Abstract
The human heart is the first organ to develop during embryogenesis and is arguably the most essential organ for life. However, after birth, the heart has very little capacity to repair malformations such as congenital heart defects or to regenerate after an injury such as myocardial infarction. Cardiac tissue engineering addresses the need for a therapeutic biologic implant to restore cardiac structure and muscle mass. This review highlights current research in cardiac tissue engineering that uses human cardiomyocytes derived from embryonic stem cells. Other human cell sources are discussed because future human therapies will benefit from novel techniques using human-induced pluripotent stem cells and cardiomyocytes derived from direct reprogramming of somatic cells. Furthermore, this review examines the main approaches to creating engineered cardiac tissue with synthetic scaffolds, natural scaffolds, or no exogenous scaffold (i.e., “scaffold free”). The choice of scaffold and cells ultimately depends on the goals of the therapy, so the review considers how congenital heart defects define the design parameters for cardiac tissue engineering needed for surgical repair in pediatric cardiac patients.
Keywords: Cardiac tissue engineering, Human cardiomyocytes, Pluripotent stem cells, Therapeutic biologic implant
The field of cardiac tissue engineering has been working toward the development of biologic, cell-based therapies using knowledge from basic science research with the ultimate aim of regenerating cardiac muscle to repair the human heart. The different types of engineered cardiac tissue that exist are being tested in injury models. Most commonly, these are models of myocardial infarction due to the prevalence and global expansion of ischemic heart disease. Pediatric cardiac patients also will benefit from the development of engineered cardiac tissue to repair congenital heart defects such as ventricular and atrial septal defects, hypoplastic left heart, and the like.
Cells are the essential component of engineered tissues because the cell phenotype determines tissue function. In general, designing an engineered tissue requires creating a scaffold, incorporating the cell type or types of choice, and culturing the “tissue” in vitro, often using a bioreactor to provide perfusion or electromechanical stimulation before implantation as a therapeutic (Fig. 1).
Fig. 1.
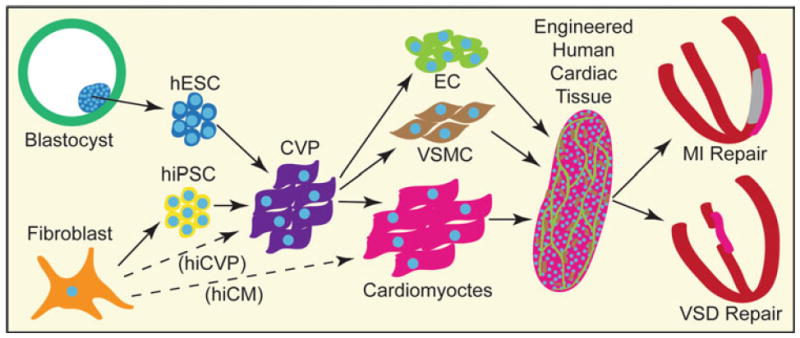
Human cardiac tissue engineering. Cell sources for human cardiac tissue engineering that are unquestionably cardiogenic include human embryonic stem cells (hESC) derived from the inner cell mass of the blastocyst and adult somatic cells (e.g., fibroblasts) reprogrammed into human-induced pluripotent stem cells (hiPSC). In the future, human-induced cardiomyocytes (hiCM) or cardiovascular progenitors (hiCVP) may become available. Directed differentiation of hESCs and hiPSCs mimics embryonic development, creates an intermediate and transient cardiovascular progenitor (CVP) population, and yields high numbers of cardiomyocytes, vascular smooth muscle cells (VSMC), and endothelial cells (EC). Human cardiomyocytes can be assembled into an engineered tissue using various methods. They can then be used for repair of the heart due to myocardial infarction (MI) or congenital heart defects such as ventricular septal defects (VSDs)
The scaffolds used for cardiac tissue engineering have evolved in recent years to include biodegradable synthetic polymers [4, 12, 14], natural matrix proteins [2, 21, 23, 37], and “scaffold-free” approaches that contain extracellular matrix produced only by the cells themselves [15, 25, 26]. Cell sourcing has included a broad range of cell types, from noncardiomyocytes or cardiomyocytes alone to (most recently) mixed populations of cardiomyocytes and vascular cells.
Because the upper limit of human cardiomyocyte proliferation appears to be approximately 1% per year at the age of 25 years (declining with age; [1]), the heart is unable to replace damaged or malformed myocardium naturally. This low proliferation rate of cardiomyocytes limits cell numbers, plasticity of cardiac tissue, and cell availability as a clinically relevant source. Recent work to derive cardiomyocytes from self-renewing human embryonic stem cells (hESCs) and human-induced pluripotent stem cells (hiPSCs; genetically reprogrammed from somatic cells) has moved research toward clinical application and shifted the focus away from nonhuman species (e.g., chicken and rat cardiomyocytes) [24].
The differentiation and maturation of hESC- and hiPSC-derived cardiomyocytes may be promoted by bioreactors, which may incorporate solution flow, electrical stimulation, or mechanical stretch. Although recent years have seen many advances in cardiac tissue engineering, a number of challenges remain including cell sourcing, matrix composition, construct dimensions, vascularization, electromechanical coupling, and bioreactor design (reviewed in reference [31]).
The promise of a human biologic implant for the heart continues to drive the development of engineered human cardiac tissue for heart repair due to ischemic injury or congenital heart defects. The presentation of these diseases in the clinic provides the basis for formulating design criteria, which include optimizing tissue construct strength, compliance, thickness, vascularization, and integration of electrical activation and contractility. Multiple approaches to meet these criteria exist in cardiac tissue engineering (reviewed in reference [36], with implications for pediatric heart surgery).
The goals of this review are to highlight current research aimed at deriving human cardiomyocytes from renewable cell sources and to describe the use of cardiomyocytes in cardiac tissue engineering. We discuss considerations for human cardiac tissue engineering in cardiac repair for patients with congenital heart defects and future challenges for cardiac tissue engineering in regenerative medicine.
Human Cell Sources
Cardiac tissue engineering, of course, requires cardiomyocytes for creating heart muscle, and the field began publishing work with human cardiomyocytes derived from hESCs in 2007 [4]. Cardiomyocytes from neonatal rat hearts, previously the leading cell type applied in engineered constructs, still are used because they are easily harvested and applied. However, no clinically relevant equivalent exists for this cell, and thus its usefulness is limited to the research arena. Direct cell transplantation for heart repair after myocardial infarction has a longer history of moving toward the clinic, and in doing so has used both human cardiac and noncardiac cell types in preclinical and clinical studies, respectively.
Numerous studies have used hESC-derived cardiomyocytes for cell transplantation in rodent models of myocardial infarction and have shown a preservation of cardiac function [6, 9-11, 30], suggesting that hESC-derived cardiomyocytes may be capable of remuscularizing the heart. Limitations of these studies include small graft size and imprecise injection locations of hESC-derived cardiomyocytes—two concerns addressed with cardiac tissue engineering.
The unquestionable cardiogenic potential of hESCs has spurred much excitement, and current practices of differentiating human pluripotent stem cells into cardiomyocytes is founded in work using hESCs [24]. Three directed differentiation schemes have been described (reviewed in reference [24]): (1) high-density monolayer directed differentiation with activin A and BMP4 developed by Laflamme and colleagues; (2) embryoid body-based directed differentiation with activin A, BMP4, the Wnt signaling inhibitor DKK1, and vascular endothelial growth factor (VEGF) developed by Keller and colleagues; and (3) co-culture of hESCs with the mouse visceral endoderm-like cells (END-2 cell line) developed by Mummery and colleagues.
Cardiomyocyte purities range from 20% to 70% with these protocols, and density gradients or selection can yield greater than 90% purity. However, limitations to the broad clinical application of hESC-derived cardiomyocytes include the ethics debate surrounding the derivation of hESCs from human embryos, the risk of contaminating cell types, tumor formation, and the need for immune suppression that accompanies transplantation of a nonautologous cell type. Creation of hESC banks with defined human leukocyte antigen (HLA) types may be a way to tailor cell therapy to individuals (without the need for autologous cells) and to reduce the need for immune suppression [28].
In addition to hESCs, both hiPSCs and induced cardiomyocytes (iCMs, see later) are renewable sources of human cardiomyocytes that could potentially be used for cardiac tissue engineering (Fig. 1). The discovery that pluripotency can be induced from human somatic cells using viral transfection of four transcription factors (either Oct4, Sox2, Klf4, c-Myc or Oct4, Sox2, Nanog, Lin28) to create hiPSCs has revolutionized stem cell research [13, 20, 27, 29, 32]. Not only do hiPSCs eliminate ethical concerns surrounding the destruction of human embryos, but hiPSCs also may address the need for an autologous cell source for clinical use.
New issues arise with the use of hiPSCs. First, the time required to derive these cells may not be compatible with their autologous use in the clinic. Second, the use of transgenes is not compatible with clinical use, although reprogramming with alternative methods is underway [24]. Finally, the cost of donor-specific hiPSC generation and differentiation may be prohibitive.
The derivation of human cardiomyocytes from hiPSCs has been accomplished using high-density monolayer directed differentiation (in our studies [unpublished results] and those of others [24, 27]), embryoid body-based directed differentiation [3], and embryoid bodies with serum induction [16, 33]. Characterization of hiPSC-derived cardiomyocytes suggest they have functional properties comparable with those of hESC-derived cardiomyocytes [33], although further studies are needed to improve the efficiency of cardiomyocyte generation in various hiPSC lines (as is true with different hESC lines).
Recently, Ieda et al. [7] reported the remarkable finding that fibroblasts from mouse heart or skin can be reprogrammed directly to a cardiomyocyte phenotype using three transcription factors (Gata4, Mef2c, and Tbx5) known to play key roles in cardiac development. These “induced cardiomyocytes” (iCMs) resemble neonatal mouse cardiomyocytes in terms of microarray gene expression patterns, formation of sarcomeres, propagation of action potentials, and synchronous beating, suggesting that pluripotent cells may not be required for producing cardiomyocytes in large numbers. Future studies likely will involve reprogramming of human fibroblasts into cardiomyocytes or tri-potential cardiovascular progenitors that can differentiate into cardiomyocytes, endothelial cells, and vascular smooth muscle cells for therapeutic use.
Engineered Human Cardiac Tissue
The process of assembling cardiac tissue in vitro has assumed different forms, from the development of complex synthetic biomaterial scaffolds with defined geometries [14] to the simple self-assembly of cells [15, 26]. Each method is valued for the lessons learned about tissue assembly. Furthermore, the field has collectively defined the characteristics and functions required for engineered cardiac tissue.
Studies currently are moving toward assessment of in vivo function, particularly with implantation in rodent models of myocardial infarction. Large animal studies will be important for the development of delivery techniques, assessment of efficacy, and identification of potential complications in larger, slower hearts on the path toward clinical trials. With these targets in mind, it is useful to categorize current research based on the scaffold used to form the engineered cardiac tissue, namely, the synthetic scaffold, the natural scaffold, or no scaffold (using only cells and their secreted extracellular matrix).
The first human cardiac tissue was engineered with a synthetic scaffold of 50% poly l-lactic acid and 50% polylactic glycolic acid in 2007 [4]. These two polymers are biodegradable, biocompatible, and already Food and Drug Act (FDA) approved because of their use in sutures and other medical devices. This suggests that these porous, sponge-like scaffolds could be more easily moved into the clinic.
Caspi et al. [4] seeded scaffolds (~3 × 3 × 1 mm3) using Matrigel (a mouse-derived basement membrane protein gel, BD Biosciences, Franklin Lakes, NJ) with 0.4 × 106 hESC-derived cardiomyocytes alone, in biculture with endothelial cells (ECs; 1:1 ratio) or a triculture with ECs and mouse embryonic fibroblasts (1:1:1 or 1:1:0.5 ratio). Histologic assessment of the in vitro constructs suggested that triculture tissues support the highest cardiomyocyte and EC proliferation rates as well as the greatest formation of vessel-like structures. Furthermore, confocal line scanning with a Ca2+ indicator dye demonstrated synchronous Ca2+ waves across adjacent cardiomyocytes in the scaffolds, suggesting electrical coupling between neighboring cardiomyocytes.
In a follow-up study, Lesman et al. [12] implanted the triculture constructs into an uninjured rat heart and demonstrated the presence of cardiomyocytes, human lumens, and some perfusion of the implant after 2 weeks. However, low cell density in this scaffold and the use of Matrigel for seeding the cells preclude the therapeutic applicability of these constructs.
Our group recently participated in a study using a different synthetic hydrogel, Poly(2-hydroxyethyl methacrylate-co-methacrylic acid (pHEMA-co-MAA) (similar to soft contact lens material), patterned with pores to promote vascular in-growth and channels to promote aligned muscle fibers [14]. When acellular scaffolds were implanted into the heart, the host angiogenic and fibrotic responses varied as a function of pore size. Pores 35 μm in size were optimal, coinciding with maximal vascular density and minimal scar capsule. Pore size also controlled the phenotype of infiltrating macrophages, leading us to hypothesize that the scaffold–macrophage interactions mediate the stromal and vascular responses. Degradable versions of these scaffolds currently are being developed and seeded with hESC-derived cardiomyocytes.
Natural scaffold materials for cardiac tissue engineering have found favor in a number of models because they are compatible with cell adhesion, can be remodeled by the cells, and are biocompatible. Zimmermann et al. [17, 37] have led this area of cardiac tissue engineering since 2000 using a collagen gel matrix mixed with cells (currently rat neonatal cardiomyocytes) and formed into rings and other shapes in molds. Mechanical stretching of these constructs has proved to be beneficial for cardiomyocyte alignment and force generation, and new methods have been developed to eliminate serum and Matrigel from their protocols to facilitate future translation to the clinic [17].
Our group is using a similar collagen gel-based method with hESC-derived cardiomyocytes and vascular cells to examine cardiomyocyte and tissue properties (unpublished data). Because these natural scaffolds are formed from gels in macroscopic molds, there is less control over the microscopic geometry. However, increased cell density throughout these constructs is a major benefit, and ongoing work is creating microstructures within natural hydrogels [2].
In an effort to create a whole heart with a natural scaffold, Ott et al. [19] decellularized a rat heart with detergent, maintaining a fully intact extracellular matrix and eliminating all cellular content. When rat neonatal cardiomyocytes were reseeded into the matrix and cultured for 8 days in a bioreactor to simulate cardiac pump function, the heart was able to produce 2% of adult rat heart function according to ex vivo assessment. This study suggests that recreating a micro- and macro-scale geometry that mimics the native heart may be essential for engineering cardiac tissue or a whole heart.
In an effort to maximize the cellularity of engineered cardiac tissue, two approaches have been developed using only cells and no exogenous scaffold. One method, pioneered by Miyagawa et al. [15], has created sheets of cells using tissue culture dishes coated with poly(N-isopropylacrylamide), a temperature-sensitive polymer that releases the confluent cells as a sheet from its surface when the temperature is reduced from 37°C to 20°C. Thin sheets (50 μm) of rat neonatal cardiomyocytes have been layered and transplanted into infarcted rat hearts and shown to improve cardiac function up to 8 weeks [15]. Our group has been able to create sheets with hESC-derived cardiomyocytes using this method (unpublished data). This simple technology shows much promise for the scaffold-free approach, including the ability to control tissue surface area, although the delicate nature of these cell sheets may require a sturdy substrate for surgical implementation in humans.
Our group has developed another scaffold-free method, and the use of hESC-derived cardiomyocytes makes it the first scaffold-free engineered human cardiac tissue [26]. With our method, hESC-derived cardiomyocytes are dispersed and put into suspension culture on an orbital shaker. After 2 days, the cells adhere to one another and form disk-shaped “patches” of cardiac tissue approximately 400 μm thick with a diameter controlled by the input number of cells (Fig. 2a). The human cardiomyocytes proliferate at a rate exceeding 5% in 2 days, with proliferation diminishing to 1% by 11 days [26]. Cardiomyocyte function is maintained, as shown by visual beating of tissue patches, Ca2+ transients that propagate across the tissue and are blocked by heptanol (a gap junction blocker), and stimulated contractions up to about 3 Hz (Fig. 2b, c). Multiple cell types can be mixed when the patches are first formed, enabling the use of triculture with cardiomyocytes, endothelial cells, and stromal/fibroblastic cells. When these three cell types are used, vascular-like structures readily form by self-assembly (Fig. 3a).
Fig. 2.
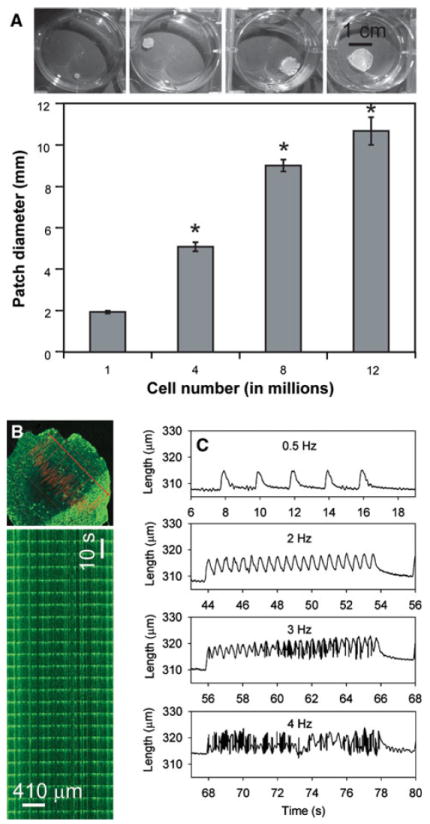
Cardiomyocytes derived from human embryonic stem cells (hESCs) form scaffold-free tissue patches with appropriate electromechanical properties. a The input cell number controls the diameter of patches (*p < 0.05). Images show patches after 2 days of culture formed from 1, 4, 8, and 12 million cells. b Waves of spontaneous Ca2+ activation (shown by fluo-4 fluorescence) propagate across a cardiac patch. A line scan taken with confocal microscopy demonstrates synchronous, regular Ca2+ waves. c Stimulated contractions measured with video edge detection at 0.5, 2, 3, and 4 Hz show that human cardiac patches can follow pacing rates up to approximately 3 Hz (or 180 bpm). Modified from references [25] and [26]
Fig. 3.
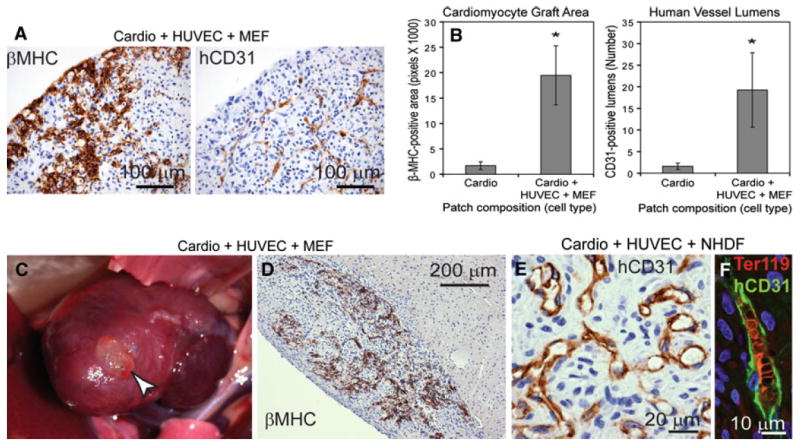
Triculture patches with human embryonic stem cell (hESC)-derived cardiomyocytes, human umbilical vein endothelial cells (HUVEC), and mouse embryonic fibroblasts (MEF) or neonatal human dermal fibroblasts (NHDF) form large, vascularized grafts. a After 8 days of in vitro culture, triculture patches show robust human cardiomyocytes by beta-myosin heavy chain (βMHC) staining and branching networks of endothelial cells by human CD31 (hCD31) staining. b In a hindlimb skeletal implant model of an athymic rat, the βMHC-positive graft area and hCD31-positive lumens both increase more than 10-fold in triculture versus cardio-only patches. c–f Implantation of triculture cardiac patches on uninjured athymic rat hearts (c) shows large βMHC-positive grafts closely apposed to the host (d) with numerous hCD31-positive lumens (e) that contain host red blood cells (marked by Ter119 [f]). Modified from reference [25]
Implantation of these engineered human cardiac tissue patches in the rat hind limb has shown more than a 10-fold increase in human cardiomyocyte graft size and more than a 10-fold increase in human lumens when vascular cells are included in the tissue patch versus a cardiomyocyte-only patch (Fig. 3b), demonstrating the importance of vascular cell co-culture in engineered cardiac patches. Importantly, when these “prevascularized” cardiac tissue patches are implanted on uninjured rat hearts (Fig. 3c, d), the large cardiac grafts develop bona fide human microvessels perfused with host red blood cells (Fig. 3e, f).
Considerations for Cardiac Repair in Pediatric Patients
With the most common cause of infant death in industrialized nations arising from congenital heart defects (29% of all infant deaths), there is good reason to address the needs of pediatric cardiac patients [5]. The most severe cardiovascular defects require surgery, often within the first weeks or months of life. The most prevalent defect is the ventricular septal defect (VSD), followed by the atrial septal defect (ASD) and other malformations of the vasculature and ventricles. Large cardiac tissue constructs are required to correct malformations of the ventricles such as hypoplastic left heart syndrome or double-inlet left ventricle, as previously suggested [36].
Patients with congenital heart defects have unique needs that require tailored approaches. For example, in many cases, the small size of infants requires only a small implant, but because children grow rapidly, the engineered cardiac tissue also needs to grow. This may be possible because engineered cardiac tissue is a biologic solution (rather than a mesh or mechanical device of fixed size) in which cell proliferation, hypertrophy/maturation, and graft integration with the host are major goals in the field.
Integration with the host tissue also may facilitate appropriate electrical conductance, potentially avoiding arrhythmogenesis that can occur with implants that cannot form electrical connections to the host. Indeed, differentiation of hESCs into specific cardiomyocyte subtypes (e.g., ventricular, atrial, and pacemaker) currently is being investigated [35] and may eventually be able to create Purkinje fibers that would be useful in repairing VSDs. The choice of cell source also is very important because using autologous or HLA-matched human cells in the pediatric population may help to avoid or at least minimize life-long immune suppression. Furthermore, because an infant’s immune system is developing, the patient’s age at the time of repair should be considered. Finally, surgical implantation methods should be considered because designing tissues with properties amenable to implantation via catheter would minimize trauma and be a great advancement over open heart surgery in infants.
Future Challenges
Many challenges remain before engineered cardiac tissue can be translated to the clinic. In addition to the regulatory requirements, choice of cell source, and scaffold and cell composition, the field will need to address issues of cardiomyocyte growth and production (e.g., scalability and sterility), as well as surgical or catheter-based implementation. Large-scale production of human cardiomyocytes will require more sophisticated bioreactors with precise control over culture conditions such as oxygen levels and growth factors for directed cardiac differentiation, as are currently being developed [18]. Purity of the input cardiomyocyte or cardiovascular progenitor population will need careful control, particularly if differentiation from pluripotent stem cells and use of fluorescent reporters [8] or cell surface markers [22] proves to be beneficial for sorting the cell population of interest.
Limited cardiomyocyte proliferation will continue to be a challenge, which can be addressed in the choice of cell source. For example, immature cardiomyocytes (derived from hESCs or hiPSCs) retain some proliferative capacity and hypertrophy, with maturation potentially facilitating integration with the host and increased graft size after implantation. Also, controlling cardiomyocyte proliferation with gene therapy [34] may allow for controlled expansion of graft size as pediatric patients grow. Finally, tailoring engineered cardiac constructs for each type of repair (such as VSD, ASD, or large ventricular malformations) will require modulation of construct size, stiffness/durability, cardiomyocyte subtype, and implantation methods. The ultimate challenge will be to devise a biologic cardiac implant that improves patient outcomes compared with current surgical repair techniques for pediatric cardiac patients.
Conclusions
Although research in cardiac tissue engineering has focused primarily on restoring cardiac function after myocardial infarction, the principles for designing cardiac tissue can be tailored to address the needs of pediatric patients with congenital heart defects. Engineering of human cardiac tissue has taken multiple approaches including the use of synthetic scaffold materials, natural extracellular matrix proteins, and no exogenous scaffold. The rapid pace of discovery in stem cell research is facilitating the development of human cell sources for cardiomyocytes that include hESCs, hiPSCs, and potentially, human-induced cardiomyocytes. Culturing techniques may promote cardiomyocyte maturation (e.g., electrical and mechanical preconditioning) and proliferation (e.g., co-culture with endothelial cells and stromal cells), and the addition of vascular cells to engineered cardiac tissue both promotes in vivo vascularization and recapitulates the diversity of cells found in the heart.
Engineered cardiac tissue can be used for studying the basic biology of cell–cell and cell–matrix interactions (such as occurs with vascular formation) and for drug discovery and testing. Yet the primary goal of cardiac tissue engineering continues to be the development of an implantable tissue for use in surgical repair of the heart in cases of myocardial infarction and congenital heart defects.
Acknowledgments
We thank Dr. Lil Pabon and Sharon Paige for comments on the manuscript. We gratefully acknowledge funding support from National Institutes of Health grants R01HL084642, R01HL64387, and P01HL094374 (to C.E.M.), Experimental Pathology of Cardiovascular Disease Training Grant T32 HL007312 (to K.L.K.), and the UW’s Mouse Metabolic Phenotyping Center U24 DK076126.
Contributor Information
Kareen L. Kreutziger, Center for Cardiovascular Biology, Institute for Stem Cell and Regenerative Medicine, University of Washington, Box 358050, 815 Mercer Street, Brotman 454, Seattle, WA 98109, USA Department of Pathology, University of Washington, Box 357470, Seattle, WA 98195, USA.
Charles E. Murry, Email: murry@uw.edu, Department of Pathology, University of Washington, Box 357470, Seattle, WA 98195, USA; Center for Cardiovascular Biology, Institute for Stem Cell and Regenerative Medicine, University of Washington, Box 358050, 815 Mercer Street, Brotman 453, Seattle, WA 98109, USA; Department of Bioengineering, University of Washington, Box 355061, Seattle, WA 98195, USA; Department of Medicine/Cardiology, University of Washington, Box 356422, Seattle, WA 98195, USA.
References
- 1.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian W, Liau B, Badie N, Bursac N. Mesoscopic hydrogel molding to control the 3D geometry of bioartificial muscle tissues. Nat Protoc. 2009;4:1522–1534. doi: 10.1038/nprot.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465:808–812. doi: 10.1038/nature09005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 5.Congenital Cardiovascular Defects–Statistics (ICD/10 Q20-Q28; ICD/9 745-747) Statistical Fact Sheet–Miscellaneous/Disease 2010 Update. American Heart Association; Dallas, TX: [Google Scholar]
- 6.Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, Graichen RE, Kay GL, Jyrala AJ, Colman A, Davidson BP, Pera M, Kloner RA. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol. 2007;43:504–516. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kita-Matsuo H, Barcova M, Prigozhina N, Salomonis N, Wei K, Jacot JG, Nelson B, Spiering S, Haverslag R, Kim C, Talantova M, Bajpai R, Calzolari D, Terskikh A, McCulloch AD, Price JH, Conklin BR, Chen HS, Mercola M. Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes. PLoS One. 2009;4:e5046. doi: 10.1371/journal.pone.0005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kofidis T, Lebl DR, Swijnenburg RJ, Greeve JM, Klima U, Robbins RC. Allopurinol/uricase and ibuprofen enhance engraftment of cardiomyocyte-enriched human embryonic stem cells and improve cardiac function following myocardial injury. Eur J Cardiothorac Surg. 2006;29:50–55. doi: 10.1016/j.ejcts.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 11.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E, Itskovitz-Eldor J. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–1284. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesman A, Habib M, Caspi O, Gepstein A, Arbel G, Levenberg S, Gepstein L. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng Part A. 2010;16:115–125. doi: 10.1089/ten.TEA.2009.0130. [DOI] [PubMed] [Google Scholar]
- 13.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human-induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci USA. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyagawa S, Sawa Y, Sakakida S, Taketani S, Kondoh H, Memon IA, Imanishi Y, Shimizu T, Okano T, Matsuda H. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. Transplantation. 2005;80:1586–1595. doi: 10.1097/01.tp.0000181163.69108.dd. [DOI] [PubMed] [Google Scholar]
- 16.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A, Laugwitz KL. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med. 2010;363(15):1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 17.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation. 2006;114:I72–I78. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 18.Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 19.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 20.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, Ince TA, Lerou PH, Lensch MW, Daley GQ. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 21.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rust W, Balakrishnan T, Zweigerdt R. Cardiomyocyte enrichment from human embryonic stem cell cultures by selection of ALCAM surface expression. Regen Med. 2009;4:225–237. doi: 10.2217/17460751.4.2.225. [DOI] [PubMed] [Google Scholar]
- 23.Shachar M, Tsur-Gang O, Dvir T, Leor J, Cohen S. The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering. Acta Biomater. 2011;7(1):152–162. doi: 10.1016/j.actbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 24.Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Curr Pharm Des. 2009;15:2791–2806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA. 2009;106:16568–16573. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–2025. doi: 10.1016/S0140-6736(05)67813-0. [DOI] [PubMed] [Google Scholar]
- 29.Tulloch NL, Pabon L, Murry CE. Get with the (re)program: cardiovascular potential of skin-derived induced pluripotent stem cells. Circulation. 2008;118:472–475. doi: 10.1161/CIRCULATIONAHA.108.791442. [DOI] [PubMed] [Google Scholar]
- 30.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, den Ouden K, Ward-van Oostwaard D, Korving J, Tertoolen LG, van Echteld CJ, Doevendans PA, Mummery CL. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 2010;16:169–187. doi: 10.1089/ten.teb.2009.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human-induced pluripotent stem cells. Circ Res. 2009;104:e30–e41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Nuglozeh E, Toure F, Schmidt AM, Vunjak-Novakovic G. Controllable expansion of primary cardiomyocytes by reversible immortalization. Hum Gene Ther. 2009;20:1687–1696. doi: 10.1089/hum.2009.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu WZ, Xie Y, Moyes KW, Gold JD, Askari B, Laflamme MA. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.1110.223917. Published online 29 July. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmermann WH, Cesnjevar R. Cardiac tissue engineering: implications for pediatric heart surgery. Pediatr Cardiol. 2009;30:716–723. doi: 10.1007/s00246-009-9405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T. Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes. Biotechnol Bioeng. 2000;68:106–114. [PubMed] [Google Scholar]


