coecke S, Hartung T, Bal-Price A. Highly purified lipoteichoic acid induced pro-inflammatory signalling in primary culture of rat microglia through Toll-like receptor 2: selective potentiation of nitric oxide production by muramyl dipeptide. J Neurochem. 2006;99(2):596–607. doi: 10.1111/j.1471-4159.2006.04085.x. [DOI] [PubMed] [Google Scholar]
- Li D, Shirakami G, Zhan X, Johns RA. Regulation of ciliary beat frequency by the nitric oxide-cyclic guanosine monophosphate signaling pathway in rat airway epithelial cells. Am J Respir Cell Mol Biol. 2000;23(2):175–81. doi: 10.1165/ajrcmb.23.2.4022. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marcato LG, Ferlini AP, Bonfim RC, Ramos-Jorge ML, Ropert C, Afonso LF, Vieira LQ, Sobrinho AP. The role of Toll-like receptors 2 and 4 on reactive oxygen species and nitric oxide production by macrophage cells stimulated with root canal pathogens. Oral Microbiol Immunol. 2008;23(5):353–9. doi: 10.1111/j.1399-302X.2008.00432.x. [DOI] [PubMed] [Google Scholar]
- Masamune A, Kikuta K, Watanabe T, Satoh K, Satoh A, Shimosegawa T. Pancreatic stellate cells express Toll-like receptors. J Gastroenterol. 2008;43(5):352–62. doi: 10.1007/s00535-008-2162-0. [DOI] [PubMed] [Google Scholar]
- Moss M, Burnham EL. Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit Care Med. 2003;31(4 Suppl):S207–12. doi: 10.1097/01.CCM.0000057845.77458.25. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176(12):7628–35. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Oleru UG. Pulmonary impairment in a cotton textile factory in Nigeria: is lifetime alcohol intake with low cigarette smoking a confounding factor? Arch Environ Health. 1987;42(4):197–203. [PubMed] [Google Scholar]
- Phulwani NK, Esen N, Syed MM, Kielian T. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol. 2008;181(6):3841–9. doi: 10.4049/jimmunol.181.6.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Ethanol suppresses cytokine responses induced through Toll-like receptors as well as innate resistance to Escherichia coli in a mouse model for binge drinking. Alcohol. 2004;33(2):147–55. doi: 10.1016/j.alcohol.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Ryu YH, Baik JE, Yang JS, Kang SS, Im J, Yun CH, Kim DW, Lee K, Chung DK, Ju HR, Han SH. Differential immunostimulatory effects of Gram-positive bacteria due to their lipoteichoic acids. Int Immunopharmacol. 2009;9(1):127–33. doi: 10.1016/j.intimp.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Saitz R, Ghali WA, Moskowitz MA. The impact of alcohol-related diagnoses on pneumonia outcomes. Arch Intern Med. 1997;157(13):1446–52. [PubMed] [Google Scholar]
- Sisson JH. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am J Physiol. 1995;268(4 Pt 1):L596–600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- Sosroseno W, Bird PS, Seymour GJ. Nitric oxide production by a human osteoblast cell line stimulated with Aggregatibacter actinomycetemcomitans lipopolysaccharide. Oral Microbiol Immunol. 2009;24(1):50–5. doi: 10.1111/j.1399-302X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- Spurzem JR, Veys T, Devasure J, Sisson JH, Wyatt TA. Ethanol treatment reduces bovine bronchial epithelial cell migration. Alcohol Clin Exp Res. 2005;29(4):485–92. doi: 10.1097/01.alc.0000158830.21657.bb. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Marletta MA. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985;82(22):7738–42. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suadicani P, Hein HO, Meyer HW, Gyntelberg F. Exposure to cold and draught, alcohol consumption, and the NS-phenotype are associated with chronic bronchitis: an epidemiological investigation of 3387 men aged 53-75 years: the Copenhagen Male Study. Occup Environ Med. 2001;58(3):160–4. doi: 10.1136/oem.58.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno K, Kato K, Atsumi T, Suzuki T, Yoshitake J, Morita H, Ohara S, Kotake Y, Shimosegawa T, Yoshimura T. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1004–12. doi: 10.1152/ajpgi.00096.2007. [DOI] [PubMed] [Google Scholar]
- Wu HS, Zhang L, Chen Y, Guo XJ, Wang L, Xu JB, Wang CY, Zhang JH. Effect of nitric oxide on toll-like receptor 2 and 4 gene expression in rats with acute lung injury complicated by acute hemorrhage necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int. 2005;4(4):609–13. [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol. 2003;163(3):1157–66. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275(4 Pt 1):L827–35. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- Zhao YT, Guo JH, Wu ZL, Xiong Y, Zhou WL. Innate immune responses of epididymal epithelial cells to Staphylococcus aureus infection. Immunol Lett. 2008;119(1-2):84–90. doi: 10.1016/j.imlet.2008.05.002. [DOI] [PubMed] [Google Scholar]