Abstract
There is currently great need for high-quality, low-cost, point-of-care diagnostics that can benefit patients in resource-limited settings, and correspondingly growing interest in the diagnostic utility of microfluidic platforms based on paper. We describe the development, early clinical testing, and potential clinical impact of a novel paper-based, multiplexed microfluidic assay designed for rapid, semi-quantitative measurement of AST and ALT in a fingerstick specimen. This device ultimately holds promise for providing universal access to affordable point-of-care screening for drug-induced liver injury in resource-limited settings, and opens the door to development of similar point-of-care clinical assays for other important analytes.
Technological Primer
In developed nations, monitoring the status of the liver via measurements of serum transaminases (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) is a standard part of medical care, particularly for individuals who either have underlying liver disease or who are taking medications that can cause hepatotoxicity. Monitoring for drug-induced liver injury (DILI) via serial transaminase measurements is particularly important in patients on therapy for HIV and/or tuberculosis (TB) 1, 2. However, despite the need, transaminase monitoring in resource-limited settings is often limited or precluded by logistical and practical concerns. Testing typically requires collecting whole blood by venipuncture, centrifuging to separate serum, and testing the serum on a large automated platform. Such systems are expensive and require highly trained technicians for testing and maintenance, making them impractical for use and scale-up in developing countries. Testing is often done in centralized or regional laboratories in these settings, which can delay obtaining and acting on results. Moreover, many patients have strong negative feelings about venipuncture itself, which can be a barrier to care 3. Because of these obstacles, in many resource-limited settings patients on potentially hepatotoxic medications receive minimal or no monitoring during treatment.
Towards the ultimate goal of providing universal access to affordable point-of-care (POC) screening for DILI, we have recently described the development and early clinical testing of a paper-based, multiplexed microfluidic assay designed for rapid, semi-quantitative measurement of AST and ALT in a fingerstick specimen 4. Our device is a representative of an emerging class of microfluidic platforms based on paper 5–18. Paper-based microfluidic devices consist of hydrophilic paper channels defined by patterning of hydrophobic barriers 5, 7, 8 or by cutting 11–15, 18. Using these defined channels, fluid flow (drawn by wicking) can be directed towards specific detection zones and operations, such as mixing, splitting, and filtration, can be performed autonomously.
Unlike lateral flow tests (traditional POC “paper-based devices”), which typically run small numbers of tests in series (requiring that reagents and buffers for each test be compatible and that assays not cross-react), paper-based microfluidic devices have the capacity to split a single, low-volume (<40 µl) sample into multiple separate portions which can each be assayed in parallel. This allows for high-level multiplexing of independently optimized assays and avoids cross-reactivity between assays. Like lateral flow tests, paper-based microfluidic devices require no power or instrumentation and are portable and disposable.
Prior proof-of-principle studies 6–19 have demonstrated the potential for clinical application of paper-based microfluidic technology by demonstrating the ability to conduct clinical chemistry, enzymatic, and immunoassay tests on patterned paper, visually and quantitatively (latter through the use of cell phone cameras), and the viability of both single layer 7, 9, 11–19 and 3D 8 patterned paper devices. Our recent work 4 advanced this field by presenting the first validation of a prototype paper-based microfluidic device using actual clinical specimens and the first demonstration of a field-ready clinical test for transaminase monitoring.
Findings
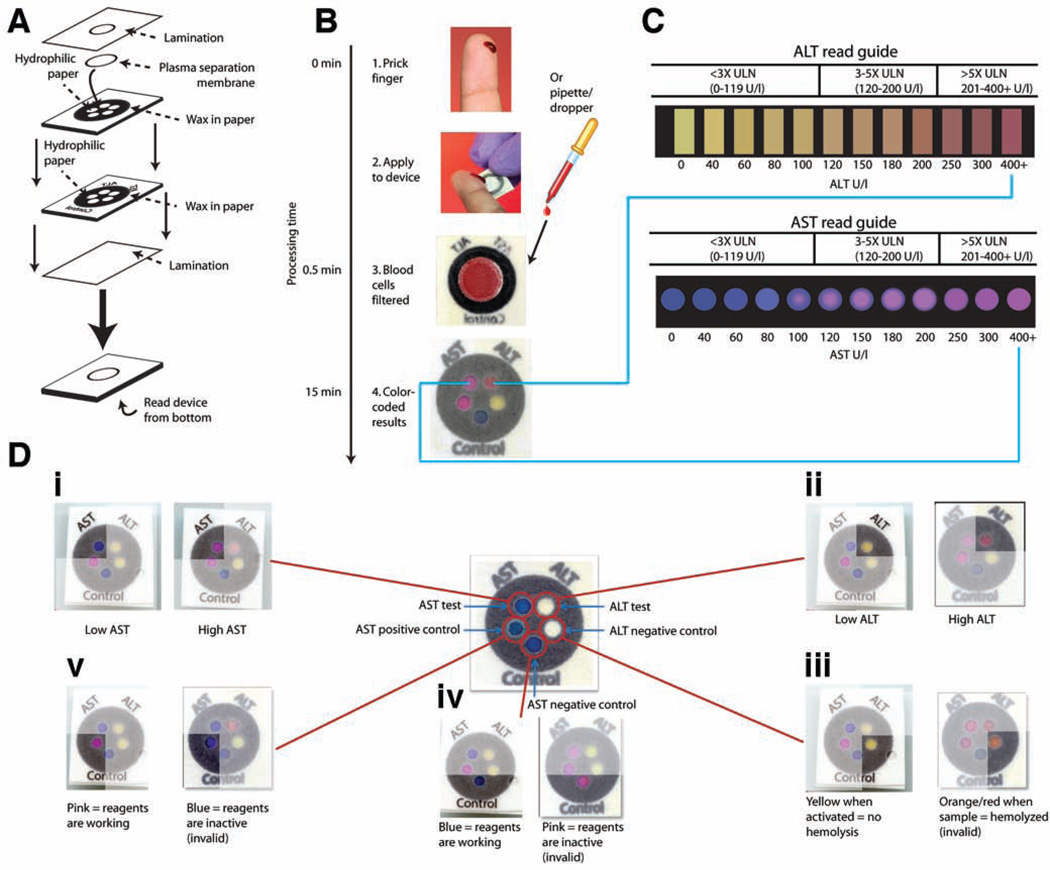
Our transaminase test is an example of a 3D device made from layering patterned paper (Fig. 1A). The postage stamp-sized device performs two separate tests on a single clinical sample in 15 minutes: one zone measures AST and another measures ALT (Fig. 1, B and C). The test also contains three control zones to ensure proper device performance (Fig. 1D). Each of the 5 test zones has a unique environment (reagents, buffers, pH) that ensures specificity. The AST assay chemistry, based on the sulfonation of methyl green, results in a visual transformation from blue to pink (Fig. 1C). The ALT assay, based on peroxidase chemistry, generates a red dye in the presence of elevated ALT levels. (Fig. 1C).
Fig. 1.
Schematic of the paper-based AST/ALT test design and protocol. (A) The device consists of two layers of similarly patterned paper, a plasma separation membrane, and a laminated cover of polyester film. (B and C) A drop of whole blood (either a fingerstick specimen or 30 µl of a specimen obtained by venipuncture) is applied to the back of the device. Red and white blood cells are filtered out by the plasma separation membrane, whereas plasma wicks to the five detection zones through patterned hydrophobic channels in the paper (B). After 15 min, the AST and ALT test zones are matched to a color read guide (C) to obtain a concentration value. Results are interpreted as being within one of three bins of values: <3× ULN (ULN defined as 40 U/liter); 3–5× ULN; or >5× ULN. (D) Detailed schema of the paper-based transaminase test and possible colorimetric readouts for the 5 zones (i to v). A schematic of test and control zones (before receiving a sample) is shown in the center of the figure. (i) AST test zone: low/normal AST values (<80 U/liter) result in a dark blue color, whereas high AST values (>200 U/liter) result in a bright pink color. (ii) ALT test zone: low/normal ALT values (<60 U/liter) result in a yellow color, whereas high ALT values (>200 U/liter) result in a deep red color. (iii) ALT negative control zone: a change from white to yellow indicates appropriate device activation; in the event of sample hemolysis, the zone becomes orange/red and the device is read as “invalid.” (iv) AST negative control zone: the baseline blue color remains unchanged if dye chemistry is functioning properly, whereas the zone becomes bright pink in the event of nonspecific dye reaction and the device is read as “invalid.” (v) AST positive control zone: the zone changes from blue to pink if AST reagents are functioning properly, but remains dark blue if either the reagents are not functioning or the zone is not activated, in which case the device is read as “invalid.”
From Pollock NR, et al, A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci Transl Med 2012; 4:152ra29. Reprinted with permission from AAAS.
The test was carefully engineered for visual readout, in that the AST/ALT test zones provide a strong color change across the target clinical range (Fig. 1, C and D). We specifically optimized these color changes to correspond to the cutoffs currently used for clinical management decisions per TB treatment guidelines in the US 1. Thus the results of the test are interpreted as being within one of the following three “bins”: <3× the upper limit of normal (ULN, here defined as 40 U/L) (0–119 U/l), 3–5× ULN (120–200 U/l), or >5× ULN (>200 U/l)(Fig. 1C). By using the additional color gradation within each bin on the read guide, the reader can approximate where within the bin the result lies, allowing semi-quantitative readout. The three control zones notify the user of insufficient sample volume, hemolysis, or damaged reagents (Fig. 1D); each zone is interpreted as “valid” or “invalid” and an “invalid” result in any control zone invalidates the entire device.
After demonstrating satisfactory analytical performance, including assessment of test linearity (linear across the target clinical range, i.e. 40 to 200 U/l), limit of detection (ALT, 53 U/l; AST 84 U/l), and repeatability (CV values <10% for both AST and ALT tests in serum and blood samples), we proceeded to evaluate the performance of the device with 223 clinical specimens obtained by venipuncture. We tested 30 µL aliquots of paired whole blood and serum specimens drawn simultaneously from patients within the previous 5 h for routine clinical testing, and for which results of automated serum transaminase testing were available. 15 min after sample application, each device was analyzed by three blinded readers who independently recorded a result in U/l (rounded to the nearest 10 U/l) using the read guide. Results of the paper-based test for paired whole blood and serum specimens were compared to the results of automated serum transaminase testing as the gold-standard to evaluate “bin placement accuracy”: whether the result of the paper-based assay was in the same bin (<3× ULN, 3–5× ULN, or >5× ULN) as the gold-standard result.
Overall accuracies for the device (data for all three bins in aggregate) were ≥90% for both AST and ALT in both serum and whole blood (Table 1). “Per bin” accuracies were calculated by dividing the number of correctly binned samples in each bin by the total number of samples in that bin. We observed lower ALT accuracies in whole blood than in serum, which we ascribe to the age of the whole blood at the time of testing (whole blood samples with normal baseline ALT levels begin to yield artificially high ALT values on the paper test after aging for >6 h, which we believe is due to red blood cell release of lactate (subsequently converted to pyruvate which activates the ALT assay)). The device also performed well with whole blood obtained via fingerstick from 10 healthy volunteers. Overall, our results suggested clinically acceptable agreement between the semi-quantitative paper test and the gold-standard automated assay method. We found that the test performed well in specimens taken from a clinically diverse population, including many patients who were critically ill with multiple abnormal laboratory values. This finding was consistent with the results of our direct evaluation of analytes with potential for assay interference; in particular, we showed that bilirubin (<10 mg/dl), creatinine (<15 mg/dl), and lactate (<200 mg/dl) did not interfere with either the ALT or the AST assay.
Table 1.
Bin placement accuracies for visual measurements in clinical specimens made using the paper-based transaminase test. 95% confidence intervals around the overall accuracy estimates are shown. For the bins, X = 40 U/l = ULN.
| Test | Specimen | Bin |
n samples in bin |
n correctly placed |
“Per bin” accuracy (%) |
Overall accuracy (%) |
|---|---|---|---|---|---|---|
| ALT | Serum | <3× | 89 | 88 | 99 | 95±4 |
| 3–5× | 12 | 11 | 92 | |||
| >5× | 19 | 15 | 79 | |||
| Blood | <3× | 70 | 66 | 94 | 90±6 | |
| 3–5× | 7 | 4 | 57 | |||
| >5× | 11 | 9 | 82 | |||
| AST | Serum | <3× | 88 | 85 | 97 | 91±5 |
| 3–5× | 26 | 18 | 69 | |||
| >5× | 14 | 14 | 100 | |||
| Blood | <3× | 69 | 68 | 99 | 94±5 | |
| 3–5× | 17 | 13 | 76 | |||
| >5× | 8 | 7 | 88 |
From Pollock NR, et al, A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci Transl Med 2012; 4:152ra29. Reprinted with permission from AAAS.
Importance of findings
We have shown that our simple and inexpensive paper-based assay can, in 15 minutes, provide visual measurements of AST and ALT in whole blood or serum which allow the user to place those values into one of three readout “bins” (<3× ULN, 3–5× ULN, and >5× ULN, corresponding to familiar clinical thresholds) with >90% accuracy. Our test performed well compared to automated methods, even in specimens that were obtained from critically ill patients with multiple derangements in other analytes, and that were up to 5 hours old at the time of testing. These experiments firmly established proof-of-concept and clinical relevance and set the stage for clinical field studies as outlined below.
Two FDA-approved devices exist that could potentially be used for rapid POC testing, but both are relatively expensive and rely on complex electronics and electricity/battery. The Roche Refletron Plus device requires venous blood draw and costs approximately $6,000 for the reader and an additional $4 per test. The Cholestech LDX is capable of using fingerstick samples, but costs approximately $3,000 for the reader and $4 per test. Manufacturing costs for our device are dependent on several key variables, including location, making them difficult to calculate accurately a priori. We anticipate, however, that our device can ultimately be produced at a cost of less than $0.10 per test.
Our platform offers several technical advantages over other platforms developed with paper-based microfluidic technology. The 3D, multi-layered design of our platform allows it to split plasma from a 30–35 µl sample of whole blood into 5 separate streams for parallel testing with independently optimized assays at ambient temperature. There is no requirement for pre-analytical sample processing, additional reagents, or equipment to read the device; operation of the device requires only tools to obtain and apply the blood sample (i.e. a lancet and potentially a capillary tube).
Translation of findings into routine clinical diagnosis and treatment
The next step in this work is to test the device for fingerstick use in a target patient population and clinical environment. We have recently completed such a study in a large HIV clinic population in Vietnam, in collaboration with PATH (Program for Alternative Technology in Health; Seattle, WA), the Harvard Medical School AIDS Initiative in Vietnam (HAIVN), and the Hospital for Tropical Diseases (Ho Chi Minh City, Vietnam). The goals of this study were to assess operational feasibility, inter-operator variability, lot-to-lot variability, device failure rate, and device accuracy, with the intention to utilize results to modify the device for further field testing as needed. The results of this study are currently being analyzed.
An important question to consider as the device is optimized for ultimate clinical use is which ALT and AST bin cutoffs would have the highest utility world-wide given existing country-specific and disease-specific clinical management guidelines. Thus far our test has been optimized for detection of AST and ALT values >3× and >5× ULN, in light of US TB treatment guidelines 1 that emphasize these cutoffs (in concert with symptoms of hepatotoxicity) for making management decisions. U.S. HIV treatment guidelines 2 do not recommend strict AST and ALT cut-offs for clinical management of DILI, but do note that some experts stop drug treatment when the ALT level rises to more than 5–10× ULN. The WHO HIV treatment guidelines 20 use a grading scale for ALT and AST identical to the AIDS Clinical Trial Group (ACTG) adverse event scale 21, with cut-offs at 2.5×, 5×, and 10× ULN. Minor ALT elevations, defined as less than 5× ULN, can be managed with observation and continued treatment. Only elevations above 5× ULN prompt discontinuation of antiretroviral therapy (ART). Many developing countries, including South Africa, India, and Vietnam, have adopted the WHO grading scale for liver function monitoring in their national HIV treatment guidelines 22–24. While we hope that optimization of visual resolution in the 3–5× ULN range will allow utility across these varied guidelines, we are also evaluating the possibility of a “triage” use scenario, in which POC test values above a pre-specified threshold would prompt automated quantitative testing by venipuncture. Performance of the device at various bin cutoffs is being closely examined during real-time fingerstick testing.
An additional question is whether the final device should include both AST and ALT tests or only an ALT test. While most US clinicians tend to order ALT and AST together, US guidelines for monitoring for DILI during TB treatment 1 focus recommendations on ALT levels, while noting that AST can provide adjunctive information (e.g. alcohol-related transaminitis); WHO guidelines 25 mention only ALT. Most international HIV treatment guidelines recommend only ALT for routine monitoring of ART. The Southern African HIV Clinicians Society explicitly cites cost considerations in their advice to use only ALT measurement for the monitoring of hepatoxicity 22. Similarly, the WHO and Vietnam national HIV treatment guidelines recommend only ALT monitoring but without noting any underlying rationale 20, 24.
Successful development of this device for clinical use will require iterative device optimization, thorough exploration of manufacturing conditions, material quality control, and device stability, and field testing under varied environmental conditions. Given that the device is designed to be read by eye, an understanding of the minimal training requirements for novice users will be key to understanding in which clinical environments this test can be used—whether that be in centralized clinics by trained staff, in decentralized clinical settings by minimally trained health-care workers, or even at home by the patients themselves.
Roadblocks and/or limitations
Our paper-based microfluidic transaminase test is essentially the first device of its class to come this far down the pathway towards clinical use, and thus there are no clear precedents for performance standards--every aspect of its design and performance are effectively being evaluated for the first time. In fact, even the question “how accurate does this device need to be in order to be useful” does not have a straightforward answer, and we anticipate that this discussion will accompany our applications for regulatory approval. In an ideal world, this device would simply be as accurate as automated testing performed on venipuncture blood. However, it can be argued that it is not reasonable to expect the performance of a paper-based test to perfectly match that of an automated testing platform. The benefits of POC use, simplicity, and extremely low cost may in fact allow a slightly less accurate paper-based device to have higher overall clinical impact than a more accurate but technically complicated and expensive test not available at POC.
Conclusions
Hepatotoxicity is a major adverse event associated with both TB and HIV therapy, and monitoring for DILI is accordingly a major priority in the care of these patients. The overall incidence of clinically relevant hepatotoxicity on TB therapy (typically due to the medications isoniazid, rifampin, and pyrazinamide) ranges from 2 to 33%, and risk may be increased by multiple factors, such as pre-existing liver disease (e.g. hepatitis B and/or C), alcohol use, and increasing age 1, 26. Hepatotoxicity rates associated with nevirapine-based HIV therapy (widely used in the developing world) can exceed 13%, depending on underlying risk factors and treatment 2, 27, 28. Simultaneous treatment for both TB and HIV further complicates matters, and sometimes generates additive risk of hepatotoxicity 29, 30. There are also other important and common global conditions (such as epilepsy) for which treatment can be associated with substantial hepatotoxicity. In practice, unfortunately, it is difficult to predict accurately which patients on treatment will actually develop hepatotoxicity 31, and thus active monitoring to detect DILI in at-risk patients is essential.
We anticipate that our device, once development is complete, will make extremely inexpensive and minimally invasive transaminase testing available at POC for all who need it, providing distinct advantages over current automated methods using venipuncture. Given that aversion to venipuncture can be a barrier to optimal care 3, our fingerstick test could conceivably improve treatment adherence. Finally, we anticipate that successful development of this paper-based platform will facilitate the development of similar POC clinical assays for other important analytes.
Acknowledgments
Funding: The referenced work (published in Science Translational Medicine) was supported in part by a grant from the Department of Defense/Center for Integration of Medicine and Innovative Technology (W81XWH-09-2-0001). N.R.P. is supported by a National Institute of Health K23 grant (5 K23 AI074638-04). J.P.R. is supported by a Harvard subcontract of a grant from the Bill & Melinda Gates Foundation (51308 – Zero Cost Diagnostics).
Dr. Rolland is listed as an inventor on a patent application filed pertaining to results: U.S. Provisional Patent Application No. 61/555,977 "Quantitative Microfluidic Devices."
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Drs. Pollock and Colby have no relevant conflicts of interest.
REFERENCES
- 1.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174(8):935–952. doi: 10.1164/rccm.200510-1666ST. Epub 2006/10/06. [DOI] [PubMed] [Google Scholar]
- 2.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed December 28, 2012]. pp. 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 3.Shieh FK, Snyder G, Horsburgh CR, et al. Predicting non-completion of treatment for latent tuberculous infection: a prospective survey. Am J Respir Crit Care Med. 2006;174(6):717–721. doi: 10.1164/rccm.200510-1667OC. Epub 2006/07/01. [DOI] [PubMed] [Google Scholar]
- 4.Pollock NR, Rolland JP, Kumar S, et al. A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci Transl Med. 2012;4(152) doi: 10.1126/scitranslmed.3003981. 152ra29.Epub 2012/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez AW, Phillips ST, Whitesides GM, et al. Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem. 2010;82(1):3–10. doi: 10.1021/ac9013989. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- 6.Martinez AW, Phillips ST, Carrilho E, et al. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem. 2008;80(10):3699–3707. doi: 10.1021/ac800112r. Epub 2008/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez AW, Phillips ST, Butte MJ, et al. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed Engl. 2007;46(8):1318–1320. doi: 10.1002/anie.200603817. Epub 2007/01/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez AW, Phillips ST, Whitesides GM. Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc Natl Acad Sci U S A. 2008;105(50):19606–19611. doi: 10.1073/pnas.0810903105. Epub 2008/12/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng CM, Martinez AW, Gong J, et al. Paper-based ELISA. Angew Chem Int Ed Engl. 2010;49(28):4771–4774. doi: 10.1002/anie.201001005. Epub 2010/06/01. [DOI] [PubMed] [Google Scholar]
- 10.Nie Z, Deiss F, Liu X, et al. Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip. 2010;10(22):3163–3169. doi: 10.1039/c0lc00237b. Epub 2010/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn JL, Lutz B, Fu E, et al. Microfluidics without pumps: reinventing the Tsensor and H-filter in paper networks. Lab Chip. 2010;10(20):2659–2665. doi: 10.1039/c004821f. Epub 2010/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu E, Lutz B, Kauffman P, et al. Controlled reagent transport in disposable 2D paper networks. Lab Chip. 2010;10(7):918–920. doi: 10.1039/b919614e. Epub 2010/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz BR, Trinh P, Ball C, et al. Two-dimensional paper networks: programmable fluidic disconnects for multi-step processes in shaped paper. Lab Chip. 2011;11(24):4274–4278. doi: 10.1039/c1lc20758j. Epub 2011/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu E, Kauffman P, Lutz B, et al. Chemical signal amplification in two-dimensional paper networks. Sens Actuators B Chem. 2010;149(1):325–328. doi: 10.1016/j.snb.2010.06.024. Epub 2010/08/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan MS, Thouas G, Shen W, et al. Paper diagnostic for instantaneous blood typing. Anal Chem. 2010;82(10):4158–4164. doi: 10.1021/ac100341n. Epub 2010/04/27. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Tian J, Garnier G, et al. Fabrication of paper-based microfluidic sensors by printing. Colloids Surf B Biointerfaces. 2010;76(2):564–570. doi: 10.1016/j.colsurfb.2009.12.023. Epub 2010/01/26. [DOI] [PubMed] [Google Scholar]
- 17.Dungchai W, Chailapakul O, Henry CS. Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal Chim Acta. 2010;674(2):227–233. doi: 10.1016/j.aca.2010.06.019. Epub 2010/08/04. [DOI] [PubMed] [Google Scholar]
- 18.Fenton EM, Mascarenas MR, Lopez GP, et al. Multiplex lateral-flow test strips fabricated by two-dimensional shaping. ACS Appl Mater Interfaces. 2009;1(1):124–129. doi: 10.1021/am800043z. Epub 2009/01/28. [DOI] [PubMed] [Google Scholar]
- 19.Vella SJ, Beattie P, Cademartiri R, et al. Measuring markers of liver function using a micropatterned paper device designed for blood from a fingerstick. Anal Chem. 2012;84(6):2883–2891. doi: 10.1021/ac203434x. Epub 2012/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antiretroviral therapy for HIV infection in adults and adolescents in resource-limited settings: recommendations for a public health approach, 2010 revision. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 21.AIDS Clinical Trials Group. Table of grading severity of adult adverse experiences. Rockville, MD: Division of AIDS, National Institute of Allergy and Infectious Diseases; 1996. [Google Scholar]
- 22.Meintjes G, Maartens G, Boulle A, et al. Guidelines for antiretroviral therapy in adults. S Afr J HIV Med. 2012;13(3):114–133. [Google Scholar]
- 23.Antiretroviral Therapy Guidelines for HIV-infected Adults and Adolescents Including Post-exposure Prophylaxis. India Ministry of Health and Family Welfare; 2007. [Google Scholar]
- 24.Vietnam Ministry of Health. Guidelines on the Diagnosis and Treatment of HIV/AIDS. Hanoi: 2009. [Google Scholar]
- 25.Treatment of Tuberculosis guidelines 4th edition. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 26.Tostmann A, Boeree MJ, Aarnoutse RE, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23(2):192–202. doi: 10.1111/j.1440-1746.2007.05207.x. Epub 2007/11/13. [DOI] [PubMed] [Google Scholar]
- 27.McKoy JM, Bennett CL, Scheetz MH, et al. Hepatotoxicity associated with long- versus short-course HIV-prophylactic nevirapine use: a systematic review and metaanalysis from the Research on Adverse Drug events And Reports (RADAR) project. Drug Saf. 2009;32(2):147–158. doi: 10.2165/00002018-200932020-00007. Epub 2009/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez E, Blanco JL, Arnaiz JA, et al. Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy. AIDS. 2001;15(10):1261–1268. doi: 10.1097/00002030-200107060-00007. Epub 2001/06/27. [DOI] [PubMed] [Google Scholar]
- 29.Shipton LK, Wester CW, Stock S, et al. Safety and efficacy of nevirapine- and efavirenz-based antiretroviral treatment in adults treated for TB-HIV co-infection in Botswana. Int J Tuberc Lung Dis. 2009;13(3):360–6. Epub 2009/03/12. [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann CJ, Charalambous S, Thio CL, et al. Hepatotoxicity in an African antiretroviral therapy cohort: the effect of tuberculosis and hepatitis B. AIDS. 2007;21(10):1301–1308. doi: 10.1097/QAD.0b013e32814e6b08. Epub 2007/06/05. [DOI] [PubMed] [Google Scholar]
- 31.Severe isoniazid-associated liver injuries among persons being treated for latent tuberculosis infection --- United States, 2004--2008. MMWR Morb Mortal Wkly Rep. 59(8):224–229. Epub 2010/03/06. [PubMed] [Google Scholar]