Abstract
Introduction
Anatomic anterior cruciate ligament (ACL) reconstruction has proven to be a reliable method to restore knee stability. However, the risk of physeal arrest with transphyseal tunnel placement in skeletally immature patients has raised concern regarding this technique. Conservative nonoperative management also has its limitations resulting in meniscal and chondral damage that may lead to degenerative joint disease and poor return to sport. Researchers have used animal models to study the threshold of physeal damage producing growth deformity. The purpose of this study was to examine the distal femoral and proximal tibial physes and determine the damage produced by drilling transphyseal tunnels. In addition, we attempted to find a reproducible angle at which to drill the tibial tunnel for safe interference screw placement. To do this, we used a custom software module.
Methods
A custom software package designed by our team was used: Module for Adolescent ACL Reconstructive Surgery (MAARS). This module created a 3-dimensional model of the distal femur and proximal tibia. The data required for MAARS were sagittal and coronal T1 magnetic resonance imagings of at least 1.5T. Thirty-one knee magnetic resonance imaging studies from patients aged 10 to 15 years old were used. The physes were segmented out to obtain volumetric measurements. Transphyseal tunnels were simulated based on the anatomic trajectory of the native ACL. The module calculated volume of physis was removed with the use of an 8-mm tunnel and the optimum angle for trajectory.
Results
Average volume of the tibial and femoral physis was 12,683.1 μL and 14,708.3 μL, respectively. The volume increased linearly with age. Average volume removed from the tibial and femoral physis was 318.4 μL and 306.29 μL, respectively. This represented 2.4% of the distal femoral physis and 2.5% of the proximal tibial physis. The volume percent removed decreased linearly with age.
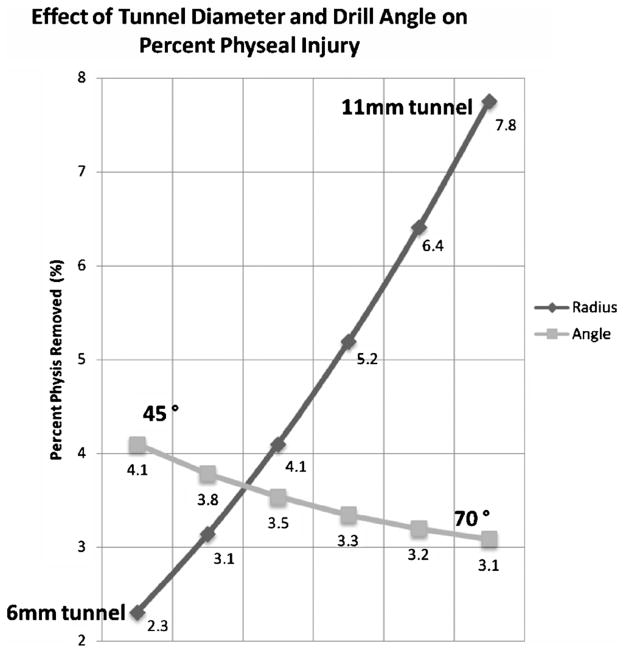
Manipulation of the variables demonstrates graft radius is the most critical parameter affecting the volume of physeal injury. Variation of graft diameter from 6 mm to 11 mm will increase volume percent removed from 2.3% to 7.8%, which averages 1.1% for every 1 mm increase. Increasing tunnel drill angle from 45 degrees to 70 degrees will decrease volume percent removed from 4.1% to 3.1% which averages 0.2% removed for each 5 degrees increase in drill angle. The average angle to maintain a distance of 20 mm from the proximal tibial physis was 65 degrees with a range of 40 degrees to 85 degrees.
Discussion
Less than 3% injury occurs when drilling an 8-mm tunnel across the physis. A vertical tunnel has minimal effect, but the tunnel diameter is critical. Interference screws can be placed safely to avoid the physis but requires careful planning. The MAARS module may be helpful in preoperative planning.
Level of Evidence
Diagnostic, level IV.
Keywords: anterior cruciate ligament, reconstruction, knee, physis, pediatrics, ACL, magnetic resonance imaging, MRI, 3-dimensional model
BACKGROUND
Heightened demands and increasing numbers of young athletes participating in organized sports have transcended into a growing number of sports related injuries. This is especially true for anterior cruciate ligament (ACL) injuries in adolescent athletes participating in high demand sports. Despite the increased awareness of this problem, many questions surround the optimal treatment algorithm for ACL reconstruction in these patients
Nonoperative management of skeletally immature patients with ACL rupture can result in persistent instability, meniscal damage, and early onset osteoarthritis; all of which lead to poor return to sport.1–6 Because success of non-operative management has been poor, more surgeons are choosing reconstructive techniques despite the risk of iatrogenic injury to the physes.
Drilling tunnels across an open physis during ACL reconstruction has the potential to cause premature closure leading to leg-length discrepancies and angular deformities. However, reports of these complications have been infrequent. Two reports in the literature,7,8 as well as a survey of the Herodicus Society and the ACL Study Group,9 have shown that these complications are more than theoretical, although have primarily resulted from technical error such as placing hardware across the physis.
Over the past decade, surgeons have developed partial transphyseal, 10,11 physeal sparing,12–14 as well as transepiphyseal, physeal sparing15 techniques to limit injury to the growth plate. Although these methods may prevent injury to the physis, the long-term results are unknown. Furthermore, it is believed that these techniques may not reproduce the normal kinematics of the knee.12,16–18 Transphyseal ACL reconstruction has an established track record. Several studies have shown that anatomic reconstruction using a soft tissue graft through a transphyseal tibial tunnel has not been shown to cause early physeal closure, limb-length discrepancy, or angular deformity.19–23
Many researchers have investigated damage to the physis with drill holes. Termed “threshold of injury,” investigators have tried to predict the amount of injury producing growth arrest. It has been found in animal studies that reducing damage to the physis to below 7% of the total physeal volume can prevent physeal closure.24,25 Guzzanti et al26 reported that in human patients, drill holes, which produced less than 6.6% injury to the total volume of the distal femoral physis, resulted in no negative sequela.
The purpose of this study was to examine the distal femoral and proximal tibial growth plates and determine the damage produced by drilling transphyseal tunnels during ACL reconstruction. In addition, we attempted to find a reproducible angle at which to drill the tibial tunnel so that interference screws could be placed to avoid the physis. To do this, we used a custom software module; Module for Adolescent ACL Reconstructive Surgery (MAARS). This software allowed us to recreate 3-dimensional (3-D) models of the knee using standard magnetic resonance imaging (MRI). From these images, the growth plates were segmented out to provide precise volumetric measurements of the distal femoral and proximal tibial growth plates. It was our hypothesis that injury to the growth plates would be less than threshold limits reported by previous researchers.
METHODS
Data and Segmentation
The data required for analysis using MAARS were sagittal and coronal T1 MRI scans of at least 1.5T with a sufficient view of the tibial and femoral physes, and the distal femur, proximal tibia, and the physes should be absent of pathology. After the Institutional Review Board approval, 31 MRI studies of adolescent knees were acquired from surrounding institutions. Radiology department records were reviewed to obtain a list of adolescents aged 10 to 15 years who had received a lower extremity MRI in the past 8 years. These MRIs were reviewed, and 31 studies were selected that included knee MRIs with both sagittal and coronal T1 images and absence of pathology. Of these 31 patients, 21 were female and 10 male. No other information regarding the patients was obtained. Identifiers were then removed from the studies.
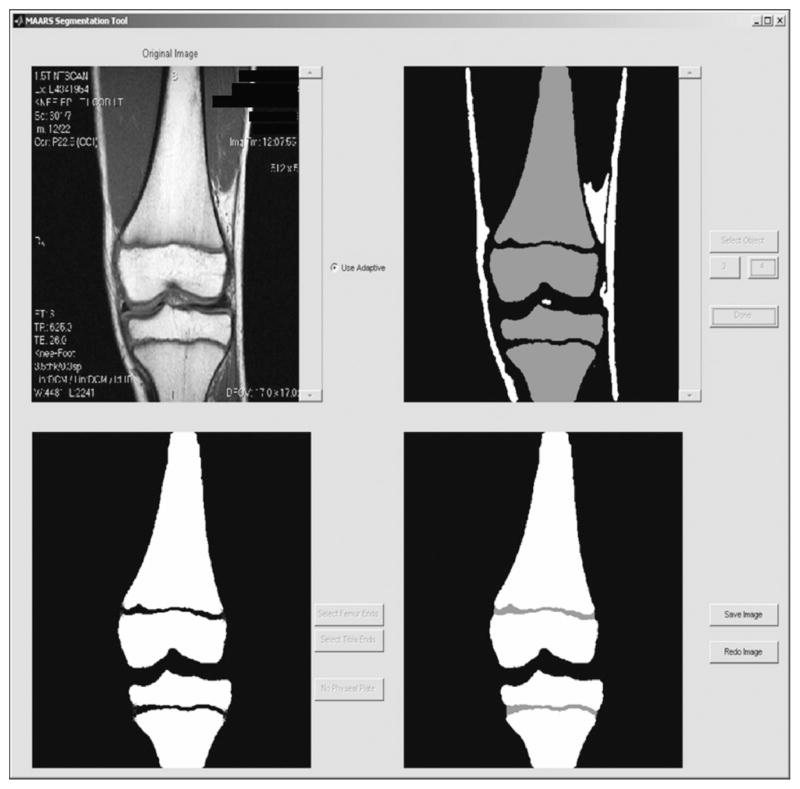
Our software used threshold-based segmentation. This technique filters pixels of similar gray scale intensity from standard MRI scans create homogeneous regions. The user first selects a rough threshold to remove the background of the scan. Then a second more precise threshold is then chosen to separate the bone and physis. To isolate the physes, the user labels the medial and lateral boundaries with a marker. The physis is then automatically closed by finding and connecting the corners of the respective surrounding bones using a shape-preserving piecewise cubic interpolation. In Figure 1, we indicate the starting and resultant image after the above steps have been performed. There does not appear to be much discrepancy in the segmentation between users, but to minimize any possible discrepancies, 2 specific team members focused on the segmentation and data processing. The segmentation interpolation program is able create a constant reliability between users.
FIGURE 1.
The segmentation process of the physes. Thresholds are used to create homogeneous regions. Once these regions are created, the data can be interpolated to create a 3-D model.
Data Preprocessing
After the data are segmented and appropriate label-maps created, a preprocessing stage is performed to create the necessary 3-D model in physical space. Thus, the following data must be specified: the sagittal and coronal mm/pixel ratio, the coronal scan order (anterior to posterior or posterior to anterior), and the desired scaling ratio. This scaling ratio will determine the final size of the images before interpolation. The users of this program were familiar with knee MRI anatomy and were able to accurately perform the manual preprocessing. Next, the volume is linearly interpolated on its slice axis to create cubic physical voxels (ie, the voxels have the same physical dimension on all sides). Finally, the volume is smoothed using a 3-D Gaussian filter with a kernel size of 9 × 9 × 9. Figures 2A and B are examples of 3-D images produced from MAARS.
FIGURE 2.
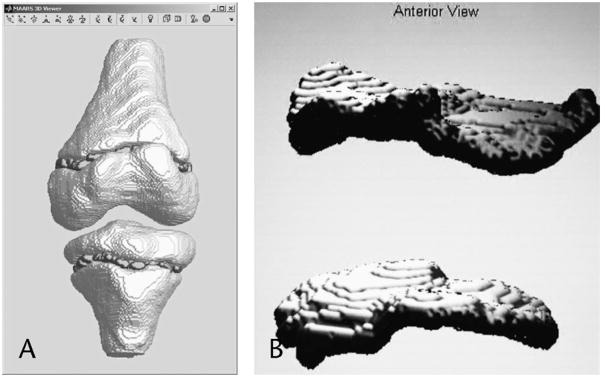
(A and B) 3-D model of the knee, distal femoral, and proximal tibial physes created with the MARRS module.
ACL Anatomical Selections
In order to recreate the anatomic trajectory of the native ACL, the user cycles through the scans and places a marker centrally in the native ACL footprint in the coronal axis. A second marker in the coronal axis is then placed on any physical point containing the central ACL. These 2 points are then combined to designate the angle of the ACL in the coronal plane.
Of importance is the angle of the ACL in the sagittal plane. This must be measured with respect to the tibia plateau, not the horizontal of the image. Thus, a tangent line is drawn with respect to the tibia plateau in this axis to identifying this plane. All sagittal angle measurements are then measured relative to this plane. Finally, 2 points on the ACL sagittal footprint are identified to specify the angle of the ACL in the sagittal plate, again measured with respect to the reference plane determined earlier. Figure 3 demonstrates a screenshot of the ACL anatomical selection portion of the MAARS module. After selection of these points, the module will reconstitute the intraarticular trajectory of the ACL.
FIGURE 3.
Screenshot of the ACL anatomical selection portion of MAARS.
MAARS 3-D Viewer and Main Module
From the volume created in the preprocessing phase, the surface of the bone and physes was extracted using the marching cubes algorithm. This surface was used for visualization and selection of surface anatomical points. Next, the intraarticular tibial anchor point selected earlier must be projected to the surface of the distal tibial cortex. This represents the starting point of the tibial tunnel.
The optimal trajectory for the tibial tunnel was determined using an algorithm to satisfy input parameters set by the user. The optimal trajectory is the sagittal angle, which is to be used for drilling the tibial tunnel. The following criteria are considered “optimal”: first, the tunnel must be of sufficient length to allow interference screw fixation that would not violate the physis; and second, the surgical path must be as anatomically close to the true ACL as possible. Tunnel length was specified by input parameters and is adjustable. For example, if a 20-mm tunnel is required for interference fixation, the algorithm will satisfy this by determining a route that will allow 20 mm of bone tunnel before the physis is contacted.
The algorithm uses the point of projection from the native ACL onto the distal tibial cortex as a starting point. The point then moves out radially until the criteria is met. While the tibial tunnel entry point moves distally from physis, more space becomes available for the interference screw and the sagittal angle increases. The radial movement allows the entry point to move along 2 dimensions of the surface, thus placing the anatomically closer points at a higher priority.
Finally, to complete the tunnel route, the femoral exit portal was selected on the surface of the femur. No optimal tunnel trajectory algorithm was used for the femoral side because of the assumption that interference fixation would not be used. However, femoral tunnel exit was manually selected to allow for sufficient closed loop fixation. Using a fixed input graft radius, a volume was then created surrounding the trajectory of the tunnel that was within the substance of the physis. Figure 4 demonstrates the tunnel with fixed input radius generated around the simulated trajectory. This volume was then divided by the total physeal volume to give the percent of the physis removed.
FIGURE 4.
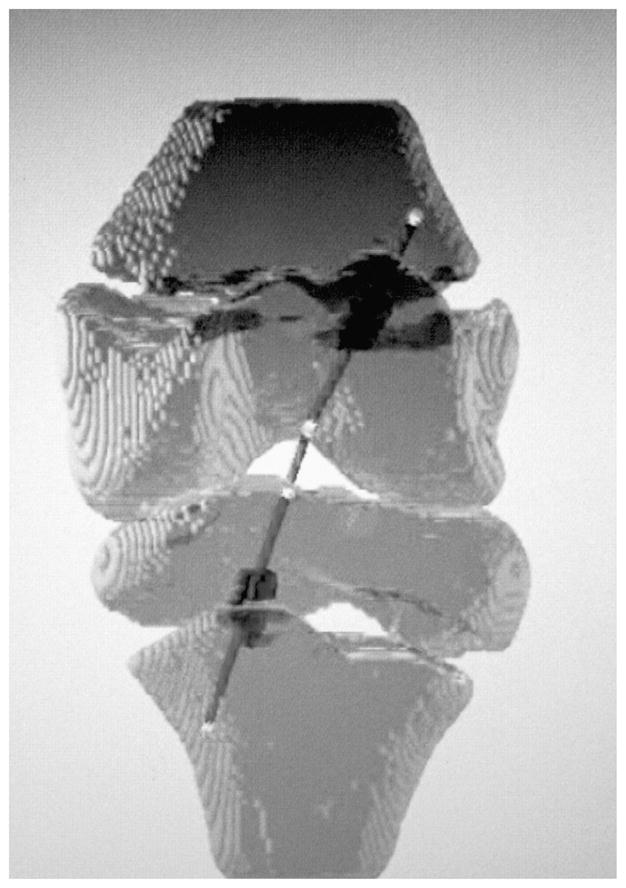
Projection of the knee with simulated tunnel trajectories. The volume with fixed radius generated around the intraphyseal portion of the tunnels.
RESULTS
The average volume of the tibial physis was 12,683.1 μL with a range of 7080 μL to 29,739 μL. The average volume of the femoral physis was 14,708.3 μL with a range of 8296.3 μL to 23,893 μL. The average volume removed from the tibial physis was 318.4 μL, and the average volume removed from the femoral physis was 306.29 μL. This represented 2.4% of the distal femoral physis and 2.5% of the proximal tibial physis. The total volumes increased linearly with age as shown in Figure 5, and the volume percent removed decreased linearly with age as demonstrated in Figure 6.
FIGURE 5.
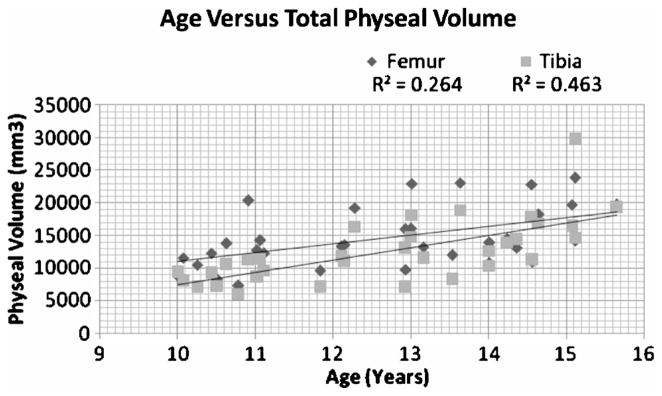
Graph depicting the linear relationship of physeal volumes to age.
FIGURE 6.
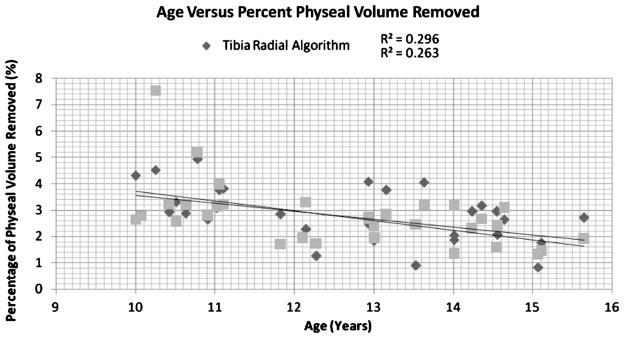
Graph depicting the relationship of age to physeal volume removed.
Manipulation of the variables demonstrates graft radius is the most critical parameter affecting the volume of physeal injury, as volume increases by the square of the radius (r2). Variation of graft diameter from 6 mm to 11 mm will increase volume percent removed from 2.3% to 7.8%. This is not linear, however, on average the volume percent removed by increasing graft diameter by 1 mm is 1.1%. Increasing tunnel drill angle from 45 degrees to 70 degrees will decrease volume percent removed from 4.1% to 3.1%, this is also not linear, however averages to a 0.2% decrease in volume removed for each 5 degrees increase in drill angle. This relationship is shown in Figure 7.
FIGURE 7.
Manipulation of the variables demonstrates graft diameter is the most critical parameter affecting the volume of physeal injury. The volume of a cylinder increases by the square of the r2 and the height is a function of the angle at which the graft crosses the physis (1/sin θ). sin θ varies from 0 to 1 as you increase to 90 degrees.
The average sagittal angle to maintain a distance of 20 mm from the proximal tibial physis was 65 degrees with a range of 40 degrees to 85 degrees.
DISCUSSION AND CONCLUSIONS
Several studies have used segmentation and data interpolation for 3-D reconstructions of the physes about the knee.27–31 However, few have examined growth plate volumes in the context of ACL reconstruction. Craig et al29 used 3-D MRI to model and estimate volume and surface area of the distal femoral and proximal tibial physes. They studied 14 patients using a large age range of 3.8 to 15.6 years old. Femoral physeal volume was reported to vary from 2.1 cm3 to 12.6 cm3, and tibial volume varied from 1.9 cm3 to 13.2 cm3. By collapsing 3-D images into 2-dimensional images, the surface areas were also estimated.
In our study, we found physeal volumes to be similar, averaging 14.7 cm3 for the femur and 12.7 cm3 for the tibia. The volumes obtained in our study were somewhat larger, but likely because our population contained patients focused in the adolescent age range. It was also found that the volumes increased linearly with age which was consistent with the findings of Craig et al.29 Seemingly counterintuitive, this finding can be explained by the increasing width of the physis. Because volume is dependent on r2, volume of the growth plate will still increase in the face of decreasing height.
Surface area was not calculated in our study. It is likely that the measure is potentially useful for predicting physeal quantity as each is related to the height and radius of the physis. Craig et al29 stated in their article that decreased variability in their measured values for surface area made this a more predictable estimate of physeal quantity. We feel that this may not be the case as the authors removed the inherent variability from the 3-D physes by collapsing data from their images into 2 dimensions. In addition, predictable measurement of the percent damage to the physis may be the most important factor pertaining to growth plate injury.
Many researchers have investigated physeal injury produced with drill holes. It has been suggested that the physis can tolerate a certain amount of destruction before provoking premature closure. This has been termed the “threshold of injury.”24–26,32
In 1988, Makela et al24 studied the effect of trauma to the lower physeal plate in rabbits. They placed 2 mm and 3.2 mm drill holes in the central portion of femoral growth plates in 2 groups of skeletally immature rabbits. At 3, 6, 12, and 24 weeks, specimens from the growth plates were analyzed using radiographic and histological techniques. It was found that destruction of 7% of the cross-sectional area of the growth plate resulted in growth disturbances of the femur.
Guzzanti et al32 performed intraarticular ACL reconstruction using semitendinosus tendons in 2 mm tunnels in 21 skeletally immature rabbits. Using computed tomography, they determined that 11% damage in the frontal plane and 3% injury of the cross-sectional area of the distal femoral physis resulted in no alterations of growth. However, in the tibia, 12% damage to the physis in the frontal plane and 4% of the cross-sectional area resulted in shortening and angular deformity. The authors noted the difficulty in determining the true area of the growth plate, but suggested a need for evaluating the percentage of physeal damage before using intraarticular methods for reconstruction of the anterior cruciate ligament in adolescents.
In a more recent paper, Janarv et al25 studied the influence of transphyseal drilling and tendon grafting in rabbits. They used a digital image analyzer to estimate the physeal area based on sequential histological sections. They reported that injury to 7% to 9% of the distal femoral physis resulted in growth retardation; however, injury to 4% to 5% of the physis was of no consequence. Interestingly, the authors’ mention the difficulty of measuring the area of the physis, but the drill injury formula given in the article represented an equation for calculating volume.
By simulating ACL drill tunnels crossing the physes with an 8-mm graft, we have found that the actual injury to the distal femoral physis averaged 306.3 μL, which represented 2.4% of the distal femoral physis. Injury to the proximal tibial physes averaged 318.4 μL, which was 2.5% of the total physeal volume. Guarino et al30 published an article on 3-D modeling of the proximal tibial physis. This article contained a single example of a model produced from an MRI of a patient of unknown age. They found that a “correctly” positioned 8 mm drill tunnel removed 221.02 μL from the proximal tibial physis, which represented 3.5% of the total volume. We have been unable to identify any other published data on physeal injury measurement using 3-D MRI data. Percent injury to the physes decreases linearly with age. Because the radius of the tunnel is fixed, we now see a reduction in volume because of the decreasing height of the physis. Based these findings, we believe it is likely that the actual injury produced from ACL reconstruction tunnels are below threshold values for physeal closure reported previously. This may provide an explanation for successful trans-physeal ACL reconstruction resulting in no negative sequala.
There is a growing body of evidence to suggest that with careful planning and particular technique, transphyseal anatomic reconstruction can be used safely. 19,20,23,33,34 Recently, Fuchs et al19 reviewed the results of 10 skeletally immature patients ranging from 9 to 15 years, and Shelbourne et al20 reported on 16 patients who were tanner stages 3 and 4. Both groups underwent transphyseal intraarticular ACL reconstruction using patellar tendon grafts without negative consequence. It was concluded that with meticulous attention to technique, transphyseal reconstruction could be successful in patients with open physes. In particular, care must be taken to avoid inadvertent placement of hardware across the physis as this type of error has led to the majority of reported complications.7–9
To examine the tibial tunnel relationship to the physis, the MAARS module simulated the tibial tunnel trajectory based on the anatomy of the native ACL and criteria set by the user. Our input criteria included a tunnel trajectory that would maintain a bony tunnel of 20 mm for the use of interference screw fixation. We found that our simulated tunnel trajectories averaged 65 degrees in the sagittal plane. The entrance for these tunnels was distal and slightly anterior to a point projected from the native ACL onto the distal tibial cortex. However, the angles ranged from 40 degrees to 85 degrees. This variability makes it difficult to predict a safe tunnel angle for interference screw fixation without careful preoperative planning or fluoroscopic assistance.
In summary, we have demonstrated with 3-D MRI reconstructions, there is significantly less injury to the physes than previously reported threshold values for premature physeal closure. A vertical tunnel angle will decrease physeal damage; however, tunnel radius has a much larger contribution to the zone of injury. Careful planning should be undertaken before the use of interference screw fixation to avoid placement across the physis. It must be noted that our determination of physeal volume removed from drill tunnels does not make any predictions on the actual threshold of injury.
Footnotes
None of the authors received financial support for this study.
References
- 1.Kannus P, Jarvinen M. Knee ligament injuries in adolescents: eight year follow-up of conservative management. J Bone Joint Surg Br. 1988;70:772–776. doi: 10.1302/0301-620X.70B5.3192578. [DOI] [PubMed] [Google Scholar]
- 2.Barrack RL, Bruckner JD, Kneisl J, et al. The outcome of nonoperatively treated complete tears of the anterior cruciate ligament in active young adults. Clin Orthop Relat Res. 1990;259:192–199. [PubMed] [Google Scholar]
- 3.Bonamo JJ, Fay C, Firestone T. The conservative treatment of the anterior cruciate deficient knee. Am J Sports Med. 1990;18:618–623. doi: 10.1177/036354659001800611. [DOI] [PubMed] [Google Scholar]
- 4.Mizuta H, Kubota K, Shiraishi M, et al. The conservative treatment of complete tears of the anterior cruciate ligament in skeletally immature patients. J Bone Joint Surg Br. 1995;77:890–894. [PubMed] [Google Scholar]
- 5.Behr CT, Potter HG, Paletta GA. The relationship of the femoral origin of the anterior cruciate ligament and the distal femoral physeal plate in the skeletally immature knee: an anatomic study. Am J Sports Med. 2001;29:781–787. doi: 10.1177/03635465010290061801. [DOI] [PubMed] [Google Scholar]
- 6.Aichroth PM, Patel DV, Zorilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents: a prospective review. J Bone Joint Surg Br. 2002;84:38–41. doi: 10.1302/0301-620x.84b1.11773. [DOI] [PubMed] [Google Scholar]
- 7.Lipscomb AB, Anderson AF. Tears of the anterior cruciate ligament in adolescents. J Bone Joint Surg Am. 1986;68:19–28. [PubMed] [Google Scholar]
- 8.Koman JD, Sanders JO. Valgus deformity after reconstruction of the anterior cruciate ligament in a skeletally immature patient: a case report. J Bone Joint Surg Am. 1999;81:711–715. doi: 10.2106/00004623-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Kocher MS, Saxon HS, Hovis WD, et al. Management and complications of anterior cruciate ligament injuries in skeletally immature patients: survey of the Herodicus Society and the ACL Study Group. J Pediatr Orthop. 2002;22:452–457. [PubMed] [Google Scholar]
- 10.Andrews M, Noyes FR, Barber-Westin SD, et al. Anterior cruciate ligament allograft reconstruction in the skeletally immature athlete. Am J Sports Med. 1994;22:48–54. doi: 10.1177/036354659402200109. [DOI] [PubMed] [Google Scholar]
- 11.Lo IK, Kirkley A, Fowler PJ, et al. The outcome of operatively treated anterior cruciate ligament disruptions in the skeletally immature child. Arthroscopy. 1997;13:627–634. doi: 10.1016/s0749-8063(97)90191-2. [DOI] [PubMed] [Google Scholar]
- 12.Brief LP. Anterior cruciate ligament reconstruction without drill holes. Arthroscopy. 1991;7:350–357. doi: 10.1016/0749-8063(91)90003-g. [DOI] [PubMed] [Google Scholar]
- 13.Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents. J Bone Joint Surg Am. 2005;87:2371–2379. doi: 10.2106/JBJS.D.02802. [DOI] [PubMed] [Google Scholar]
- 14.Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents: surgical technique. J Bone Joint Surg Am. 2006;88(Suppl 1 Pt 2):283–293. doi: 10.2106/JBJS.F.00441. [DOI] [PubMed] [Google Scholar]
- 15.Anderson AF. Transepiphyseal replacement of the anterior cruciate ligament using quadruple hamstring grafts in skeletally immature patients. J Bone Joint Surg Am. 2004;86-A(Suppl 1 Pt 2):201–209. doi: 10.2106/00004623-200409001-00010. [DOI] [PubMed] [Google Scholar]
- 16.Nottage WM, Matsuura PA. Management of complete traumatic anterior cruciate ligament tears in the skeletally immature patient: current concepts and review of the literature. Arthroscopy. 1994;10:569–573. doi: 10.1016/s0749-8063(05)80016-7. [DOI] [PubMed] [Google Scholar]
- 17.Stanitski CL. Anterior cruciate ligament injury in the skeletally immature patient: diagnosis and treatment. J Am Acad Orthop Surg. 1995;3:146–158. doi: 10.5435/00124635-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Ramaniraka NA, Saunier P, Siegrist O, et al. Biomechanical evaluation of intra-articular and extra-articular procedures in anterior cruciate ligament reconstruction: a finite element analysis. Clin Biomech (Bristol, Avon) 2007;22:336–343. doi: 10.1016/j.clinbiomech.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Fuchs R, Wheatley W, Uribe JW, et al. Intra-articular anterior cruciate ligament reconstruction using patellar tendon allograft in the skeletally immature patient. Arthroscopy. 2002;18:824–828. doi: 10.1053/jars.2002.36136. [DOI] [PubMed] [Google Scholar]
- 20.Shelbourne KD, Gray T, Wiley BV. Results of transphyseal anterior cruciate ligament reconstruction using patellar tendon autograft in tanner stage 3 or 4 adolescents with clearly open growth plates. Am J Sports Med. 2004;32:1218–1222. doi: 10.1177/0363546503262169. [DOI] [PubMed] [Google Scholar]
- 21.Aronowitz ER, Ganley TJ, Goode JR, et al. Anterior cruciate ligament reconstruction in adolescents with open physes. Am J Sports Med. 2000;28:168–175. doi: 10.1177/03635465000280020601. [DOI] [PubMed] [Google Scholar]
- 22.Gaulrapp HM, Haus J. Intraarticular stabilization after anterior cruciate ligament tear in children and adolescents: results 6 years after surgery. Knee Surg Sports Traumatol Arthrosc. 2006;14:417–424. doi: 10.1007/s00167-005-0698-6. [DOI] [PubMed] [Google Scholar]
- 23.Edwards PH, Grana WA. Anterior cruciate ligament reconstruction in the immature athlete: long-term results of intra-articular reconstruction. Am J Knee Surg. 2001;14:232–237. [PubMed] [Google Scholar]
- 24.Makela EA, Vainionpaa S, Vihtonen K, et al. The effect of trauma to the lower femoral epiphyseal plate: an experimental study in rabbits. J Bone Joint Surg Br. 1988;70:187–191. doi: 10.1302/0301-620X.70B2.3346285. [DOI] [PubMed] [Google Scholar]
- 25.Janarv PM, Wikstrom B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18:149–154. [PubMed] [Google Scholar]
- 26.Guzzanti V, Falciglia F, Gigante A, et al. The effect of intra-articular ACL reconstruction on the growth plates of rabbits. J Bone Joint Surg Br. 1994;76:960–963. [PubMed] [Google Scholar]
- 27.Craig JG, Cramer KE, Cody DD, et al. Premature partial closure and other deformities of the growth plate: MR imaging and three-dimensional modeling. Radiology. 1999;210:835–843. doi: 10.1148/radiology.210.3.r99mr20835. [DOI] [PubMed] [Google Scholar]
- 28.Ecklund K, Jaramillo D. Patterns of premature physeal arrest: MR imaging of 111 children. AJR Am J Roentgenol. 2002;178:967–972. doi: 10.2214/ajr.178.4.1780967. [DOI] [PubMed] [Google Scholar]
- 29.Craig JG, Cody DD, Van Holsbeeck M. The distal femoral and proximal tibial growth plates: MR imaging, three-dimensional modeling and estimation of area and volume. Skeletal Radiol. 2004;33:337–344. doi: 10.1007/s00256-003-0734-x. [DOI] [PubMed] [Google Scholar]
- 30.Guarino J, Tennyson S, Barrios Y, et al. Modeling the growth plates in the pediatric knee: implications for anterior cruciate ligament reconstruction. Comput Med Imaging Graph. 2004;28:419–424. doi: 10.1016/j.compmedimag.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Shea KG, Appel PJ, Pfeiffer AP, et al. The anatomy of the proximal tibia in pediatric and adolescent patients: implications for ACL reconstruction and prevention of physeal arrest. Knee Surg Sports Traumatol Arthrosc. 2007;15:320–327. doi: 10.1007/s00167-006-0171-1. [DOI] [PubMed] [Google Scholar]
- 32.Guzzanti V, Falciglia F, Stanitski CL. Preoperative evaluation and anterior cruciate ligament reconstruction technique for skeletally immature patients in tanner stages 2 and 3. Am J Sports Med. 2003;31:941–948. doi: 10.1177/03635465030310063301. [DOI] [PubMed] [Google Scholar]
- 33.McCarroll JR, Shelbourne KD, Porter DA, et al. Patellar tendon graft reconstruction for midsubstance anterior cruciate ligament rupture in junior high school athletes: an algorithm for management. Am J Sports Med. 1994;22:478–484. doi: 10.1177/036354659402200407. [DOI] [PubMed] [Google Scholar]
- 34.Matava MJ, Siegel MG. Arthroscopic reconstruction of the ACL with semitendinosus-gracilis autograft in skeletally immature adolescent patients. Am J Knee Surg. 1997;10:60–69. [PubMed] [Google Scholar]