Abstract
Injectable materials often have shortcomings in mechanical and drug-eluting properties that are attributable to their high water contents. A water-free, liquid four-armed PEG modified with dopamine end groups is described which changed from liquid to elastic solid by reaction with a small volume of Fe3+ solution. The elastic modulus and degradation times increased with increasing Fe3+ concentrations. Both the free base and the water-soluble form of lidocaine could be dissolved in the PEG4-dopamine and released in a sustained manner from the cross-linked matrix. PEG4-dopamine was retained in the subcutaneous space in vivo for up to 3 weeks with minimal inflammation. This material’s tailorable mechanical properties, biocompatibility, ability to incorporate hydrophilic and hydrophobic drugs and release them slowly are desirable traits for drug delivery and other biomedical applications.
Keywords: Biomedical Applications, Biomimetics, Drug Delivery, Hydrogels, Polymeric Materials
1. Introduction
Implantable biomaterials are important in many fields of medicine, including drug delivery, tissue reconstruction, and tissue engineering.[1] Injectable biomaterials have distinct advantages over materials that require a surgical procedure for placement.[2] The properties that make them injectable also make them easy to apply, especially on complex surfaces or via the relatively limited lumens of the tools used in minimally invasive procedures. However, injectable materials are often less than ideal as drug delivery systems. For example, low viscosity will result in rapid drug release – a problem frequently encountered with hydrogel systems.[3] Such problems can be addressed to some degree by in situ cross-linking or gelation, where the injectate is non-viscous while being administered, then becomes firm within the body in response to a variety of cues or chemical reactions[4]. The relatively high water content of injectable hydrogels will still have several inherent drawbacks attributable to their high water content.[5] The combination of high water content, gel porosity and weak structural integrity often leads to rapid drug release kinetics.[6] Regardless of the desired release pattern, drugs with poor water solubility may be hard to contain in an aqueous based hydrogel. Furthermore, the elastic modulus of soft mammalian tissues ranges from 100 Pa for the softest tissues such as the brain, to around 100 kPa for muscle.[7] Injectable materials, with typical elastic moduli of 5–15 KPa lack adequate mechanical strength and are therefore only suitable for the lower end of that range.[8] These deficiencies in mechanical properties can limit materials’ usefulness in biomedical applications where they are in close apposition with living tissues, such as tissue engineering.
Here, we have designed an injectable neat (no water required as solvent) liquid prepolymer that cross-links to form a matrix with a high elastic modulus in the presence of ferric cations (Fe3+) and that can incorporate and release hydrophilic and hydrophobic drugs. The high elastic modulus is expected[9] since this material will contain only the amount of water necessitated by the addition of Fe(NO3)3 for cross-linking, which is much less than what is found in a typical hydrogel (90–99% water[10]). The prepolymer of our material was a four-armed polyethylene glycol with a pentaerythritol core and succinimidyl glutarate terminations (PEG4: 2,000 Da) modified with the catecholamine dopamine (PEG4-dopamine). We chose PEG4 because it is liquid at room temperature,[11] possesses a higher number of potentially reactive end-groups per molecule than a linear polymer, and has low immunogenicity and toxicity.[12] The cross-linking via dopamine moieties is related to the means by which the marine blue mussel, Mytilus edulis[13] secretes protein solution that solidifies quickly ex vivo in a wet environment.[14] The rapid cross-linking of the secreted protein solution has been attributed to interactions between adjacent catecholic amino acid L-3,4-dihydroxyphenylalanine (DOPA) residues.[15]
2. Results and Discussion
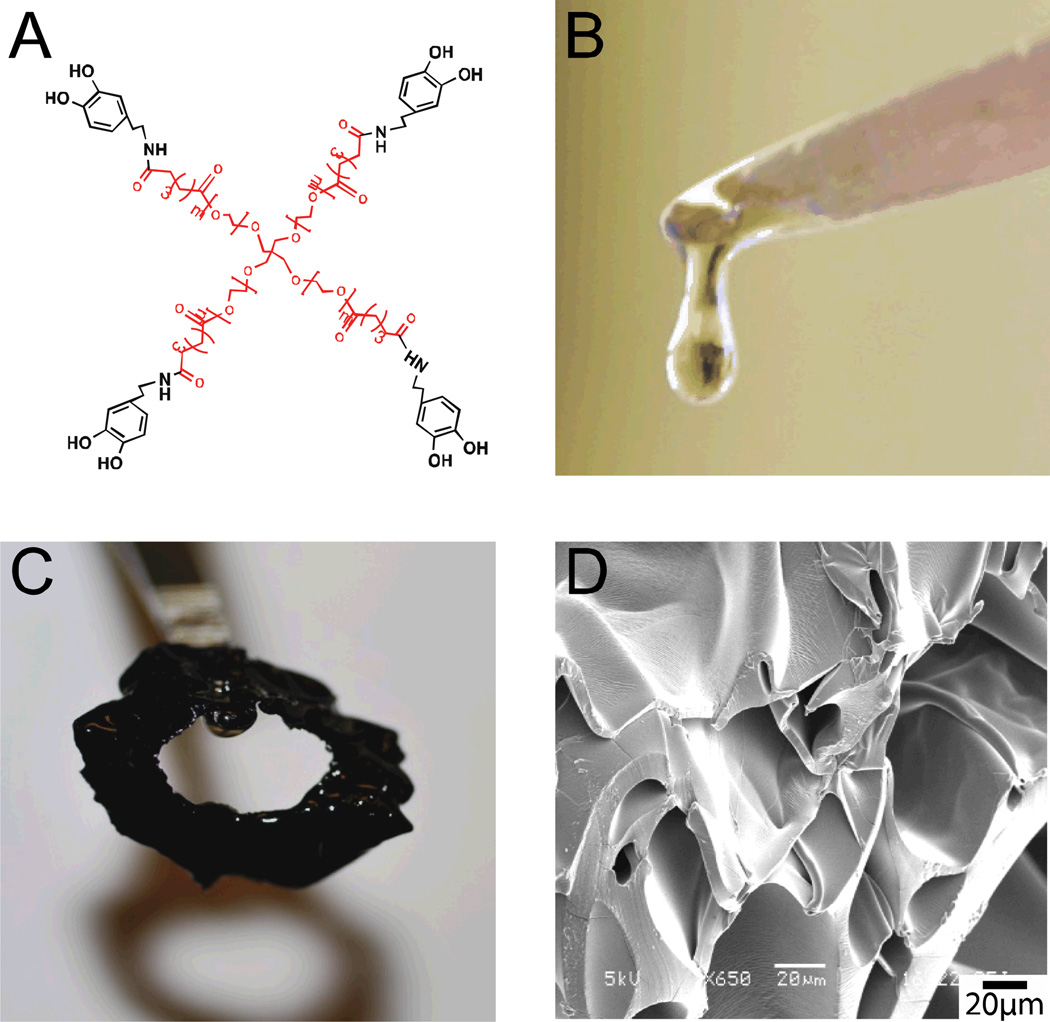
PEG4 was reacted with dopamine hydrochloride solution overnight (4°C, pH 5.9). The reaction solution was dialyzed (1000 MWCO) against acidic water (pH 3.5–4.0) and lyophilized to yield PEG4-dopamine (Fig. 1A). This material was viscous and sticky (Fig. 1B). 76% of the PEG end groups were functionalized with dopamine as confirmed by 1H NMR (Fig. S1 in the Supporting Information), comparable to efficiencies reported with functionalization of multi-armed PEGs polymers with higher molecular weights.[16] When 20 mg (18.2 µL, ~7 µmol) of the PEG4-dopamine were mixed with 6 µL of 2 M Fe3+ solution (12 µmol), a tough rubbery green-black material was formed within a few seconds (Fig. 1C). For reference, the molar corresponding between Fe3+ ions and the dopamine components is about 1:2 (2M), 1:4 (1M) and 1:8 (0.5M). The microscale morphology of this material, observed by scanning electron microscopy (SEM) (Fig. 1D) showed a rough surface with pores of varying sizes and shapes.
Figure 1.
(A) Chemical structure of PEG4-dopamine (pentaerythritol-PEG4 in red, dopamine in black). (B and C) Photograph of PEG4-dopamine (20 mg) before (B) and after (C) the introduction of 2 M aqueous Fe3+ solution (6 µL). (D) SEM image of the material in (C).
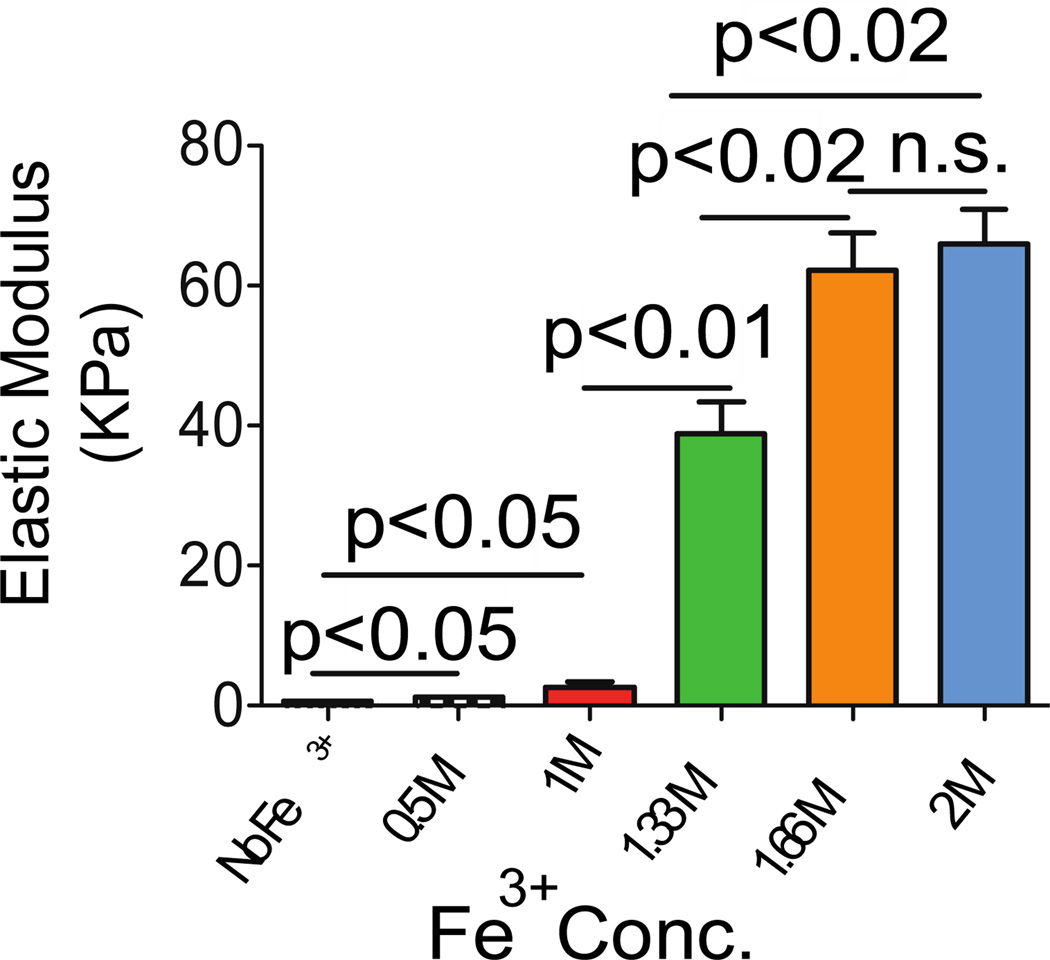
To be suitable for injection and administration through minimally invasive devices, but be useful for drug delivery and as a structural biomaterial, PEG4-dopamine should have low viscosity, but should rapidly harden after injection. We measured the modulus of PEG4-dopamine before and after mixing with Fe3+ using atomic force microscopy (Fig. 2).[17] The addition of 0.5 and 1 M Fe3+ solutions (6 µL) to the PEG4-dopamine (20 mg) increased the elastic modulus slightly (to stiffness values that can be formed with conventional injectable hydrogels). However, 1.33, 1.66 and 2 M Fe3+ solutions increased it greatly, from 0.6 to 39, 62 and 66 kPa respectively, suggesting intermolecular cross-linking.[18] Our material’s morphology may have played an important role in creating its high elastic moduli in comparison to other cross-linked systems[8]. Unlike hydrogel systems, which are composed of polymeric matrix within a continuous water phase, in our system water droplets were likely entrapped within the polymer matrix after being mixed. This phenomenon lead to the formation of an elastomer with thick-walled pores throughout the specimen.[19] Such a morphology has been found to increase the mechanical properties of cross-linked material.[20] The elastic modulus values of the PEG4-dopamine correlated well with Fe3+ concentration (R2= 0.99) in the range of 1 to 1.66 M, suggesting that this system can easily be tailored to desired mechanical properties, e.g. in situations where matching the mechanical properties of tissues is required.[21]
Figure 2.
The influence of Fe3+ concentrations (6 µL) on the mechanical properties of PEG4-dopamine (20 mg), as measured by atomic force microscopy. Data are means ± SD, n = 6.
The cross-linking of PEG4-dopamine moieties was studied by UV-Vis spectroscopy (Fig. 3A). Catechol-catechol binding can be formed by two mechanisms: (1) oxidation of the catechol (e.g. by metals or periodate) to a highly reactive quinone intermediate, which further reacts to form covalent phenol-phenol bridges[22]; (2) creating stable catechol-metal ion complexes; metal ions such as Fe3+ have been used for this purpose. Catechol coupling via bis or tris complexes requires neutral or basic pH values.[23] Since the pH value of our system is acidic (due to the Fe3+ solution) this route is likely not applicable to our study.
Figure 3.
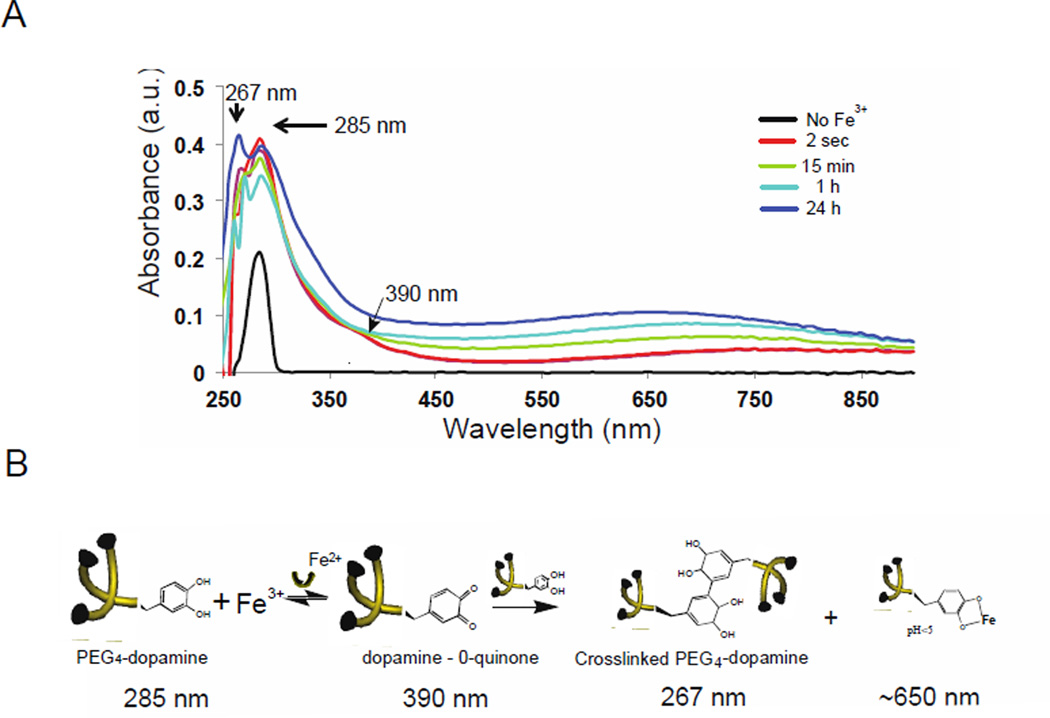
Cross-linking mechanism of PEG4-dopamine. (A) UV/Vis spectra for the oxidation of 5 mg PEG4-dopamine with 1.5 µL of 2 M Fe3+.) The arrows show peaks assigned to the covalent dopamine-dopamine conjugate 5,5’-di(3,4-dihydroxyphenylalanine) (267 nm), dopamine (285 nm), dopamine-o-quinone (390 nm) and dopamine-metal ion (1:1) complexes (~650) (B) The structures of PEG4-dopamine before and after the addition of Fe3+: Covalent dopamine-dopamine bridges are formed after oxidation by ferric ions, as well as dopamine-metal ion complexes (1:1). The wavelengths associated with each structure are given (see panel A).
Five milligrams of PEG4-dopamine were dissolved in 100 µL methanol to allow a uniform film to be cast on a glass slide. After methanol evaporation, a single peak at 285 nm was observed, which was attributable to dopamine moieties in the sample.[24] In order to examine the effect of Fe3+, 1.5 µL of 2 M Fe3+ aqueous solution (pH ~ 1) were mixed with 100 µL methanol and placed on top of the PEG4-dopamine film. Methanol was allowed to evaporate for one minute in the hood. The UV-Vis spectrum (Fig. 3A) developed new peaks at around 390 nm, attributable to the quinone intermediate of dopamine,[25] and at around 267 nm, suggesting the formation of 5,5’-di(3,4-dihydroxyphenylalanine), the covalent dopamine-dopamine conjugate at low pH.[26] A broad elevation in absorption above 650 nm was attributable to mono Fe-catechol complexes (1:1 ratio),[13] which are favored to occur at acidic pH. These results imply that both mechanisms of metal-catechol reaction mentioned above occurred (Fig. 3B): 1) oxidization (390 nm) forming covalent bonds between adjacent dopamine moieties (267 nm), and 2) complexation via 1:1 Fe3+:dopamine reversible coordination bonds (650 nm). The formation of covalent bonds between dopamine moieties correlated well (R2=0.97) with Fe3+ concentrations (Fig. S2). That Fe3+ ions were responsible for the former is consistent with its known redox activity leading to catechol oxidation and subsequently to covalent cross-linking.[27]
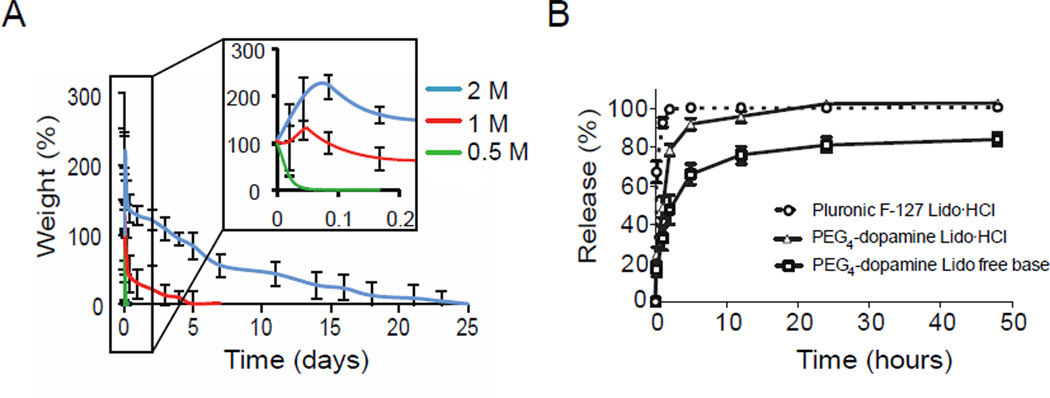
The swelling and erosion behavior of 20 mg PEG4-dopamine (mixed with 6 µL of various concentrations of Fe3+ solutions) when immersed in phosphate buffer (pH 7.4) is shown in Fig. 4A. PEG4-dopamine cross-linked by 1 and 2 M Fe3+ solutions started swelling immediately after being immersed in the solution, reaching a maximum weight after approximately 1.1 and 2.2 h, respectively. The 2 M group swelled more than the 1 M group (130 vs. 30%, respectively). PEG4-dopamines cross-linked with 1 and 2 M Fe3+ degraded and/or dissolved completely over 7 and 25 days, respectively. In contrast, the 0.5 M group started to dissolve upon immersion and was completely dissolved within 2 h. The slower degradation rate of the more cross-linked gels can be explained by decreased accessibility of water to the hydrolytically degradable ester linkage on the PEG4-dopamine backbone. This linkage has been reported to be responsible for the degradation of comparable PEGs.[28]
Figure 4.
Swelling of and drug release from cross-linked PEG4-dopamine. (A) Water absorption and erosion of PEG4-dopamine cross-linked by various concentrations of Fe3+ aqueous solutions in PBS (pH 7.4) at 37 °C. (B) Release profiles for lidocaine free base and lidocaine hydrochloride from cross-linked PEG4-dopamine (20 mg PEG4-dopamine /6 µL, 2 M Fe3+) and from Pluronic F-127. Data are means ± SD, n = 4.
To assess the performance of cross-linked PEG4-dopamine as a drug delivery system, the in vitro drug release profiles from 20 mg of PEG4-dopamine cross-linked with 6 µL of 2 M Fe3+ solutions were studied (Fig. 4B). This combination was chosen since 2 M Fe3+ caused considerably longer degradation times (Fig. 4A). Lidocaine free base or lidocaine hydrochloride (1 mg) were dissolved in 30 mg of PEG4-dopamine (3.33 % w/w) followed by mixing with Fe3+ (1.5 µm) and immersion in 0.1 M phosphate buffered saline (PBS) pH 7.4 at 37°C. Drug release into the PBS was measured by HPLC (Fig. 4B), and was compared to that of lidocaine HCl from 20% w/v Pluronic F-127 (drug loading was the same as that used with PEG4-dopamine). While it is obviously impossible to select a single formulation that can be said to be representative of the drug-eluting, mechanical, etc. properties of all hydrogels, Pluronic-F127 has been a commonly used injectable vehicle to deliver lidocaine HCl.[29] Lidocaine free base could not be dissolved in the Pluronic. The release rate was affected by the hydrophobicity of the drug (free base vs. hydrochloride) and the drug delivery vehicle used. With the Pluronic, 90% of the lidocaine HCl was released within 1 h; only 49% was released from the PEG4-dopamine during the same time period, and 33% when lidocaine free base was used. The solubility of the hydrophilic and hydrophobic forms of lidocaine in the PEG4-dopamine, is consistent with the solvent properties of short PEGs,[30]. Furthermore, PEG4-dopamine demonstrated a more sustained release pattern compared to the Pluronic hydrogel, which can be attributed to the relatively high polymer mass per unit volume.[31] The lower amplitude of the release burst observed with PEG4-dopamine can be, in part, explained by its morphology. Pores formed from more highly concentrated solutions are more likely to reduce burst release from swellable systems, due to thicker walls and smaller pores.[32]
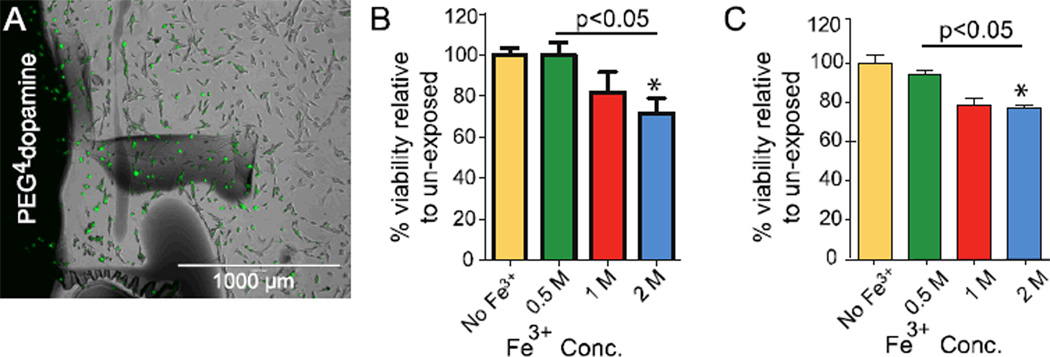
To evaluate the cytotoxicity of PEG4-dopamine in vitro in NIH 3T3 fibroblast cell lines, cells were exposed to 5 mg of PEG4-dopamine cross-linked by 1.5 µL of various concentrations of Fe3+. The cross-linked PEG4-dopamine was positioned either in the center of the wells of culture plates, covering about 5% of the surface area (where they would be in direct contact with cells, Fig. 5A) or in Transwell inserts where they would be isolated from cells (indirect contact with soluble elements). Polymers were allowed to cross-link for 1 hour, followed by sterilization with 70% ethanol aqueous solution then washing with sterile media. After 48 hours of cell exposure to polymers, cytotoxicity was assessed by the MTS assay.[33] In both the direct and indirect assays viability (as a percentage relative to cells not exposed to PEG4-dopamine) decreased slightly with increasing Fe3+ concentration (Fig. 5B and C) suggesting a possible release of unreacted Fe3+ from the gel to the media. The potential role of Fe3+ in that reduction in cell viability was seen in the severe cytotoxicity of 1.5 µL of the corresponding Fe3+ concentrations when added without PEG4-dopamine (Fig. S3). PEG4-dopamine without Fe3+ dissolved immediately upon addition to media but had no effect on cell viability. The toxicity produced by Fe3+ in biological systems can be attributed to the enhanced production of oxidants capable of initiating and propagating lipid peroxidation processes, oxidizing proteins, and damaging DNA.[34]
Figure 5.
NIH 3T3 fibroblast cell viability 48 hours after exposure to cross-linked PEG4-dopamine. (A) Fluorescence microscopy of the margins of the cross-linked PEG4-dopamine (black area to left of panel) 48 h after incubation with the cells (green). (B) MTS assay of cells exposed directly to cross-linked PEG4-dopamine formed with varying Fe3+ concentrations. (C) MTS assay of cells exposed indirectly to PEG4-dopamine formed with varying Fe3+ concentrations. Asterisks denote statistical significance compared to unexposed cells. Data are means ± SD, n = 4.
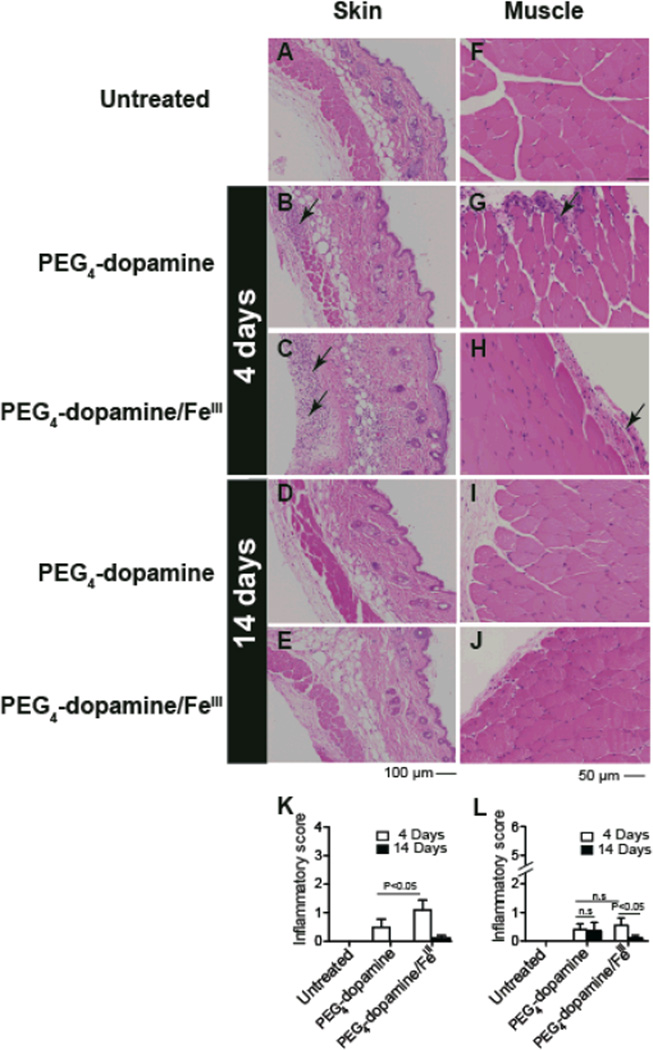
Tissue reaction to PEG4-dopamine was determined by injecting male Balb/c mice subcutaneously in the left flank with 0.1 mL PEG4-dopamine, with or without co-administration of Fe3+ solution. A Fe3+ concentration of 1.33 M was chosen based on a trade-off between the effects of Fe3+ on the elastic modulus and on cytotoxicity (both increase with increasing concentration, Fig. 2 and 5). Mice were euthanized on days 4 and 14 (n = 5 at each time point), and tissues from the injected area were harvested for histological analysis (Fig. 6). Inflammation was assessed with a scoring system,[35] (see experimental section) with scores ranging from 0–6 for muscle and 0–4 for skin. Tissues from the contralateral untreated flank area of each mouse were also examined (untreated, Fig. 6 A and F).
Figure 6.
Tissue reaction to 100 µl of cross-linked and un-cross-linked (no Fe3+) PEG4-dopamine 4 and 14 days after subcutaneous administration, shown on hematoxylin-eosin staining of skin (A–E) and muscle (F–J) tissue sections harvested from the vicinity of the implant. (A, F) Untreated animals show normal morphology. (K, L) Histological scores for inflammation (data expressed as means ± SD; n = 5 per group) were compared by 1-way ANOVA with Tukey post hoc comparison (*P < 0.05). Arrows indicate areas of inflammation. Photographs are representative views.
After 4 days, histological analysis of skin and muscle demonstrated sparsely scattered neutrophils and macrophages without other signs of tissue injury (Fig. 6). Inflammation scores from skin and muscle samples harvested from animals exposed to PEG4-dopamine/Fe3+ were low; the skin scores were higher than those from animals receiving PEG4-dopamine without Fe3+ (Fig. 6K). These scores reflect the finding that inflammatory cells were limited to the superficial panniculus carnosus muscle and advential layer in the skin (Fig. 6B, C). After 14 days, all skin and muscle sections exhibited almost normal morphology (similar to untreated controls) with minimal inflammation (Fig. 6L).
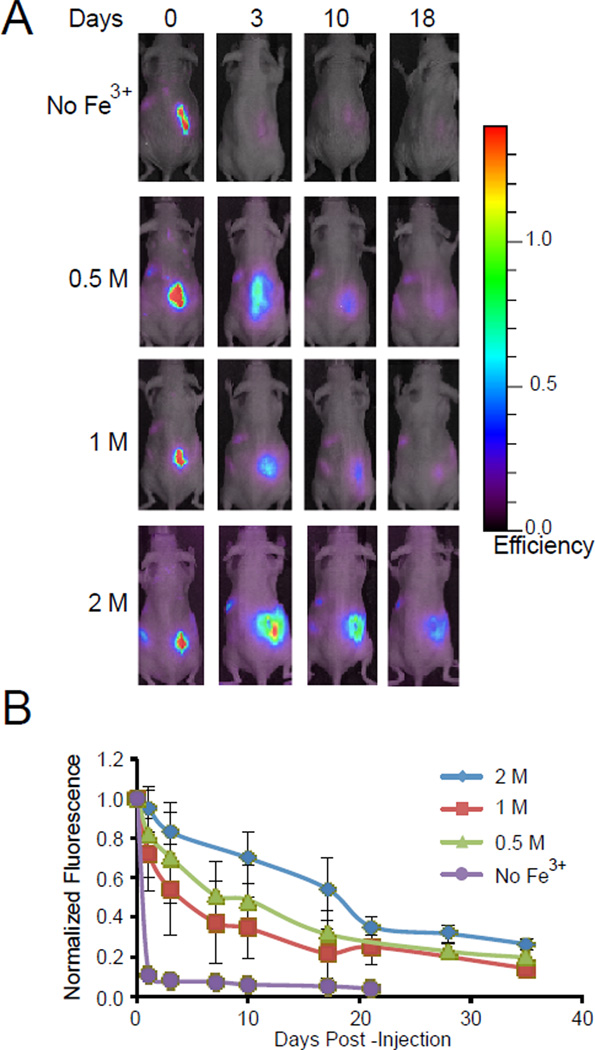
The time course of degradation in vivo was studied by whole body imaging using PEG4-dopamine labeled with an amine-reactive Alexa Fluor 647 (PEG4AF-dopamine). Twenty mg of PEG4AF-dopamine with 6 µL of Fe3+ solutions or with 6 µL PBS (no Fe3+) were injected subcutaneously on the right posterior flank of female SKH1 mice. In the animals injected with PEG4AF-dopamine (no Fe3+), 90% of the fluorescence intensity was lost by one day after administration (Fig. 7A and B). In contrast, the relative fluorescence intensity of the PEG4-dopamine mixed with 0.5, 1, and 2 M Fe3+ solutions decreased by 50% after 3, 7, and 20 days, respectively. The degradation rates in vivo were consistent with in the vitro data on degradation (Fig. 4A).
Figure 7.
Real time whole-body animal imaging was used to assess the retention of subcutaneously injected fluorescently-labeled PEG4-dopamine with or without various concentrations of Fe3+ solution. Images (A) and quantification of fluorescent signal (B) indicated rapid degradation in the group without Fe3+, improved retention for implants formed with 0.5 M Fe3+ solution, and maximal retention for implants formed with 2 M Fe3+ solution. Data are means ± SD; n=3.
3. Conclusions
We have developed a liquid four-armed PEG-based pre-polymer modified with dopamine end groups. When this material reacted with Fe3+ solution it changed from a liquid to an elastic solid where modulus increased with Fe3+ concentration. Fe3+ concentrations greater than 1 M formed stiffer matrices than can be formed with injectable hydrogels. The absence of water in the PEG4-dopamine and the solvent capabilities of PEG allowed the dissolution of water soluble and of poorly water soluble drugs. Drug release was slower than from a commercially available hydrogel. The mechanical and the degradation properties could easily be tuned by varying the Fe+3 concentration. Cytotoxicity was minimal and the polymers were retained in the subcutaneous space in vivo for several weeks with minimal evidence of local inflammation at the concentrations tested. The injectable nature of the formulation, and the fact that it cross-linked in situ – allowing facile conformal application within the body – are attractive features for use topically and internally. This flexible system has potential for applications in drug delivery, tissue reconstruction and for other areas of biomedicine.
4. Experimental
Materials and Methods
All chemicals were obtained from commercial sources. Ferric nitrate nonahydrate 98%, HCl, NaOH, dopamine hydrochloride, phosphate buffer saline, methanol and ethanol were purchased from Sigma-Aldrich, Inc. (St Louis, MO). Four-armed PEG, succinimidyl glutarate terminated and pentaerythritol core (2000 Da) was purchased from Polymer Source Inc. (Montreal, Canada). Spectra/Por® dialysis membranes (MWCO: 1000 Da) were purchased from Spectrum Labs (Rancho Dominguez, CA). Deuterium oxide was purchased from Cambridge Isotope Laboratories, Inc. (Cambridge, MA). Water used in polymerization and dialysis (DDW) was distilled and purified by using a Nanopure® water purification system (Thermo Scientific, Bremen, Germany).
PEG4-dopamine synthesis and characterization
1.0 g four-armed PEG and 2.5 g dopamine hydrochloride were separately dissolved in 20 and 30 mL DDW, respectively. The polymeric solution was added dropwise to the dopamine solution and the pH was adjusted to 5.9 ± 0.1 by 0.1 M NaOH. The solution was left to stir for 1 hour at 4 °C and the pH was retained at 5.9 ± 0.1 followed by mixing overnight at 4 °C. The solution was dialyzed six times (1000 MWCO dialysis membrane, Spectra/Por®, Rancho Dominguez, CA) against 3L of DDW containing a few drops of 0.1 M HCl (pH 3.5–4.0) followed by lyophilization. The chemical structures of the molecule as well as the efficiency of the modification were determined by 1H NMR spectroscopy using a Varian Mercury (Palo Alto, CA) 300 MHz spectrometer in D2O (Fig. S1). The dopamine content was determined by comparing the integral value of pentaerythritol methylene protons at δ = 3.9 to the hydroxyl protons of the dopamine at δ = 6.6.
Mechanical Properties
Force-distance curves were obtained using an Asylum Research AFM, model MFP-3D (Santa Barbara, CA). Tested materials were allowed to cross-link for 1 h before mechanical testing was applied. Experiments were performed at room temperature. A doped Force Modulation etched Silicon Probe (FESP) cantilever (Bruker, CA; nominal spring constant 2.8 N/m) was used. Force-distance curves were obtained by using a silicon tip cantilever (spring constant 2.8 N/m). The force applied was calculated by:
where F is force (nN), k is the spring constant of the cantilever (N/m), and Δz is the deflection distance of the tip (nm). The elastic modulus for a conical tip geometry [36] was calculated by:
where F is the calculated force, E is the elastic modulus, δ is the indentation depth (equal to the difference between the piezo height, z, and the cantilever deflection (zd)), γ is the Poisson ratio, (we used a value of 0.5 which is commonly used for rubbers and gels [37]), and α is the opening angle of the cone tip.
Swelling
20 mg of PEG4-dopamine were mixed with 6 µL of Fe3+ solutions of various concentrations. After 1 hour, the materials were placed in the bottom of pre-weighed 2 mL Eppendorf tubes (one specimen per tube), and 1 mL phosphate buffer saline was added. The tubes were kept at 37 °C with constant shaking (75 rpm). At each time point, supernatant medium was aspirated and the tube and PEG4-dopamine were weighed after residuals of PBS were blotted dry with filter paper. Fresh PBS was added before tubes were returned to the incubator for the next time point.
In vitro biocompatibility
NIH 3T3 fibroblast cells were grown at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Gibco-Invitrogen Corp., Grand Island, NY). Cultures were maintained in a 95% air/5% carbon dioxide atmosphere, at 95% relative humidity. For the indirect assay, gels were placed in 6.5 mm in diameter Transwell tissue culture plate inserts (Corning Incorporated, Corning, NY). PEG4-dopamines were allowed to cross-link at room temperature for 1 hour before sterilization was carried out using 70% ethanol aqueous solution for 10 minutes. Residuals of ethanol were removed by washing with sterile media. Cell were exposed to the materials in the Transwell inserts for 48 hours, then cytotoxicity was assessed using the MTS assay [38] (CellTiter 96® Aqueous kit, Promega, Madison, WI). Four replicates were seeded for each of the tested group, as well as for the control. Evos fl (AMG, Bothell, WA) was used at 200× magnification to image cells after incubation in 2 µM calcein acetoxymethyl (Live/Dead fluorescence viability kit, Molecular Probes, Eugene, OR).
In vivo studies
Animals were cared for in compliance with protocols approved by the Massachusetts Institute of Technology Committee on Animal Care, in conformity with the NIH guidelines for the care and use of laboratory animals (NIH publication 85-23, revised 1985).
Experimental materials were injected subcutaneously on the flanks of male Balb/c mice weighing 19–21 g, under anesthesia with 1% isoflurane (Iso-Thesia, Rockville, NY) in oxygen. The same anesthetic was used to keep animals still for imaging. In selected experiments, tissues were harvested at predetermined time points for histology after euthanasia with carbon dioxide.
Histological images were acquired using an Eclipse 50i microscope (Nikon Instruments Inc., Melville NY) and a SPOT Insight 4 Meg FW Color Mosaic camera with SPOT4.5.9.1 software (Diagnostic Instruments Inc., Sterling Heights, MI). For histological analysis, skin and muscle samples were scored for the severity of inflammation ranging from 0–4 for skin and 0–6 for muscle. Skin inflammatory scores represented 0: normal morphology, 1: inflammatory cells limited only to the panniculus carnosus muscle and the adventia, 2: inflammatory cells were present in the subcutaneous tissue but not beyond, 3: inflammatory cells extended till the reticular dermis and 4: severe inflammation with inflammatory cells extending completely into the papillary dermis. The muscle inflammatory scores represented 0: normal, 1: perifascicular internalization, 2: deep internalization (>5 cell layers), 3: perifascicular regeneration, 4: deep regeneration, 5: hemifascicular regeneration, and 6: holofascicular regeneration.
For dynamic imaging of polymer degradation in vivo, PEG4-dopamine was labeled with an amine-reactive Alexa Fluor 647 (Invitrogen, Grand Island, NY) by adding Alexa Fluor 647 (1 mol% compared to the dopamine HCl) to the dopamine solution before synthesis. Prior to injections, 20 mg PEG4-dopamine was mixed with 6 µL of Fe3+ solution of various concentrations or DDW (control). Excitation and emission wavelengths used for Alexa Fluor 647 imaging on the IVIS were 640 and 680 nm, respectively.
Statistics
All data were presented as mean ± SD. The Student t-test or ANOVA with Tukey post hoc comparison was used to groups. p values < 0.05 were considered to reflect statistical significance.
Supplementary Material
Acknowledgements
This work was funded by NIDCD R21 DC 009986 (to DSK). B.P.T. acknowledges an NIH Ruth L. Kirschstein National Research Service Award (no. F32GM096546) and NIH R01 EB00244 (DGA and RL).
Footnotes
Supporting Information is available online from Wiley InterScience or from the author.
References
- 1.a) Hollister SJ. Nat. Mater. 2005;4:518. doi: 10.1038/nmat1421. [DOI] [PubMed] [Google Scholar]; b) Langer R, Tirrell DA. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]; c) Lutolf MP, Hubbell JA. Nat. Biotech. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 2.a) Zhang Z, Grijpma DW, Feijen J. Macromol. Chem. Physic. 2004;205:867. [Google Scholar]; b) Amsden B. Soft Matter. 2007;3:1335. doi: 10.1039/b707472g. [DOI] [PubMed] [Google Scholar]; c) Homicz MR, Watson D. Facial Plast Surg. 2004;20:21. doi: 10.1055/s-2004-822955. [DOI] [PubMed] [Google Scholar]
- 3.a) Hoare T, Bellas E, Zurakowski D, Kohane DS. doi: 10.1002/jbm.a.32420. [DOI] [PubMed] [Google Scholar]; b) J. Biomed. Mater. Re.s A. 2010;92:586. doi: 10.1002/jbm.a.32420. [DOI] [PubMed] [Google Scholar]; c) Hoare TR, Kohane DS. Polymer. 2008;49:1993. [Google Scholar]
- 4.Hudson SP, Langer R, Fink GR, Kohane DS. Biomaterials. 2010;31:1444. doi: 10.1016/j.biomaterials.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ito T, Yeo Y, Highley CB, Bellas E, Benitez CA, Kohane DS. Biomaterials. 2007;28:975. doi: 10.1016/j.biomaterials.2006.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Biomaterials. 2009;30:2724. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Calvert P. Adv. Mater. 2009;21:743. [Google Scholar]
- 6.Ruel-Gariepy E, Chenite A, Chaput C, Guirguis S, Leroux JC. Int. J. Pharm. 2000;203:89. doi: 10.1016/s0378-5173(00)00428-2. [DOI] [PubMed] [Google Scholar]
- 7.Levental I, Georges PC, Janmey PA. Soft Matter. 2007;3:299. doi: 10.1039/b610522j. [DOI] [PubMed] [Google Scholar]
- 8.a) Khetan S, Burdick JA. Biomaterials. 2010;31:8228. doi: 10.1016/j.biomaterials.2010.07.035. [DOI] [PubMed] [Google Scholar]; b) Wang S, Cai L. Int. J. Pol. Sci. 2010:2010. [Google Scholar]; c) Zhang X, Wu D, Chu C-C. Biomaterials. 2004;25:4719. doi: 10.1016/j.biomaterials.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Mizrahi B, Walden C, Kohane DS. In: Active Implants and Scaffolds for Tissue Regeneration. Zilberman M, editor. Vol. 8. Berlin: Springer; 2011. p. 39. [Google Scholar]
- 10.Gérentes P, Vachoud L, Doury J, Domard A. Biomaterials. 2002;23:1295. doi: 10.1016/s0142-9612(01)00247-2. [DOI] [PubMed] [Google Scholar]
- 11.Younes HM, Bravo-Grimaldo E, Amsden BG. Biomaterials. 2004;25:5261. doi: 10.1016/j.biomaterials.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Burke SA, Ritter-Jones M, Lee BP, Messersmith PB. Biomed. Mater. 2007;2:203. doi: 10.1088/1748-6041/2/4/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDowell LM, Burzio LA, Waite JH, Schaefer J. J. Biol. Chem. 1999;274:20293. doi: 10.1074/jbc.274.29.20293. [DOI] [PubMed] [Google Scholar]
- 14.Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Ann. Rev. Mater. Res. 2011;41:99. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Filpula DR, Lee S, Link RP, Strausberg SL, Strausberg RL. Biotechno. Progr. 1990;6:171. doi: 10.1021/bp00003a001. [DOI] [PubMed] [Google Scholar]
- 16.Huang K, Lee BP, Ingram DR, Messersmith PB. Biomacromolecules. 2002;3:397. doi: 10.1021/bm015650p. [DOI] [PubMed] [Google Scholar]
- 17.Costa KD, Yin FCP. Biomech. Eng. 1999;121:462. doi: 10.1115/1.2835074. [DOI] [PubMed] [Google Scholar]
- 18.Amsden B, Wang S, Wyss U. Biomacromolecules. 2004;5:1399. doi: 10.1021/bm034538j. [DOI] [PubMed] [Google Scholar]
- 19.a) Chirila TV, Chen YC, Griffin BJ, Constable IJ. Polym. Int. 1993;32:221. [Google Scholar]; b) Clayton AB, Chirilaa TV, Dalton PD. Polymer International. 1997;42:45–56. [Google Scholar]
- 20.Ramay HR, Zhang M. Biomaterials. 2003;24:3293–3302. doi: 10.1016/s0142-9612(03)00171-6. [DOI] [PubMed] [Google Scholar]
- 21.Artzi N, Shazly T, Baker AB, Bon A, Edelman ER. Adv. Mater. 2009;21:3399. doi: 10.1002/adma.200900340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu M, Hwang J, Deming TJ. J Am. Chem. Soc. 1999;121:5825. [Google Scholar]
- 23.Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KY, Waite JH. Proc. Natl. Acad. Sci. U S A. 2011;108:2651. doi: 10.1073/pnas.1015862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barreto WJ, Barreto SRG, Santos MA, Schimidt R, Paschoal FMM, Mangrich AS, deOliveira LFC. J. Inorg. Biochem. 2001;84:89. doi: 10.1016/s0162-0134(00)00207-5. [DOI] [PubMed] [Google Scholar]
- 25.a) Shultz MD, Reveles JU, Khanna SN, Carpenter EE. J Am. Chem. Soc. 2007;129:2482. doi: 10.1021/ja0651963. [DOI] [PubMed] [Google Scholar]; b) Clarke SJ, Hollmann CA, Zhang Z, Suffern D, Bradforth SE, Dimitrijevic NM, Minarik WG, Nadeau JL. Nat. Mater. 2006;5:409. doi: 10.1038/nmat1631. [DOI] [PubMed] [Google Scholar]
- 26.Bruce PL, Dalsin LJ, Messersmith PB. Polymer Preprints. 2001:42. [Google Scholar]
- 27.a) Sever MJ, Weisser JT, Monahan J, Srinivasan S, Wilker JJ. Ange. Chem. Int. Edit. 2004;43:448. doi: 10.1002/anie.200352759. [DOI] [PubMed] [Google Scholar]; b) Cortlandt G P. Coordin. Chem. Rev. 2001 Jun;216–217:99. [Google Scholar]
- 28.a) Zhao X, Harris J Milton. J. Pharm. Sci. 1998;87:1450. doi: 10.1021/js980065o. [DOI] [PubMed] [Google Scholar]; b) Cho E, Kutty JK, Datar K, Lee J Soo, Vyavahare NR, Webb K. J. Biomed. Mater. Res. A. 2009;90A:1073. doi: 10.1002/jbm.a.32172. [DOI] [PubMed] [Google Scholar]
- 29.a) Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. J. Pharm. Pharm. Sci. 2006;9:339. [PubMed] [Google Scholar]; b) Paavola A, Yliruusi J, Rosenberg P. J. Con. Rel. 1998;52:169. doi: 10.1016/s0168-3659(97)00206-x. [DOI] [PubMed] [Google Scholar]
- 30.a) Kodera Y, Inada Y. Seikagaku. 1988;60:1005. [PubMed] [Google Scholar]; b) Seedher N, Bhatia S. AAPS PharmSciTech. 2003;4:36. doi: 10.1208/pt040333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang XZ, Wu DQ, Chu CC. Biomaterials. 2004;25:3793. doi: 10.1016/j.biomaterials.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 32.Huang X, Brazel CS. J. Con. Rel. 2001;73:121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 33.Cory AH, Owen TC, Barltrop JA, Cory JG. Cancer Commun. 1991;3:207. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 34.Halliwell B, Gutteridge JMC. Biochemical Journal. 1984;219:1. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padera RF, Tse JY, Bellas E, Kohane DS. Muscle Nerve. 2006;34:747. doi: 10.1002/mus.20618. [DOI] [PubMed] [Google Scholar]
- 36.Last JA, Russell P, Nealey PF, Murphy CJ. Invest. Ophthalmol Vis. Sci. 2010;51:6083. doi: 10.1167/iovs.10-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanabria-DeLong N, Crosby AJ, Tew GN. Biomacromolecules. 2008;9:2784. doi: 10.1021/bm800557r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cory AH, Owen TC, Barltrop JA, Cory JG. Cancer Commun. 1991;3:207. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.