Abstract
Background
The artificial urinary sphincter (AUS) is a well-established treatment for male stress urinary incontinence.
Objective
We aimed to characterize the surgical learning curve for reoperation rates after AUS implantation.
Design, Setting, and Participants
The study cohort consisted of 65,602 adult males who received an AUS 1988–2008, constituting close to 90% of all operations conducted during that time. Data on reoperations were obtained from the manufacturer, which requires documentation for warranty coverage.
Measurements
Surgeon experience was calculated as the number of original AUS implants performed prior to the index patient’s surgery. Multivariable, logistic regression models were used to examine the association between experience and reoperative rates, adjusted for case-mix.
Results and Limitations
There was a slow but steady decrease in reoperative rates with increasing surgeon experience (p=0.020), showing no plateau through 200 procedures. The risk of reoperation for a surgeon with 5 prior cases was 24.0%, which decreased to 18.1% for a surgeon with 100 prior implants (absolute risk difference 5.9%; 95%CI 1.3%, 10.1%) and to 13.2% for a surgeon with 200 prior implants absolute risk difference 10.7%; 95%CI 2.6%, 16.6%). Two-thirds of contemporary patients (2000 – 2008) saw a surgeon who had done 25 or fewer prior AUS implants; only 9% saw a surgeon with 100 or more prior procedures.
Conclusions
There is a clear and obvious disparity between the learning curve for AUS surgery and typical surgeon experience, suggesting a considerable burden of avoidable reoperations. Efforts to flatten the learning are urgently needed.
Keywords: Artificial Urinary Sphincter, Urinary Incontinence, Surgical Technique
Introduction
The artificial urinary sphincter (AUS) is a well-established treatment for male stress urinary incontinence1, 2 including that resulting from radical prostatectomy. The AUS system consists of multiple components, including at least a urethral cuff, a pressure regulating balloon, and a control pump. The urethral cuff and the pressure regulating balloon come in multiple sizes allowing significant operative variability1.
The AUS is a complex prosthetic procedure requiring intraoperative measurements. It is plausible that operative outcomes improve with increasing surgeon experience. The object of this study was to study the learning curve for AUS surgery, measured in terms of reoperation rates, in contrast to the typical experience levels of surgeons in contemporary practice.
Patients and Methods
Study Cohort and Data Sources
Data were obtained from American Medical Systems, Inc., the manufacturer of the artificial urinary sphincter. All data were originally collected as part of routine care and were fully de-identified before download. There were 72,908 total unique, complete records for male AUS procedures. Those treated before 1988 (n= 5,833) were excluded because major modifications were made to the AUS in 1987, after which point there has been little modification to the device. These modifications have been associated with significantly better outcomes3, 4. For the purpose of our analyses, we excluded pediatric cases (n=1,473). The study cohort consisted of 65,602 males over the age of 18 who received an AUS after 1987. Each original surgery was performed by one (n= 60,135; 92%), two (n= 5,339; 8%) or three (n=128; <1%) of the 8,497 surgeons in this data set. All patients were treated in the United States.
Outcome ascertainment
The AUS is made by only one manufacturer, American Medical Systems, Minnetonka, MN. The company requires all surgeons to submit a patient information form after every implant in order to for the device to be under warranty coverage, and has kept data on each procedure since its introduction in the 1970s. This form contains information about indications for procedure, technical questions related to surgical technique, including location of urethral cuff and surgical approach used. The estimated compliance rate for form completion is 89%, based on 3938 responses2 compared to 4426 AUS units sold5 in 2005. Reoperative surgery for removal of an AUS represents a situation where the operating surgeon does not replace a device and therefore does not submit a form. In these cases, the manufacturer creates a patient information form to capture the removal either through a complaint mechanism or through field sales representatives. In a 5 year audit of reimplanted AUS, 99% of the reimplants had a record of previous removal in the patient information database, suggesting high capture of reoperative surgery to remove an AUS.
Statistical Methods
Surgeon experience was calculated as the number of original AUS implants performed on males prior to the patient’s surgery. If more than one surgeon participated in a surgery, each surgeon was credited in terms of experience, with the patient indicator for experience coded by randomly selecting one of the surgeons.
The censoring structure of the data is unknown: a patient with no subsequent surgery may have survived for many years with a functioning AUS, or may have died or emigrated at any point. Accordingly, rather than time-to-event analyses we used binary outcomes at fixed follow-up times after surgery, such as reoperative surgery within five years. The study database was closed in 2008, and so we excluded patients treated after 2003 for the main analysis.
A learning curve requires a complete or near complete surgical history for each surgeon: we therefore specified that, to be eligible, we must have data on one of the surgeon’s first 10 surgeries. This subset served as our main cohort and included 6,868 surgeons who treated 89% (40,347 of 45,207 patients) of the full cohort between 1988 and 2003, the cutoff year for the primary analysis of any correctional surgery within 5 years.
Our overall aim was to examine the association between experience and the rate of any of the four reoperations (revisions, replacements, removals and reimplants). We hypothesized that the learning curve would be gradual and require 100 – 200 operations to plateau. Since the expected lifetime of the current model AUS is over 10 years, we chose a primary endpoint of five years since it is reasonable that any intervention within this time frame be attributable to the surgery and not device malfunction.
For our main analysis, we created a multivariable, logistic regression model in which surgeon experience was entered as a continuous variable with age and residency as covariates. Age was included using restricted cubic splines with knots at the tertiles; residency was used as a covariate on the grounds that patients living outside the United States may be less likely to return for a reoperation. We saw no evidence of non-linearity in the association between experience and outcome, and so all principal analyses use only the linear term for experience.
Since data from different patients seen by the same surgeon are not independent, we incorporated within-surgeon clustering into our analyses using a generalized estimating equations approach by specifying the cluster option in Stata statistical software (version 11; Stata Corp, College Station, TX). To produce a learning curve, we used the mean value of covariates to calculate the probability of requiring reoperation within 5 years predicted by the model for each level of surgical experience.
Results
Patient and procedure characteristics of the 65,602 males over the age of 18 who received an artificial urinary sphincter (AUS) after 1987 are given in Table 1. Age and etiology of incontinence (post-RP and neurogenic) did not differ importantly by surgeon experience.
Table 1. Characteristics of men 18 and older who received an Artificial Urethral Sphincter (AUS) between 1988 and 2008, for surgeons with full case histories. Categorization of patients into different levels of surgeon experience is for illustrative purposes only (n=59,266).
Data are given as median (quartiles) or frequency (%).
| Overall | Surgeon experience (no. of cases before the incident case) | P* | |||
|---|---|---|---|---|---|
| <5 | 5–20 | >20 | |||
| Patient and surgical characteristics | N=59,266 | N=21,984 | N=23,972 | N=13,310 | |
| Age at implantation | 70 (64, 75) | 69 (64, 74) | 70 (64, 75) | 69 (63, 75) | 0.7 |
| Treated in the US | 40,879 (69%) | 15,537 (71%) | 16,826 (70%) | 8,516 (64%) | 0.3 |
| Reason for incontinence | |||||
| Post RP | 41,629 (70%) | 15,243 (69%) | 17,152 (72%) | 9,234 (69%) | 0.9 |
| Neurogenic | 3,456 (6%) | 1,319 (6%) | 1,348 (6%) | 789 (6%) | 0.17 |
| Cuff size (cm) | |||||
| <=5 | 50,965 (86%) | 17,942 (82%) | 21,095 (88%) | 11,928 (90%) | 0.032 |
| 5.5–7.5 | 2,235 (4%) | 693 (3%) | 850 (4%) | 692 (5%) | |
| >=8 | 785 (1%) | 212 (1%) | 284 (1%) | 289 (2%) | |
| Missing | 5,281 (9%) | 3,137 (14%) | 1,743 (7%) | 401 (3%) | |
| Location of cuff | 0.017 | ||||
| Urethral | 53,200 (90%) | 18,635 (85%) | 21,945 (92%) | 12,620 (95%) | |
| Bladder neck | 785 (1%) | 212 (1%) | 284 (1%) | 289 (2%) | |
| Missing | 5,281 (9%) | 3,137 (14%) | 1,743 (7%) | 401 (3%) | |
| Unadjusted Outcomes | |||||
| Removal | 1,746 (3%) | 647 (3%) | 769 (3%) | 330 (2%) | - |
| Reimplantation | 916 (2%) | 371 (2%) | 400 (2%) | 145 (1%) | - |
| Revision | 7,787 (13%) | 3,234 (15%) | 3,174 (13%) | 1,379 (10%) | - |
| Replacement | 3,239 (5%) | 1,261 (6%) | 1,386 (6%) | 592 (4%) | - |
| Any Correction | 11,483 (19%) | 4,631 (21%) | 4,779 (20%) | 2,073 (16%) | - |
Associations between patient characteristics and surgeon experience were tested using linear regression (age) and logistic regression (etiology of incontinence: radical prostatectomy or neurogenic, and location of AUS: urethral or bladder neck), and ordinal logistic regression (cuff size).
The majority of surgeons included in our analyses performed in total fewer than 5 AUS implants (n=4,757; 59%); 23% (n=1,813) had a lifetime history 10 or more prior cases and <2% (n=134) performed 50 or more AUS implants (Table 2).
Table 2. Distribution of experience at the time of surgery for men 18 and older who received an Artificial Urethral Sphincter (AUS) between 1988 and 2008.
Includes only surgeons with full case history (defined as at least one of the first 10 cases on record).
| By surgeon | By Patient | ||
|---|---|---|---|
| Total lifetime number of AUS implant procedures | No. of surgeons (%) | Surgeon experience at time of initial implant | No. of patients (%) |
| <5 | 4,757 (59) | <5 | 21,984 (37) |
| 5–9 | 1,443 (18) | 5–9 | 11,696 (20) |
| 10–49 | 1,679 (21) | 10–49 | 21,395 (36) |
| 50–99 | 106 (1) | 50–99 | 3,144 (5) |
| 100–199 | 25 (<1) | 100–199 | 904 (2) |
| ≥200 | 3 (<1) | ≥200 | 143 (<1) |
| Total | 8,013 | Total | 59,266 |
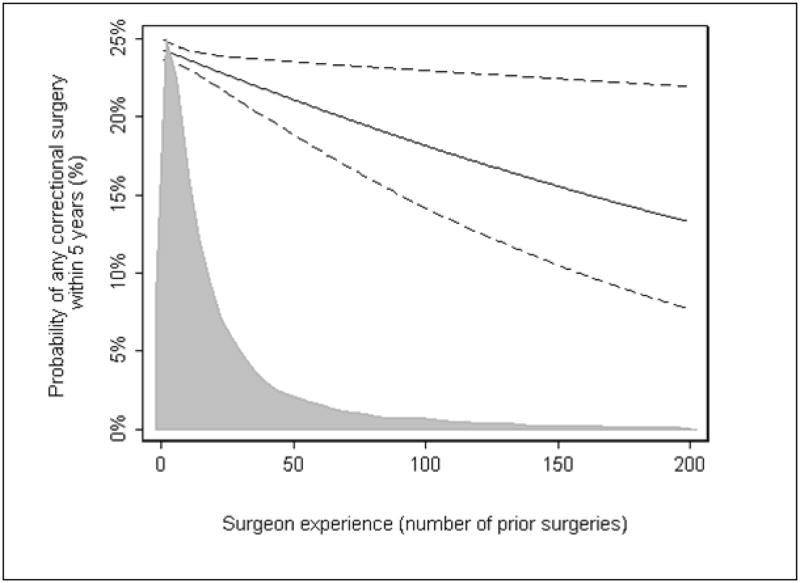
The learning curve for reoperation after AUS surgery is shown in figure 1. There is a slow but steady decrease in the risk of reoperation with increasing surgeon experience (p=0.020). The risk of reoperative surgery for an inexperienced surgeon, with 5 prior cases, was 24.0% which decreased to 18.1% for an experienced surgeon with 100 prior operations (absolute risk difference 5.9%; 95%CI 1.3%, 10.1%) and 13.2% for a surgeon with 200 prior operations (risk difference 10.7%; 95%CI 2.6%, 16.6%).
Figure 1.
The surgical learning curve for reoperative surgery required after implantation of AUS. Predicted probability (solid) and 95% confidence intervals (dashed) of reoperative surgery at 5 years after original device implantation are plotted against surgeon experience. Probabilities are given for a typical patient, i.e. Average age and US residency. Includes only surgeons with full case history (defined as at least one of the first 10 cases on record). The overlaying density plot shows the distribution of patients by surgeon experience in a contemporary cohort (treated between 2000 and 2008).
Figure 1 overlays the distribution of surgeon experience of contemporary patients undergoing AUS, defined as those undergoing surgery in 2000 or after. There is a clear and obvious disparity between the learning curve and typical surgeon experience: 67% of patients treated between 2000 and 2008 were treated by a surgeon who had done 25 or fewer prior AUS implants; only 9% saw a surgeon with a prior experience of 100 or more.
Analyses for reoperations occurring within 6 months and 2 years are shown in figure 2. Trends are very similar to the principal analysis. The association between experience and outcome is significant at 6 months (p=0.018), but just fails to meet conventional levels of statistical significance for the analysis at two years (p=0.07), possibly due to the lower event rate in this analysis.
Figure 2.
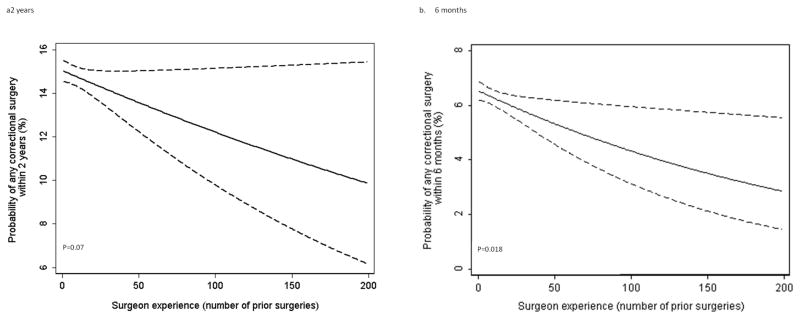
The surgical learning curve for reoperative surgery required after implantation of AUS. Predicted probability (black) and 95% confidence intervals (dashed) of reoperative surgery at a. 2 years, b. 6 months after original device implantation are plotted against surgeon experience. Probabilities are given for a typical patient, i.e. Average age, and US residency and includes only surgeons with full case history (defined as at least one of the first 10 cases on record
In an analysis by type of correctional procedure, we saw no significant difference between correctional surgery subtypes (p=0.4). Table 3 shows a number of sensitivity analyses conducted to examine the robustness of our findings. The relationship between surgeon experience and outcome was statistically significant for all sensitivity analyses, with estimates of the effect of experience largely falling within the 95% confidence intervals of the main analysis.
Table 3.
Sensitivity analyses. Estimates of learning curve given various assumptions.
| Adjusted 5-year reoperative rates* | ||||||
|---|---|---|---|---|---|---|
| Analysis | Number of surgeons+ | Number of patients+ | Surgeons with 5 prior operations | Surgeons with 100 prior operations | Difference between reoperative rates | p value† |
| Main analysis | 6,868 | 40,347 | 24.0% | 18.1% | 5.9% (95%CI 1.3%, 10.1%) | 0.020 |
| Adjusting for cuff size | 6,868 | 40,347 | 24.0% | 18.0% | 6.0% | 0.013 |
| Surgeons with at least 25 total cases | 336 | 12,257 | 24.9% | 16.9% | 8.0% | 0.015 |
| Surgeons with at least 50 total cases | 64 | 4,229 | 27.5% | 17.4% | 10.2% | 0.008 |
| Most experienced surgeon for patients with multiple attending surgeons | 6,404 | 38,864 | 23.9% | 16.6% | 7.3% | <0.001 |
| Least experienced surgeon for patients with multiple attending surgeons | 7,083 | 39,914 | 24.0% | 19.2% | 4.8% | 0.045 |
| Patients without neurogenic etiology‡ | 6,681 | 37,628 | 23.6% | 17.9% | 5.7% | 0.031 |
| Surgeons with complete case history defined as data on 1st lifetime case | 6,249 | 32,743 | 23.6% | 17.3% | 6.3% | 0.004 |
| Surgeons with complete case history defined as data on 25th lifetime case | 6,908 | 42,186 | 24.0% | 19.7% | 4.3% | 0.039 |
| Excluding removals | 6,868 | 40,347 | 22.7% | 16.9% | 5.8% | 0.018 |
Adjusted probabilities are for a patient of average age with residency in the United States.
Test for the association between experience and outcome in the multivariable model (two-sided P value).
Incontinence of neurogenic etiology includes neurologic disorder, other neurogenic, meningomyelocele, sacral agenesis, spinal cord trauma/tumor/surgery, and DESD (detrusor external sphincter dyssynergia).
The number of surgeons and patients may differ from table 2 for two reasons: 1) Table 2 includes patients through 2008 whereas the main analysis includes only those through 2003. 2) In the case of analyses using different criteria to select surgeons for patients with multiple attending surgeons, selection of different surgeons affected the number with full history of cases.
As a “negative control”, we examined reoperative rates after 10 years since these have been shown to be a function of the device and not surgeon technique. We adjusted for the same covariates as the main analysis as well as year of initial procedure, on the grounds that the likelihood of device failure increases with time since surgery. In the 16,968 patients who were event free at 10 years, but who were followed for at least 10 years, 5% of patients eventually received a reoperation. Surgeon experience was associated with an increased, rather than a decreased, likelihood of reoperation (4.6% vs 8.8%. for surgeons with 5 and 100 prior cases p=0.036). This analysis therefore provides no evidence that case mix or selective reporting explained our principal findings.
Discussion
We have found a long learning curve for the standard AUS operation, with no plateau even after 200 procedures. The learning effect is large, with a near halving of reoperation rates for the most experienced surgeons. Yet the vast majority of contemporary patients are treated by surgeons with relatively little surgical experience: fewer than 1 in 10 patients were seen by a surgeon with a prior experience of 100 or more; two-thirds were treated by surgeons at the very early part of the learning curve, where reoperation rates were highest.
There are a number of reasons to believe why surgical experience might have a strong effect on the outcome of AUS surgery. The operation involves three different surgical components – a urethral cuff, a pressure regulating balloon, and a control pump1. Urethral cuff size is determined by measurements performed intra-operatively and varies from 4.0 cm to 11 cm increasing in 0.5 cm increments, and the pressure regulating balloon is available in three different pressures − 55 cm of water, 65 cm of water, and 75 cm of water. Surgical technique also varies with respect to location of the urethral cuff, the number of urethral cuffs used, the amount of fluid placed in the system, and the type of surgical approach used6–8.
Once the AUS is active, it has a high satisfaction rate, but also high rates of reoperation9–13. Early reoperations are usually due to infection, recurrent incontinence, or urethral erosion14, 15, all of which are likely affected by surgical technique. Another cause for reoperation is device failure. This has been reduced significantly since multiple device modifications were made in 19873, 4, and the current analysis focuses only on AUS performed after this time. We used a 5-year reoperative rate as a marker of surgical quality. This allows us to focus on reoperations resulting from inadequate surgical technique since reoperation due to device malfunction occurs at a median of 68 months15. There are two different standard surgical approaches – perineal and transverse scrotal - with possibly differing outcomes. However, the transverse scrotal approach was popularized in the middle of the last decade, limiting the number of patients with 5-year followup, and not allowing adequate comparison between the two different approaches in this series.
Unlike the learning curve for recurrence after radical prostatectomy16, we failed to see a plateau in our learning curve, about which improvements in outcome with increasing experience are reduced. This may be because the plateau occurs at some level of experience above 200 cases, or because such a small proportion of cases are conducted by experienced surgeons that the confidence interval around the learning curve is wide at high levels of experience.
Our analysis involves prospectively collected data, but is not randomized and is therefore subject to the possibility of reporting bias and unmeasured confounding. Yet this is not an analysis comparing a group of experienced surgeons with a group of those less experienced, but a comparison of surgeon’s results between the early and later parts of their career. It seems implausible that reporting or an unmeasured confounder would change systematically along the course of all surgeons’ careers. Furthermore, we saw little evidence of large differences in measured covariates by surgical experience, making important unmeasured confounding unlikely. This is especially because measured confounding was in the opposite direction to our main result: for example, more experienced surgeons saw slightly younger patients, but it was younger patients who were more likely to undergo reoperation. This finding would also argue against the possibility that experienced surgeons refer on high risk cases to less experienced colleagues. Moreover, it is difficult to believe that an unmeasured confounder, or differences in reporting around the very high average of 89%, would have the large effect seen here, with an approximate halving of reoperation rates along the learning curve. The findings from the negative control – rates of reoperations taking place after 10 years – fail to provide evidence that differences in case mix or reporting explain our results: indeed they suggest a greater willingness to reoperate, where appropriate, with increasing surgical experience.
The rate of decrease in reoperations with surgeon experience is of clear clinical importance. It is tempting to speculate whether a similar curve might be associated with other surgical implant procedures, particularly those that require intraoperative measurements. There are also obvious health economic implications: a reoperation incurs significant costs, so the large differences in reoperation rates reported here would be associated with large cost differences.
Conclusion
The AUS is a complex prosthetic procedure requiring intraoperative measurements. We have shown that this complexity translates to a long surgical learning curve, with large decreases in reoperative rates out to 200 or more procedures. Efforts to flatten the learning curve (eg. through improved training) are urgently needed. In addition, AUS placement is rarely performed in high volume settings, with the result that most patients are treated by inexperienced practitioners. This would appear to result in avoidable complications.
Acknowledgments
Funding
This work was supported by the National Cancer Institute (P50-CA92629); the Sidney Kimmel Center for Prostate and Urologic Cancers and David H Koch through the Prostate Cancer Foundation. The study sponsors had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Authors’ contributions
The study was conceived by JS. AV and JS were responsible for the overall study design. Statistical analyses were conducted by AV and AM. All authors contributed to writing the manuscript and approved the final version. AV had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.Fishman IJ, Shabsigh R, Scott FB. Experience with the artificial urinary sphincter model AS800 in 148 patients. J Urol. 1989;141:307. doi: 10.1016/s0022-5347(17)40748-8. [DOI] [PubMed] [Google Scholar]
- 2.Lee R, Te AE, Kaplan SA, et al. Temporal trends in adoption of and indications for the artificial urinary sphincter. J Urol. 2009;181:2622. doi: 10.1016/j.juro.2009.01.113. [DOI] [PubMed] [Google Scholar]
- 3.Light JK, Reynolds JC. Impact of the new cuff design on reliability of the AS800 artificial urinary sphincter. J Urol. 1992;147:609. doi: 10.1016/s0022-5347(17)37319-6. [DOI] [PubMed] [Google Scholar]
- 4.Leo ME, Barrett DM. Success of the narrow-backed cuff design of the AMS800 artificial urinary sphincter: analysis of 144 patients. J Urol. 1993;150:1412. doi: 10.1016/s0022-5347(17)35793-2. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds WS, Patel R, Msezane L, et al. Current use of artificial urinary sphincters in the United States. J Urol. 2007;178:578. doi: 10.1016/j.juro.2007.03.146. [DOI] [PubMed] [Google Scholar]
- 6.Guralnick ML, Miller E, Toh KL, et al. Transcorporal artificial urinary sphincter cuff placement in cases requiring revision for erosion and urethral atrophy. J Urol. 2002;167:2075. [PubMed] [Google Scholar]
- 7.Wilson SK, Delk JR, 2nd, Henry GD, et al. New surgical technique for sphincter urinary control system using upper transverse scrotal incision. J Urol. 2003;169:261. doi: 10.1016/S0022-5347(05)64082-7. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor RC, Lyon MB, Guralnick ML, et al. Long-term follow-up of single versus double cuff artificial urinary sphincter insertion for the treatment of severe postprostatectomy stress urinary incontinence. Urology. 2008;71:90. doi: 10.1016/j.urology.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Elliott DS, Barrett DM. Mayo Clinic long-term analysis of the functional durability of the AMS 800 artificial urinary sphincter: a review of 323 cases. J Urol. 1998;159:1206. [PubMed] [Google Scholar]
- 10.Montague DK, Angermeier KW, Paolone DR. Long-term continence and patient satisfaction after artificial sphincter implantation for urinary incontinence after prostatectomy. J Urol. 2001;166:547. [PubMed] [Google Scholar]
- 11.Dalkin BL, Wessells H, Cui H. A national survey of urinary and health related quality of life outcomes in men with an artificial urinary sphincter for post-radical prostatectomy incontinence. J Urol. 2003;169:237. doi: 10.1016/S0022-5347(05)64076-1. [DOI] [PubMed] [Google Scholar]
- 12.Gousse AE, Madjar S, Lambert MM, et al. Artificial urinary sphincter for post-radical prostatectomy urinary incontinence: long-term subjective results. J Urol. 2001;166:1755. [PubMed] [Google Scholar]
- 13.Clemens JQ, Schuster TG, Konnak JW, et al. Revision rate after artificial urinary sphincter implantation for incontinence after radical prostatectomy: actuarial analysis. J Urol. 2001;166:1372. [PubMed] [Google Scholar]
- 14.Raj GV, Peterson AC, Toh KL, et al. Outcomes following revisions and secondary implantation of the artificial urinary sphincter. J Urol. 2005;173:1242. doi: 10.1097/01.ju.0000152315.91444.d0. [DOI] [PubMed] [Google Scholar]
- 15.Lai HH, Hsu EI, Teh BS, et al. 13 years of experience with artificial urinary sphincter implantation at Baylor College of Medicine. J Urol. 2007;177:1021. doi: 10.1016/j.juro.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 16.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]