Capsule Summary
We have identified dizygotic twins with a novel syntaxin-binding protein 2 (STXBP2) mutation, where cytotoxicity cannot be restored with IL-2. This defines STXBP2 as an absolute requirement for NK cell cytotoxic function.
Keywords: Familial hemophagocytic lymphohistiocytosis (FHL), syntaxin-binding protein 2 (STXBP2), Munc18-2, syntaxin-11
Familial Hemophagocytic Lymphohistiocytosis (FHL) is a rare, autosomal recessive, primary immunodeficiency characterized by fever, hepatosplenomegaly, and systemic inflammatory response syndrome. It is associated with pancytopenia, hypertriglyceridemia, hypofibrinogenemia, and tissue infiltration by activated macrophages and hematophagocytes. It is rapidly fatal if untreated1.
Diagnostic criteria for hemophagocytic lymphohistiocytosis (HLH) include the presence of a disease-causing mutation and/or at least 5 of the following 8 clinical criteria: prolonged fever (>7 days); cytopenias affecting ≥ 2 peripheral blood cell lineages; splenomegaly; hypertriglyceridemia and/or hypofibrinogenemia; hemophagocytosis; low or absent natural killer (NK) cell activity; hyperferritinemia; and elevated plasma sIL2Rα2.
FHL results from defective cytolytic function leading to an inability to appropriately regulate and contain certain immune responses1,3. As a result, there is excessive cytokine production and macrophage activation. FHL disease causing mutations occur in the genes encoding perforin (PRF1, FHL2), Munc13-4 (UNC13D, FHL3), syntaxin-11 (STX11, FHL4), and Munc18-2 (STXBP2, FHL5)4,5. All impair the ability of cytotoxic lymphocytes, including NK cells and cytotoxic T lymphocytes, to secrete lytic effector molecules normally contained within specialized lysosomal organelles called lytic granules1,3. Cytotoxic lymphocytes function to recognize and destroy infected or malignant cells and their role in FHL represents a new understanding as to how they function to maintain normal immunity.
The most recently described form of FHL (FHL5) results from mutation of the STXBP2 gene encoding syntaxin-binding protein 2 (also known as Munc18-2). Munc18-2 interacts with syntaxin-11, a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein and together they enable fusion of the lytic granule membrane with that of the cytotoxic lymphocyte to promote degranulation. Mutations in FLH5 abrogate the interaction between Munc18-2 and syntaxin-11 and prevent this necessary prerequisite step for cytotoxicity. Although in FHL5, NK cells are able to conjugate with and polarize lytic granules toward the target cell, they are unable to release their granule contents6. Thus, there is reduced activity of NK and cytotoxic T cells, (the former being more pronounced in FHL5 for reasons that are presently unclear)4,6-8.
Previous in vitro studies with FHL5 patient cells have demonstrated that the defect in NK cell cytotoxicity can be partially restored after short term in vitro exposure to the stimulatory cytokine IL-24,6-8. This implies the existence of a Munc18-2 -independent pathway to access SNARE function. It also suggests the potential for therapeutic intervention to restore NK cell function in FHL5.
We present the case of 19 month old dizygotic twins with FHL5, in which we studied the NK cell cytolytic function in consideration of expanded FHL5 phenotypes.
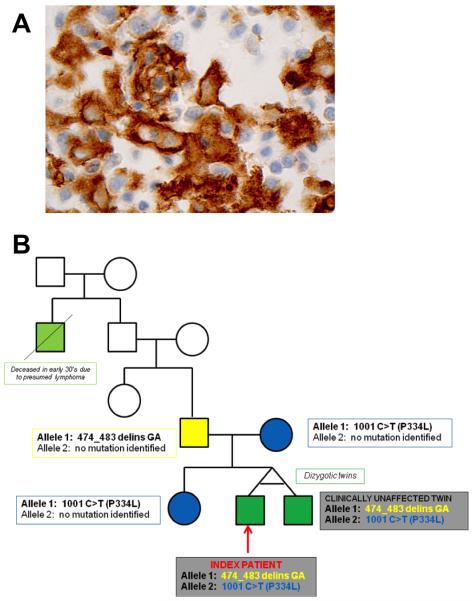
The index patient had an unremarkable medical history and was born to non-related Ashkenazi Jewish parents. At 19 months, he had two weeks of fever (Tmax 105°F), during which time his twin brother had been diagnosed with an upper respiratory tract infection. On presentation, the index patient was febrile and ill-appearing with exudative tonsillar hypertrophy, diffuse cervical lymphadenopathy, hepatosplenomegaly, and bilateral pedal edema (clinical laboratory data: white blood cell count 36.5 thousand/uL with 53% atypical lymphocytes, hemoglobin 10.3 g/dL, platelets 214 thousand/uL, alanine aminotransferase 440 U/L, aspartate aminotransferase 312 U/L, ferritin 807 ng/mL, trigylcerides 494 mg/dL, sIL2Rα 25,409 U/mL). Bone marrow examination demonstrated diffuse hematophagocytes (Figure 1A). Both the index patient and his twin were found to have novel biallelic mutations for Munc18-2 [474_483 del_insGA/1001 C>T (P334L)] (Figure 1B). They did not have mutations in the other known genes responsible for FHL or X-linked lymphoproliferative syndrome. The index patient fulfilled 8/8 HLH diagnostic criteria2, while his twin brother remained asymptomatic. PCR-based sequencing of the STXBP2 gene in 329 unrelated patients diagnosed with HLH yielded bi-allelic STXBP2 mutations in 32 unrelated families and 22 additional heterozygous mutations and sequence variants. None of these, however, shared our patient’s mutations.
Figure 1.
(A) Hemophagocytes Noted on Bone Marrow (100X magnification). Paraffin immunohistochemistry for CD163 (a marker for histiocytes) performed on the bone marrow biopsy shows numerous activated histiocytes, many of which have round nuclei and ample cytoplasm. Occasional histiocytes show multiple nuclei, or lucencies, consistent with hemophagocytosis. (B) Family Tree and STXBP2 Gene Mutation Analysis.
Informed consent was obtained for record review and experimental studies using a protocol approved by The Children’s Hospital of Philadelphia Institutional Review Board for the protection of human subjects. For experimental studies of NK cell functions, PBMCs were prepared and cytotoxicity was assessed by using 4-hour 51Cr release assays against labeled K562 erythroleukemia cells, 721.221 EBV-transformed B cells, or Raji B cells as described9. Short-term IL-2 stimulation was performed by adding 1,000 IU/ml human recombinant IL-2 (NIH AIDS reagent program), while antibody-dependent cellular cytotoxicity (ADCC) was determined by the addition of monoclonal anti-CD20 (Rituximab, Genentech Inc., San Francisco, CA) at 20 μg/mL to the 4-hour assay against Raji B target cells (which were not lysed without added antibody, data not shown). All assays were repeated at least twice by using independent blood samples.
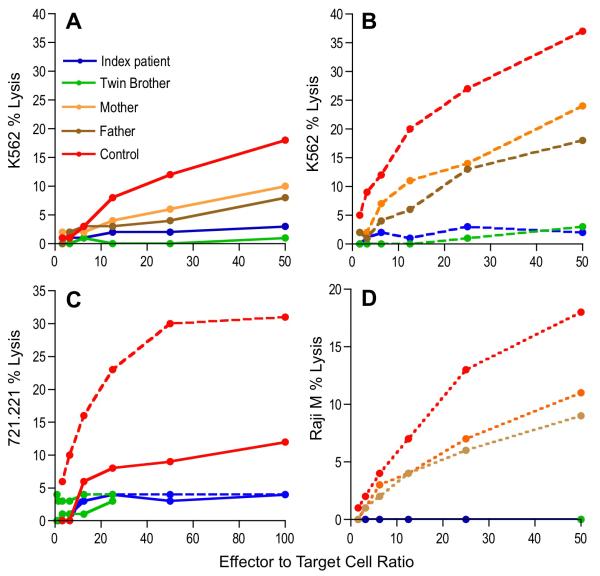
Our symptomatic patient as well his asymptomatic brother had defective NK cell cytotoxicity against the canonical NK cell target cell line, K562 erythroleukemia cells (Figure 2A). In contrast to reported results, however, both affected individuals, but not their parents or a control, failed to respond to IL-2 with an increase in NK cell cytotoxicity (Figure 2B). To ensure that the IL-2-resistant decreased NK cell function in the patients was not specific to the target cells used, we also evaluated an EBV-transformed B cell target cell (Figure 2C). Here we found that the NK cell cytotoxic activity of the two brothers was deficient and could not be enhanced by short-term IL-2 exposure. To ensure that the deficient NK cell function was not a feature of particular receptors, we also evaluated ADCC, which has not been previously studied in FHL5. The ADCC of the brothers, but not their parents, was absent (Figure 2D). In all cases the Munc18-2 mutant heterozygous parents were similar in cytolytic function, and less than that of the control.
Figure 2.
NK cell cytotoxicity. (A) NK cell cytotoxicity in resting PBMCs was assessed against K562 cells. Dashed lines (B, C) represent short-term IL-2 stimulation in vitro. ADCC was determined by the in vitro addition of Rituximab and is represented by dotted lines (D).
Prior studies have shown that the deficient cytotoxicity in FHL5 patients can be reversed after short-term in vitro exposure of PBMC to IL-24,6-8. However, our data describe a novel STXBP2 mutation, which to our knowledge represents the first reported to have a fixed pervasive deficiency of NK cell cytotoxicity that does not respond to IL-2 stimulation. Given the complete and consistent lack of response to IL-2, it is thus unlikely that IL-2 is inducing a pathway to cytotoxicity truly independent of Munc18-2 (akin to what we have found in Wiskott-Aldrich syndrome, where IL-2 can circumvent WASp altogether)9. Thus we hypothesize that IL-2 is allowing for expanded access to function via a crippled STXBP2 gene or Munc18-2 protein.
At present, the only curative option for patients with STXBP2 mutations in FHL5, as with other types of HLH, is hematopoietic stem cell transplantation1. The index patient was transplanted with hematopoietic stem cells from a matched sibling donor facilitated by reduced intensity conditioning. He is presently doing well. His twin brother remains asymptomatic. It is unclear as to why he has not developed hematophagocytosis and speaks to potential modifying genetic influences not present in his affected brother.
Given that IL-2 has been previously identified to induce NK cell cytotoxicity, it has been proposed as a potential adjunct or temporizing therapy for patients with FHL54,6-8. In light of our observations, further studies are needed to elucidate which syntaxin-11/Munc18-2 abnormalities can be augmented by IL-2 and certainly should at least prompt evaluation of the results of direct in vitro cytotoxicity testing before considering IL-2 therapeutically. Specifically, it may be effective for only a subset of FHL5 patients.
Acknowledgments
We thank: Dr. Nancy Bunin (The Children’s Hospital of Philadelphia) for care of the index patient during the post-transplant process; Dr. John Choi (The Children’s Hospital of Philadelphia) and Dr. Daniela Mihova (University of Pennsylvania) for immunohistochemistry assistance; the patients and family for their assistance in this effort.
Sources of Funding: NIH R01 AI076946 (JSO) and the Jeffrey Modell Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Filipovich AH. Hemophagocytic lymphohistiocytosis and other hemophagocytic disorders. Immunol Allergy Clin North Am. 2008 May;28(2):293–313. viii. doi: 10.1016/j.iac.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007 Feb;48(2):124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 3.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008 Sep;8(9):713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.zur Stadt U, Rohr J, Seifert W, Koch F, Grieve S, Pagel J, et al. Familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) is caused by mutations in Munc18-2 and impaired binding to syntaxin 11. Am J Hum Genet. 2009;85:482–92. doi: 10.1016/j.ajhg.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zur Stadt U, Beutel K, Kolberg S, Schneppenheim R, Kabisch H, Janka G, et al. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D, STX11, and RAB27A. Hum Mutat. 2006 Jan;27(1):62–8. doi: 10.1002/humu.20274. [DOI] [PubMed] [Google Scholar]
- 6.Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. 2009 Dec;119(12):3765–73. doi: 10.1172/JCI40732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeths M, Entesarian M, Al-Herz W, Chiang SC, Wood SM, Al-Ateeqi W, et al. Spectrum of clinical presentations in familial hemophagocytic lymphohistiocytosis type 5 patients with mutations in STXBP2. Blood. 2010 Oct 14;116(15):2635–43. doi: 10.1182/blood-2010-05-282541. [DOI] [PubMed] [Google Scholar]
- 8.Cetica V, Santoro A, Gilmour KC, Sieni E, Beutel K, Pende D, et al. STXBP2 mutations in children with familial haemophagocytic lymphohistiocytosis type 5. J Med Genet. 2010 Sep;47(9):595–600. doi: 10.1136/jmg.2009.075341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orange JS, Roy-Ghanta S, Mace EM, Maru S, Rak GD, Sanborn KB, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest. 2011 Apr 1;121(4):1535–48. doi: 10.1172/JCI44862. [DOI] [PMC free article] [PubMed] [Google Scholar]