Abstract
Glucose-regulated protein 78 (GRP-78) is one of the many endoplasmic reticulum chaperone proteins that have been shown to possess multifunctional roles. We have previously demonstrated that GRP-78 functions as a receptor for dentin matrix protein 1 (DMP1) and is required for DMP1-mediated calcium release; that it is a secreted protein and can bind to type I collagen and DMP1 extra-cellularly and aid in the nucleation of calcium phosphate. We provide evidence in this study that tyrosine phosphorylation is required for DMP1/GRP-78-mediated calcium release in mesenchymal cells. We further demonstrate that GRP-78 is localized in the nucleus of mesenchymal cells and that the cell surface GRP-78 is not associated with the G-protein Gαq in mesenchymal cells. Results from this study show that during development of mineralized tissues, increased expression of GRP-78 can be observed in condensing cartilage and mesenchymal cells of the alveolar bone, endochondral bone and dental pulp. Additionally, we show that GRP-78 is present in the mineralizing matrices of teeth, bone and in the extracellular matrix of differentiating human marrow stromal cells and dental pulp stem cells. Collectively, our observations provide a new perspective on GRP-78 with respect to mineralized matrix formation.
Keywords: GRP-78, DMP1, Mineralization, Extracellular matrix, Bone, Dentin
Introduction
Recent studies show that osteoblast differentiation and bone formation causes endoplasmic reticulum (ER) stress resulting in the up regulation of the stress chaperone proteins (Saito et al. 2011, Murakami et al. 2009). Several of these proteins are necessary for osteoblast differentiation either by regulating type I collagen expression or by being involved as a chaperone enabling folding of type I collagen and other proteins that show increased production during osteoblast differentiation (Murakami et al. 2009, Nandan et al. 1990). Glucose-regulated protein 78 (GRP-78), also known as hspa5, is a central regulator of endoplasmic reticulum (ER) homeostasis due to its multiple functional roles in protein folding, ER calcium binding, and controlling the activation of transmembrane ER stress sensors (Luo et al. 2006). Up regulation of GRP-78 expression along with other ER chaperone proteins is considered as a typical indicator for ER stress and represents a major pro survival arm of the unfolded protein response (UPR) and thus suppresses stress-induced apoptosis (Altmeyer et al. 1996; Delpino and Castelli 2002; Weist et al. 1997; Xiao et al. 1999).
The anti-apoptotic function of GRP-78 is extremely important for the survival of stressed cells and plays an important role in cancer cell proliferation, drug resistance and diabetes (Kaufman 2002; Lee 2001; Reddy et al. 2003). Mesenchymal cells such as osteoblasts and odontoblasts are under high stress due to excessive amounts of calcium being transported to the extracellular matrix (ECM) for mineralized matrix formation. Therefore, it is logical to assume that GRP-78 plays a significant role in the survival of these cells. We have published previously that GRP-78 functions as a receptor for DMP1 in odontoblasts and osteoblasts (Ravindran et al. 2008). Recent studies by our group and others show that GRP-78 expression is increased both intracellularly and in the secretome during osteoblast differentiation (Ravindran et al. 2011; Chiellini et al. 2008). GRP-78 binds calcium ions to maintain calcium homeostasis in the ER (Lièvremont et al. 1997). Although numerous studies point to multiple roles for the stress chaperones, only a few studies discuss the extracellular role of these proteins. We demonstrated an extracellular role to the calcium-binding ability of GRP-78 by showing that it is involved in the nucleation of calcium phosphate on collagen fibrils (Ravindran et al. 2011). We demonstrate in this article that the calcium release in mesenchymal cells mediated by DMP1 and GRP-78 (Eapen et al. 2010) requires tyrosine phosphorylation and that the membrane-bound GRP-78 does not interact with the G-protein Gαq as has been previously published (Misra and Pizzo 2008).
GRP-78 is highly conserved evolutionarily from yeast to humans suggesting a critical role for this protein across different species (Ting 1988). With the GRP-78 homozygous knockout mouse being embryonic lethal and the recent development on the extracellular role of this protein, there is presently a lack of knowledge on the developmental expression pattern of GRP-78 with respect to mineralized tissue formation. In this manuscript, we have addressed this issue by histologically examining the expression pattern of GRP-78 in developing mouse molars, incisors and long bones. We also show that GRP-78 is present in the ECM assembled by differentiating human dental pulp stem cells (DPSCs) and human marrow stromal cells (HMSCs) that were cultured in a three-dimensional (3D) biomimetic culture environment.
Materials and methods
Cell culture
Seven different cell types were used in this study namely DPSCs (a gift from Dr. Songtao Shi, University of southern California), HMSCs (NIH cancer center), T4-4 odontoblasts (characterized in our laboratory), MC3T3 mouse calvarial osteoblasts, C3H10T1/2 (C3) mouse embryonic pluripotent mesenchymal cells, primary mouse odonto-blasts (OD) (isolated from 3 day old wild type pups) and primary mouse calvarial cells (CAL) (isolated from 3 day old wild type pups). DPSCs, HMSCs, ODs and CALs were cultured in alpha minimum essential medium (MEM, GIBCO, Carlsbad, CA, USA) supplemented with 20 % fetal bovine serum (FBS, GIBCO, Carlsbad, CA, USA), 1 % l-glutamine and 1 % antibiotic and antimycotic solution (GIBCO, Carlsbad, CA, USA). T4-4 and MC3T3 cells were cultured in DMEM/F12 (1:1) medium with 10 % FBS and 1 % antibiotic and antimycotic solution (GIBCO). C3s were cultured in BME media containing 10 % FBS and 1 % antibiotic and antimycotic solution.
Immunohistochemistry
Embryonic day 13.5 (E13.5), embryonic day 16.5 (E15.5) whole mouse embryo, postnatal day 1 (P1), day 3 (P3), day 5 (P5), day 7 (P7) mouse heads and day 20 (P20) mouse mandible were embedded in paraffin wax. Additionally, long bones from 3-day, 5-day and 4-week-old postnatal mice were also embedded in paraffin. 5 μm thick sections were cut, deparaffinized with xylene and hydrated through graded ethanol. They were then incubated in 3 % H2O2 for 30 min to quench endogenous peroxidase activity and blocked with PBS containing serum (Vectastain ABC peroxidase kit) for 1 h at RT. Sections were then incubated overnight at 4 °C with anti- GRP-78 antibody (a gift from Dr. Sylvie Blond, UIC) (1/200) and then incubated with biotin-conjugated secondary antibody, washed 4× in PBS, incubated with peroxidase-conjugated streptavidin (Vectastain ABC peroxidase kit) and developed with the DAB kit (Vector labs, Burlingame, CA, USA). All experiments were performed in accordance with approved UIC animal protocol (assurance no: A3460.01).
Generation of ECM from HMSCs and DPSCs
HMSCs and DPSCs (2 × 106 cells/ml) were embedded in a collagen scaffold as described previously (Ravindran et al. 2010). The cells were cultured for 48 h in growth media. Subsequently, differentiation media containing 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate and 10 mM dexamethasone was used for culturing the cells for an additional 2 weeks. At the end of 2 weeks, the cells were treated with buffer 1 (10 mM sodium phosphate, 150 mM sodium chloride and 0.5 % Triton X-100) for 30 min at 37 °C in a tissue culture incubator. The buffer was then changed to buffer 2 (25 mM ammonium hydroxide) and the scaffolds were incubated for 20 min at 37 °C. Finally, the scaffolds were washed three times in Hanks balanced salt solution (HBSS) containing no calcium or magnesium. ECM scaffolds were then subjected to three freeze–thaw cycles in liquid nitrogen and 37 °C cell culture incubator, respectively. Finally, the scaffolds were washed three times in HBSS and treated with DNAse (Gibco) at 37 °C for 30 min to remove traces of DNA bound to the matrix. The scaffolds were then washed four times in HBSS, fixed in neutral buffered formalin, paraffin embedded and sectioned (5 μm). The sections were immunostained with anti-GRP-78 antibody (1/200) and anti-fibronectin antibody (1/200, Sigma, Saint Louis, MO, USA) followed by staining with the corresponding fluorescent secondary antibodies. To visualize collagen, the sections were also stained with picosirius red as per standard protocol. The sections were then imaged using a Zeiss LSM 510 Meta confocal microscope or a Zeiss Axiovert D1 fluorescent microscope equipped with the respective filtersets and the Axiovision imaging software.
Detection of GRP-78 in secreted protein pools
For these experiments, the cells were grown to complete confluence and the media was changed to serum-free regular media or differentiation media 48 h prior to completion of the specified time point. At the end of the time point, the media was collected and the cells were trypsinized and counted to ensure that the cell number did not vary significantly between the time points. 15 μl of the reconstituted protein samples obtained from media collected from a single flask at each time point were resolved on SDS-PAGE and immuno-blotting was performed with rabbit polyclonal anti-GRP-78 primary antibody (1/1,000) and a corresponding anti-rabbit HRP-conjugated secondary antibody.
Calcium release experiments
The calcium release experiments were performed as published previously (Eapen et al. 2010). Tyrosine phosphorylation was blocked by pretreatment with the inhibitor genistein (50 μM) for a period of 45 min prior to treatment with DMP1.
GRP-78 immunostaining
ODs, CALs, HMSCs and C3s were cultured on glass coverslips for a period of 48 h in growth media. The cells were then fixed in 4 % paraformaldehyde and permeabilized with 0.5 % Triton X-100. The cells were then blocked with 5 % BSA and immunostained with the GRP-78 primary antibody (1/200) followed by TRITC-conjugated rabbit secondary antibody (1/100). The coverslips were then mounted on to slides and imaged using a Zeiss LSM 510 Meta confocal microscope.
GRP-78 and Gαq colocalization
MC3T3 cells were seeded on to coverslips, fixed and permeabilized as described in the previous section. The cells were then immunostained with rabbit polyclonal GRP-78 antibody (1/200) and mouse monoclonal Gαq antibody (1/100) (BD Biosciences). The slides were then imaged using a Zeiss LSM 510 Meta confocal microscope.
Immunoprecipitation (IP)
Membrane proteins were isolated from MC3T3 cells using the PIERCE MEM-PER isolation kit as per the manufacturer's instructions. Immunoprecipitation (IP) was carried out by incubating 1 μg of antibody [GRP 78 (Santa Cruz Biotechnology) or Gαq (BD Biosciences)] with membrane proteins isolated from 2 × 106 cells, overnight at 4 °C. The proteins were then incubated with protein A beads for 1 h followed by removal of unbound proteins and washing with PBS containing 150 mM NaCl (5 times). The bound proteins were eluted out of the beads by boiling with 1× SDS loading buffer. The samples were then resolved by SDS-PAGE and immunoblotting was performed using GRP-78 antibody and Gαq antibody followed by incubation with HRP-conjugated protein A. IgG IP served as the negative control.
In situ proximity ligation assays
In situ proximity ligation assays (PLA) were performed using the Duolink II PLA kit (Olink Biosciences) on fixed and permeabilized MC3T3 cells according to the manufacturer's protocol. GRP-78 rabbit polyclonal antibody (1/200) and Gαq mouse monoclonal antibodies were used as the primary antibodies.
Results
Expression of GRP-78 during embryonic development
Localization of GRP-78 during development was assessed by immunohistochemical analysis. Figure 1 shows the expression of GRP-78 at embryonic stages 13.5 and 16.5. Even though the expression of GRP-78 is ubiquitous, increased expression was observed in mineralizing tissues. In the embryonic stage 13.5, increased expression was observed in Meckel's cartilage (Fig. 1a) and developing vertebrae (Fig. 1b). At day 16.5, GRP-78 was expressed abundantly in the alveolar bone, while less expression was observed in the Meckels cartilage (Fig. 1c). Figure 1d shows the localization of GRP-78 in a developing incisor. Distinct localization pattern was seen in the preodontoblasts and in the basement membrane between the differentiating preosteoblasts and preameloblasts. Interestingly, strong expression was seen in the undifferentiated cells of the cervical loop. The alveolar bone surrounding the matrix continued to express GRP-78 at 16.5.
Fig. 1.
Expression of GRP-78 during embryonic development. a, b Expression of GRP-78 in condensing cartilage of a developing mouse E 13.5. c, d Expression of GRP-78 in condensing cartilage of a developing mouse E 16.5. Arrows in all images represent areas of increased GRP-78 expression in bone and cartilage
Expression of GRP-78 in postnatal mice day 1 to day 7 in a developing tooth
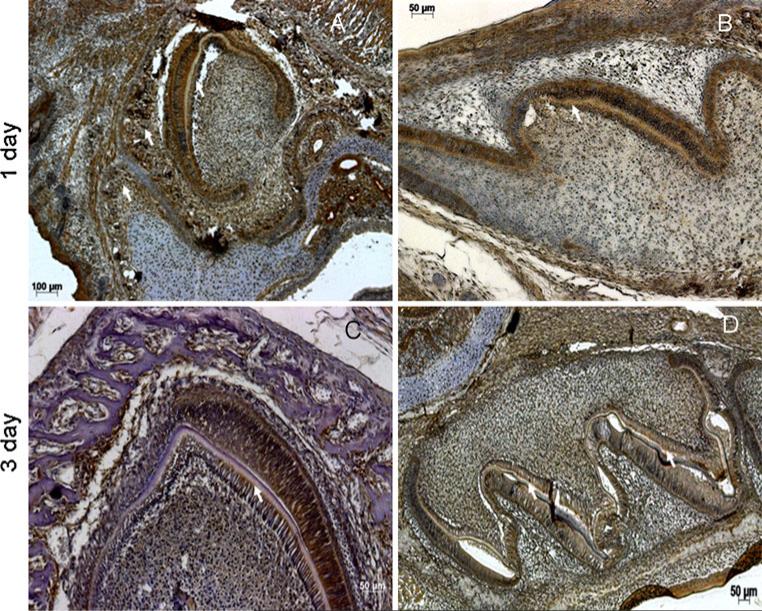
During tooth morphogenesis, postnatal GRP-78 expression followed a similar pattern to the late embryonic stage. Localization was seen in osteoblasts, odontoblasts and ameloblasts of the developing incisor at day 1 and 3. In these cells, localization was predominant in the cytoplasm (Fig. 2a, c). Correspondingly, based on the secretory nature of this protein, localization was observed in dentin and alveolar bone matrices (Fig. 2a, c and supplementary fig 1A). The white arrows in Figs. 2 and 3 represent the presence of protein in the dentin matrix. At day 1, chondrocytes expressed GRP-78 (Fig. 2a) in the Meckel's cartilage below the incisor. Increased expression in hypertrophic chondrocytes was also observed at day 3 (supplementary Fig 1C). In the molars, GRP-78 was expressed predominantly by preameloblasts and preodontoblasts (Fig. 2b, d). At day 5, GRP-78 was seen at the mineralization front of the dentin matrix in the incisors and in the alveolar bone matrix (Fig. 3a). In the pulp, a defined population of dental pulp cells expressed GRP-78 (Fig. 3b). Supplementary figure 1B is a higher magnification of Fig. 3b showing the presence of GRP-78 in the mineralization front of the dentin matrix. At day 7, GRP-78 expression was seen throughout the pulp, dentin and bone matrices as well as in the ameloblasts (Fig. 3c, d).
Fig. 2.
Expression of GRP-78 during postnatal tooth development. a, b Expression of GRP-78 in developing incisor (a) and molar (b) at postnatal day 1. c, d Expression of GRP-78 in developing incisor (a) and molar (b) at postnatal day 3. White arrows in all images indicate presence of GRP-78 in the mineralizing matrix
Fig. 3.
Expression of GRP-78 during postnatal tooth development. a, b Expression of GRP-78 in developing incisor (a) and molar (b) at postnatal day 5. c, d Expression of GRP-78 in developing incisor (a) and molar (b) at postnatal day 7. White arrows in all images indicate localization of GRP-78 in the mineralizing matrix
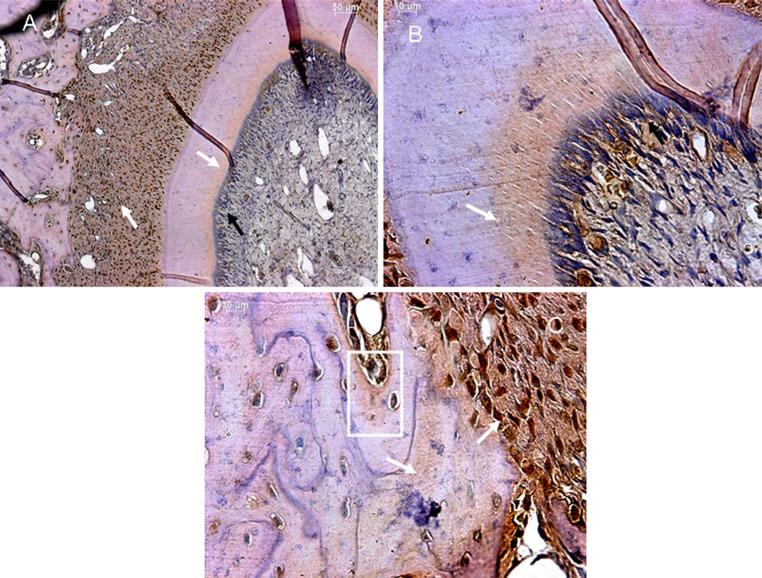
Expression of GRP-78 in the 20-day mouse mandible
An interesting observation at this time point was the abundant expression of GRP-78 in the periodontal ligament and in the bony socket surrounding the tooth (Fig. 4a, c). Generally, less expression was seen throughout the odontoblasts, with predominant expression in the odontoblasts of the cuspal region. In the matrix, GRP-78 expression was seen in the predentin region (Fig. 4a, b) and also in the alveolar bone (Fig. 4c). White arrows indicate expression in the matrix.
Fig. 4.
Expression of GRP-78 in the mandible at postnatal day 20. a Expression of GRP-78 in the periodontal ligament and dentin matrix. Arrows indicate the localization in these two tissues. b A higher magnification image showing the presence of GRP-78 in the dentin matrix and its absence in the odontoblasts. Arrow points to the presence of GRP-78 in the dentin matrix. c Expression of GRP-78 in the alveolar bone and the periodontal ligament. Arrows point to the presence of GRP-78 in these tissues. The boxed region shows the presence of GRP-78 in the alveolar bone matrix
Expression of GRP-78 in developing mouse femurs
In order to understand the expression pattern of GRP-78 during endochondral bone formation, immunohistochemical analysis was performed on long bones of 3-day, 5-day and 4-week-old mice. At 3 days, GRP-78 was present in the matrix surrounding the proliferating chondrocytes (Fig. 5a, b). At this stage of development, no significant amount of bone matrix was observed. At day 5, GRP-78 was localized to the cartilage matrix, bone matrix and in the osteoblasts (Fig. 5c, d and Supplementary figs 2A and 2B). Supplementary Fig 2B shows collagen autofluorescence overlayed on the GRP-78 immunostained image. The presence of GRP-78 in the bone matrix can be clearly seen from this image. High expression was seen in the osteocytes embedded within the cortical bone (Fig. 5d). At 4 weeks of development, no expression of GRP-78 was seen in the chondrocytes and in the cartilage matrix. However, increased GRP-78 expression was observed in the bony spicules of the trabeculae, in the cortical bone matrix and in the periosteum (Fig. 5e, f).
Fig. 5.
Expression of GRP-78 in the developing long bone. a, b Expression of GRP-78 in postnatal day 3 long bones of developing mice. Black arrows point to localization of GRP-78 in the extracellular matrix between the chondrocytes. c, d Expression of GRP-78 in postnatal day 5 long bones. Black arrow points to localization of GRP-78 in the cartilage extracellular matrix. White arrow shows the presence of GRP-78 in the mineralizing bone matrix. e, f Expression of GRP-78 in postnatal day 4 long bones. No staining was observed in the cartilage matrix at this stage. White arrow in (f) points to localization of GRP-78 in the bone matrix
Expression of GRP-78 in the ECM secreted by HMSCs and DPSCs in a biomimetic environment
In order to confirm that GRP-78 is a secretory protein, we immune-stained the ECM secreted by HMSCs and DPSCs after differentiating them on a 3D collagen scaffold. Confocal images of the immunohistochemical analysis performed on the ECM showed that the matrix producing HMSCs and DPSCs did secrete GRP-78 (Fig. 6a, e). Colocalization of GRP-78 and DMP1 was also observed on the extracellular matrix (Fig. 6a, e). Fibronectin, a major ECM protein was used as a positive control (Fig. 6b, f). Anti-rabbit secondary antibody was used as a negative control (Fig. 6c, g). The absence of cells was confirmed by staining for the cell nuclei with DAPI. Negative staining for DAPI shows the absence of intact cells or any nuclear material (Fig. 6d, h).
Fig. 6.
Localization of GRP-78 in the extracellular matrix. a, e Expression of GRP-78 and DMP1 in the biomimetic ECM secreted by differentiating HMSCs (a) and DPSCs (e). b, f Expression of fibronectin in the biomimetic ECM secreted by differentiating HMSCs (b) and DPSCs (f). c, g Secondary antibody negative controls. d, h Images showing lack of DAPI fluorescence in the sections showing absence of cellular DNA. i Representative fluorescence micrograph showing colocalization of secreted GRP-78 (green) on collagen fibrils (red) in the biomimetic ECM of differentiating HMSCs. j GRP-78 immuno-blots of the secretome from HMSCs and T44 odontoblasts after 0, 7, 14, and 21 days in differentiation media. k Densitometric quantification of the blots in figure (j) showing fold change in GRP-78 secretion. The empty bars represent GRP-78 secreted by HMSCs and the filled bars represent GRP-78 secreted by T4-4 odontoblasts
Colocalization of the secreted GRP-78 and type I collagen in the ECM of HMSCs
We have published previously that GRP-78 binds to type I collagen (Ravindran et al. 2011). To verify if the secreted GRP-78 colocalized with type I collagen, sections of the HMSC ECM biomimetic scaffold were stained with picosirius red for type I collagen. Picosirius red induces red fluorescence of type I collagen fibrils. GRP-78 was immunostained in green. Figure 6i shows a representative fluorescence image of GRP-78 localized with type I collagen fibrils. The boxed areas represent examples of colocalizing regions. Due to the enormous amount of type I collagen in the scaffolds, not all the areas that showed type I collagen staining colocalized with GRP-78. However, most of the GRP-78 immunostained areas colocalized with type I collagen.
Secretion of GRP-78 by HMSCs and T44 odontoblast cells under mineralizing conditions
We have shown earlier that the secretion of GRP-78 is increased when osteoblasts are subjected to mineralizing conditions (Ravindran et al. 2011). We show here that both HMSCs and T4-4 odontoblasts show an increase in GRP-78 secretion when subjected to differentiation media. However, the secretion pattern was markedly different. HMSCs showed an initial spike in secretion at day 7 after which the secretion dropped to levels that were only slightly higher than basal levels of GRP-78 at days 14 and 21 Fig. 6j, empty bars in Fig. 6k). On the other hand, the T44 cells showed a sustained increase beginning at day 7 until day 21 (Fig. 6j, filled bars in Fig. 6k).
Tyrosine phosphorylation is required for DMP1-GRP-78-mediated calcium release in mesenchymal cells
We have published earlier (Eapen et al. 2010) that GRP-78 is required for DMP1-mediated calcium release in MC3T3 mesenchymal cells. Cell surface GRP-78 is phosphorylated on tyrosine residues upon ligand binding (Misra et al. 2005). We examined if this tyrosine phosphorylation is important for the cell surface GRP-78 to mediate calcium release upon DMP1 binding. Blocking of tyrosine phosphorylation using a tyrosine kinase inhibitor genistein blocked the DMP1-mediated calcium release and the subsequent uptake of calcium following the release. Figure 7 shows that there is a calcium release upon DMP1 treatment and a subsequent uptake of calcium following the release upon addition of 1.5 mM calcium (Fig. 7a). This release and uptake was completely blocked when the cells were pretreated with genistein (Fig. 7c). Treatment with genistein alone (Fig. 7b) did not trigger a calcium release or calcium uptake in the cells and served as a control. In Fig. 7a, the DMP1-mediated calcium release appears small. This is due to the fact that the uptake of calcium following the release was substantially larger and made the release look small in comparison.
Fig. 7.
Tyrosine phosphorylation is required for DMP1-GRP-78-mediated calcium release. a Plot indicating calcium release upon addition of DMP1 to MC3T3-E1 cells and the subsequent calcium uptake upon addition of 1.5 mM calcium. b Control plot showing that treatment with genistein alone does not trigger calcium release or uptake. c Blocking DMP1-mediated calcium release and the subsequent uptake upon pretreatment with genistein
GRP-78 does not interact with G-protein Gαq
GRP-78 has been shown to interact with the G-protein Gαq in macrophages (Misra and Pizzo 2008). We performed colocalization experiments in MC3T3 cells using a rabbit polyclonal GRP-78 antibody and a mouse monoclonal Gαq antibody. Figure 8 shows representative confocal images of this experiment. It can be seen that although GRP-78 and Gαq appear to be in close proximity, there is no colocalization. The circled areas represent examples of non-colocalizing GRP-78 and Gαq signals. Figure 8b is an enlarged image of the boxed area in Fig. 8a. Figure 8c is another representative image.
Fig. 8.
GRP-78 does not interact with G-protein Gαq. a Confocal image showing absence of colocalization between GRP-78 and Gαq. b Enlarged image of the boxed area in (a). c Another representative confocal image showing differential expression of GRP-78 and Gαq. In all images red signal indicates GRP-78 and green signal indicates Gαq. (d) GRP-78 and Gαq immunoblots of immunoprecipitate (IP) samples showing absence of Gαq in the GRP-78 IP and GRP-78 in the Gαq IP. NSP indicates non-specific bands. In the Gαq immunoblot the NSP band is that of IgG
We also performed IP experiments (Fig. 8d) on membrane protein extracts from MC3T3 cells both with GRP-78 and Gαq antibodies. Proximity ligation assays (data not shown) to look for interaction between the two proteins were also performed. The results of both these experiments were negative. Figure 8d shows representative immunoblots of GRP-78 and Gαq IP experiments with both GRP-78 and Gαq antibodies. IgG IP was used as the control. It is evident from these blots that GRP-78 and Gαq did not coimmunoprecipitate. Therefore, our experiments failed to demonstrate an interaction between GRP-78 and Gαq in the cell types used in this study.
Localization of GRP-78 in the nucleus
GRP-78 has been reported to be present in the nucleus of cancer cells and cells subjected to DNA damage by UV radiation. GRP-78 is involved in the DNA repair process by binding directly to the DNA (Ni et al. 2011). In this study, nuclear localization of GRP-78 could be observed in several different cell types. However, not all the cells in a population showed nuclear GRP-78. Figure 9 shows the presence of GRP-78 in several mesenchymal cells namely: OD (Fig. 9a), CAL (Fig. 9b), HMSC (Fig. 9c) and C3 (Fig. 9d). Additionally, immunohistochemical analyses showed the presence of GRP-78 in the nucleus of the odontoblasts in postnatal day 20 mouse mandible (Fig. 9e).
Fig. 9.
Nuclear localization of GRP-78. a–d Representative images showing the presence of GRP-78 in primary odontoblasts, primary calvarial cells, HMSCs and C3H10 T1/2 pluripotent mesenchymal cells, respectively. In all images red signal indicates GRP-78. Arrows point to nuclear localized GRP-78. e 20-day mouse mandible section immunostained with GRP-78 showing the presence of GRP-78 in the nucleus of odontoblasts (white arrows)
Discussion
GRP-78 is a multifaceted protein based on the distinct intracellular, extracellular and plasma membrane localization pattern. Intracellularly, it is a chaperone for type I collagen and acts as a receptor for proteins and viruses (Triantafilou et al. 2002, 2003; Misra et al. 2005; Jindadamrongwech et al. 2004). It was originally assumed that an increase in GRP-78 expression was to only function as a chaperone for collagen folding during calcified matrix formation. Recently, we showed that GRP-78 is localized on the plasma membrane of osteoblasts and odontoblasts and aids in the endocytosis of DMP1 resulting in intracellular calcium signaling (Ravindran et al. 2008, 2011; Eapen et al. 2010). Extracellularly, GRP-78 influences sperm penetration at the zona pellucida by modulating calcium ion concentrations (Marín-Briggiler et al. 2009). Additionally, we have shown that extracellular GRP-78 plays a role in matrix mineralization by binding to type I collagen and DMP1 and thereby mediating nucleation of calcium phosphate polymorphs (Ravindran et al. 2011). Further, we showed that expression of GRP-78 mRNA, protein expression and secretion increased upon initiation of osteoblast differentiation. All these results suggest that GRP-78 plays an important role in mesenchymal cell differentiation and matrix mineralization.
With GRP-78 knockout mice being embryonic lethal (Luo et al. 2006), there is presently a dearth of knowledge on the developmental role of GRP-78. The heterozygous knockout mouse model showed that GRP-78 is required for maintaining the pluripotency of the inner cell mass during embryonic development (Luo et al. 2006). However, there is no information regarding the expression of GRP-78 in different tissues during development. In the present study, we focused on the mineralizing tissues and investigated the localization pattern of GRP-78 in cartilage, bone and teeth during development. Intracellular localization pattern demonstrate the presence of GRP-78 in the nucleus of some mesenchymal-derived cells that form bone and teeth. The function of nuclear GRP-78 requires further investigation.
Immunohistochemical experiments revealed that GRP-78 expression was observed in mineralizing tissues from early embryonic development. At E13.5, the condensing chondrocytes of the vertebrae showed increased expression compared to the surrounding tissue. At E16.5, expression of GRP-78 could be observed in the differentiating osteoblasts of the alveolar bone surrounding the tooth. In long bones, GRP-78 was expressed in the cells of the periosteum, osteoblasts and in the osteocytes. Interestingly, the cartilage “anlage” at days 3 and 5 express large amounts of GRP-78, however, at day 28 there was no expression of GRP-78 in the chondrocytes of the growth plate. During bone development, increased intracellular GRP-78 expression could be attributed to increased collagen synthesis by the differentiating mesenchymal cells as GRP-78 is one of the major chaperones involved in type I collagen folding. Increased GRP-78 expression was also observed in the calcifying matrix of both alveolar and long bone, indicating that GRP-78 plays a similar functional role in the ossification process of both intramembranous and endochondral bone.
One of the interesting observations is the expression of GRP-78 in odontoblasts. During early embryonic development, GRP-78 was localized in the odontoblasts and in the basement membrane. Undifferentiated cells within the cervical loop niche stained positive for GRP-78 early on during development and lower expression levels were observed with development. In the incisors, at post-natal days 1 and 3, GRP-78 was clearly localized in the odontoblasts, and in the ameloblasts. At days 5 and 7 there was distinct localization at the mineralization front implying a role in mineralization. At post-natal day 20, GRP-78 was localized mainly in the predentin. At this stage of development, the odontoblasts have fully differentiated and matrix mineralization is nearly complete, hence there are fewer requirements for GRP-78 in mineralization. However, the osteoblasts and the periodontal ligament cells continue to express increased amounts of the protein at day 20. Based on the localization pattern, we can conclude that GRP-78 expression and secretion is increased only when there is active matrix production and mineralization.
To further confirm the localization of GRP-78 in the extracellular matrix, we differentiated both HMSCs and DPSCs on biomimetic collagen scaffolds for 2 weeks after which the cells were removed and the ECM incorporated matrix was analyzed for the presence of fibronectin, GRP-78 and DMP1. GRP-78 was strongly expressed in the ECM indicating that the differentiating mesenchymal stem cells secrete GRP-78 into the ECM. The GRP-78 immunostaining also colocalized with DMP1 indicating that the two proteins interact in the ECM. In our earlier study (Ravindran et al. 2011), we had shown that immobilized GRP-78 on type I collagen-coated substrate and on demineralized dentin wafer can nucleate amorphous calcium phosphate. The data presented in this manuscript shows interaction of GRP-78 with both type I collagen and DMP1 in the ECM of mesenchymal cells. Based on these observations, we suggest that GRP-78 might sequester calcium ions in the ECM and aid in the process of type I collagen mineralization. Western blot analysis of the secretome of HMSCs that are osteoprogenitors showed increased expression at 7 days with basal level expression at 14 and 21 days. In contrast, the T4-4 odontoblastic cell line showed high levels of secreted GRP-78 after 7 days and maintained the secreted levels until 21 days. These results indicate that the requirement for GRP-78 during cell differentiation varies in mesenchymal cell types.
Another interesting finding presented in this study is the nuclear localization of GRP-78. We provide evidence for nuclear localization in both in vitro cell culture and in vivo. Interestingly, not all cell types in a given population showed nuclear GRP-78. This might indicate that nuclear localization might occur during a specific stage of differentiation in mesenchymal cells. GRP-78 has been speculated to aid in DNA repair process in cells subjected to radiation damage (Ni et al. 2011). However, at this point, we cannot speculate on the function GRP-78 might play in the nucleus of mesenchymal cells as we are still attempting to understand at what stage nuclear localization occurs.
Aside from analyzing the localization pattern of GRP-78, we shed new light on the receptor function of GRP-78 showing that tyrosine phosphorylation of GRP-78 is required for DMP1-mediated calcium release. However, GRP-78 did not appear to be a G-protein coupled receptor as published and did not interact with the G-protein Gαq (Misra and Pizzo 2008).
In conclusion, our study demonstrates that:
osteoblasts, odontoblasts and chondrocytes synthesize GRP-78 at different levels depending on their developmental stage and tissue mineralization requirements,
secretion pattern of GRP-78 varies in different mesenchymal cells during differentiation,
secreted GRP-78 colocalizes with the extracellular DMP1 in the ECM of mesenchymal cells,
GRP-78 can be localized in the nucleus of some mesenchymal cells,
tyrosine phosphorylation of GRP-78 is required for DMP1 and GRP-78-mediated calcium release,
GRP-78 does not interact with G-protein Gαq in mesenchymal cells.
Overall, our results provide critical information on the expression pattern of this multifaceted protein along with insights into several new functions. Future studies would be devoted to identifying the multiple functions of GRP-78 during the development of bone and teeth.
Supplementary Material
Acknowledgments
This work was supported by grant from National Institutes of Health DE 11657 and the Brodie Endowment Fund.
Contributor Information
Sriram Ravindran, Brodie Tooth Development Genetics and Regenerative Medicine Research Laboratory, Department of Oral Biology, University of Illinois at Chicago, Chicago, IL 60612, USA.
Qi Gao, Brodie Tooth Development Genetics and Regenerative Medicine Research Laboratory, Department of Oral Biology, University of Illinois at Chicago, Chicago, IL 60612, USA.
Amsaveni Ramachandran, Brodie Tooth Development Genetics and Regenerative Medicine Research Laboratory, Department of Oral Biology, University of Illinois at Chicago, Chicago, IL 60612, USA.
Premanand Sundivakkam, Department of Pharmacology, University of Illinois at Chicago, Chicago, IL 60612, USA.
Chinnaswamy Tiruppathi, Department of Pharmacology, University of Illinois at Chicago, Chicago, IL 60612, USA.
Anne George, Brodie Tooth Development Genetics and Regenerative Medicine Research Laboratory, Department of Oral Biology, University of Illinois at Chicago, Chicago, IL 60612, USA anneg@uic.edu.
References
- Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK. Tumor-specific cell surface expression of the-KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 1996;69:340–349. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chiellini C, Cochet O, Negroni L, Samson M, Poggi M, Ailhaud G, Alessi MC, Dani C, Amri EZ. Characterization of human mesenchymal stem cell secretome at early steps of adipocyte and osteoblast differentiation. BMC Mol Biol. 2008;9:2627. doi: 10.1186/1471-2199-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpino A, Castelli M. The 78 kDa glucose-regulated protein (GRP78/BIP) is expressed on the cell membrane, is released into cell culture medium and is also present in human peripheral circulation. Biosci Rep. 2002;22:407–420. doi: 10.1023/a:1020966008615. [DOI] [PubMed] [Google Scholar]
- Eapen A, Sundivakkam P, Song Y, Ravindran S, Ramachandran A, Tiruppathi C, George A. Calcium-mediated stress kinase activation by DMP1 promotes osteoblast differentiation. J Biol Chem. 2010;285:36339–36351. doi: 10.1074/jbc.M110.145607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindadamrongwech S, Thepparit C, Smith DR. Identification of GRP 78 (BiP) as a liver cell expressed receptor element for dengue virus serotype 2. Arch Virol. 2004;149:915–927. doi: 10.1007/s00705-003-0263-x. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Investig. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- Lièvremont JP, Rizzuto R, Hendershot L, Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+. J Biol Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;15:5688–5697. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Briggiler CI, González-Echeverría MF, Munuce MJ, Ghersevich S, Caille AM, Hellman U, Corrigall VM, Vazquez-Levin MH. Glucose-regulated protein 78 (Grp78/BiP) is secreted by human oviduct epithelial cells and the recombinant protein modulates sperm-zona pellucida binding. Fertil Steril A. 2009;15:1574–1584. doi: 10.1016/j.fertnstert.2008.12.132. [DOI] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. Heterotrimeric Galphaq11 co-immunoprecipitates with surface-anchored GRP78 from plasma membranes of alpha2M*-stimulated macrophages. J Cell Biochem. 2008;104:96–104. doi: 10.1002/jcb.21607. [DOI] [PubMed] [Google Scholar]
- Misra UK, Deedwania R, Pizzo SV. Binding of activated alpha2-macroglobulin to its cell surface receptor GRP78 in 1-LN prostate cancer cells regulates PAK-2-dependent activation of LIMK. J Biol Chem. 2005;280:26278–26286. doi: 10.1074/jbc.M414467200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Saito A, Hino S, Kondo S, Kanemoto S, Chihara K, Sekiya H, Tsumagari K, Ochiai K, Yoshinaga K, Saitoh M, Nishimura R, Yoneda T, Kou I, Furuichi T, Ikegawa S, Ikawa M, Okabe M, Wanaka A, Imaizumi K. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nat Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- Nandan D, Ball EH, Sanwal BD. Two stress proteins of the endoplasmic reticulum bind denatured collagen. Biochem Cell Biol. 1990;68:1057–1061. doi: 10.1139/o90-156. [DOI] [PubMed] [Google Scholar]
- Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochem J. 2011;434:181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Narayanan K, Eapen AS, Hao J, Ramachandran A, Blond S, George A. Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1. J Biol Chem. 2008;283:29658–29670. doi: 10.1074/jbc.M800786200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Song Y, George A. Development of three-dimensional biomimetic scaffold to study epithelial-mesenchymal interactions. Tissue Eng. 2010;16:327–342. doi: 10.1089/ten.tea.2009.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Gao Q, Ramachandran A, Blond S, Predescu SA, George A. Stress chaperone GRP-78 functions in mineralized matrix formation. J Biol Chem. 2011;286:8729–8739. doi: 10.1074/jbc.M110.179341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RK, Mao C, Baumeister P, Austin RC, Kaufman RJ, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation. J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J Biol Chem. 2011;286:4809–4818. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J, Lee AS. Human gene encoding the 78,000-dalton glucose-regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988;7:275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Triantafilou M. Lipid raft microdomains: key sites for Coxsackievirus A9 infectious cycle. Virology. 2003;317:128–135. doi: 10.1016/j.virol.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Fradelizi D, Wilson K, Triantafilou M. GRP78, a coreceptor for coxsackievirus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J Virol. 2002;76:633–643. doi: 10.1128/JVI.76.2.633-643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weist DL, Bhandoola A, Punt J, Kreibich G, McKean D, Singer A. Incomplete endoplasmic reticulum (ER) retention in immature thymocytes as revealed by surface expression of “ER-resident” molecular chaperones. Proc Natl Acad Sci USA. 1997;94:1884–1889. doi: 10.1073/pnas.94.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Chung TF, Pyun HY, Fine RE, Johnson RJ. KDEL proteins are found on the surface of NG108-15 cells. Mol Brain Res. 1999;72:121–128. doi: 10.1016/s0169-328x(99)00188-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.