INTRODUCTION
South Africa’s national antiretroviral therapy (ART) program, launched in 2004, is the world’s largest [1], with 1.8 million individuals initiated on ART by mid-2011 [2]. Initially, the threshold for ART eligibility in the national guidelines for adult ART initiation was set at a CD4 cell count below 200 cells/μl or a WHO Stage IV clinical condition [3]. In late 2009, the WHO revised their guidelines by increasing the threshold for eligibility for ART in resource-limited settings to ≤350 cells/μl [4,5]. In August 2011, South Africa officially adopted this policy [6]. It has been estimated that this policy expanded eligibility to an additional 1.06 million (95%CI: 0.88–1.29m) ART-naïve adults with CD4 values 200–349 cells/μl [2].
Patients who initiate ART with a CD4 above 200 cells/μl are at reduced risk of death and serious opportunistic infections including tuberculosis, as demonstrated in developed countries [7–9], a randomized trial in Haiti [10], and observational studies in sub-Saharan Africa [11–14]. However, if patients initiate treatment before perceiving the clinical necessity, gains from positive clinical outcomes from earlier treatment may be offset by increases in patient loss to follow-up [15]. To date, only one study has reported the effect of initiation at higher CD4 counts on loss to follow-up under routine early initiation (≤350 cells/μl) within sub-Saharan Africa and found 39% reduced loss among those initiating at CD4 cell counts >200 cells/μl versus ≤200 cells/μl (aHR 0.61, 95%CI: 0.43–0.87) [11].
Witkoppen Health and Welfare Centre (Witkoppen) is an NGO-operated clinic serving a primarily poor population from formal and informal settlements in northern Johannesburg, South Africa. In March 2010, a year before the national policy was enacted, Witkoppen began ART initiation for all adult patients at the higher threshold of ≤350 cells/μl. This provides a unique opportunity to examine the impact of initiating treatment among patients presenting at higher CD4 counts on patient loss to follow-up under routine care.
METHODS
We created a retrospective cohort of all adult (≥18 years) ART-naïve patients initiating ART at Witkoppen during April-December 2010 who presented with a baseline CD4 value eligible for ART (≤350 cells/μl). We excluded 29 patients missing a baseline CD4 value, leaving 1430 for analysis. Data were extracted from the clinic’s electronic patient record system (TherapyEdge-HIV™) in May 2012, allowing all subjects time to experience a 12-month outcome. Clinic visits following ART initiation typically are scheduled monthly until the patient is considered stable and adherent (usually 5–6 months after initiation), at which time patients may be asked to return every three to six months. Patients who provided a South African ID number (n=685, 47.9%) were matched to the National Population Register of the South African Department of Home Affairs in August 2011 to identify deaths. We conducted a sensitivity analysis restricted only to patients with a valid South African ID number but this did not change the size of our effect or our conclusions.
We sought to estimate the effect of initiating ART with a CD4 count 201–350 versus ≤200 cells/μl on loss to follow-up (primary outcome) in the first 12 months after ART initiation. We defined loss to follow-up as not returning to the clinic within 3 months of the patient’s last missed scheduled visit. For loss to follow-up, person-time began accumulating three months after ART initiation (when patients became at risk for loss) and excluded 50 patients who died or transferred in the first three months. Person-time ended at death within 12 months, or the earliest of 12 months of follow-up, loss to follow-up or transfer. For outcomes of death and incident TB, person-time began accumulating at ART initiation. We defined baseline CD4 count (≤200 and 201–350 cells/μl) as the temporally last measure between six months prior and seven days after ART initiation and grouped patients into those who presented and initiated ART with a lower (≤200 cells/μl) or higher (201–350 cells/μl) baseline CD4 value.
We produced crude Kaplan-Meier curves of time to loss by CD4 group. We used Cox proportional hazards regression to estimate the association of CD4 category and 12-month loss to follow-up and report adjusted hazard ratios (aHR) and 95% confidence intervals (95%CI). We also evaluated the impact of death on estimates of loss using a competing risks analysis and report the subdistribution hazard ratio (sHR) and 95%CI.
We identified pregnancy at ART initiation as an effect measure modifier of the relation between CD4 count and loss to follow-up by stratifying estimates of the risk of loss by CD4 group, gender and pregnancy. Based on prior knowledge and change-in-estimate evaluation, we adjusted models for age, nationality, employment and prevalent TB. The study was approved by institutional review boards at the University of North Carolina at Chapel Hill and the University of the Witwatersrand.
RESULTS
Among the 1430 patients, nearly half (48.0%) presented with a CD4 201–350 cells/μl at ART initiation (Web Appendix 1). Median (IQR) baseline CD4 by group was 105 cells/μl (55–154) versus 268 cells/μl (239–307). The higher CD4 count group was more likely to be <30 years old (31.7% vs. 24.5%) and female (75.7% vs. 63.4%), partly because there were more pregnant women (19.2% vs. 10.6%) in the higher CD4 group due to routine antenatal HIV testing. The low CD4 count group had more tuberculosis at ART initiation (16.7% vs. 5.7%).
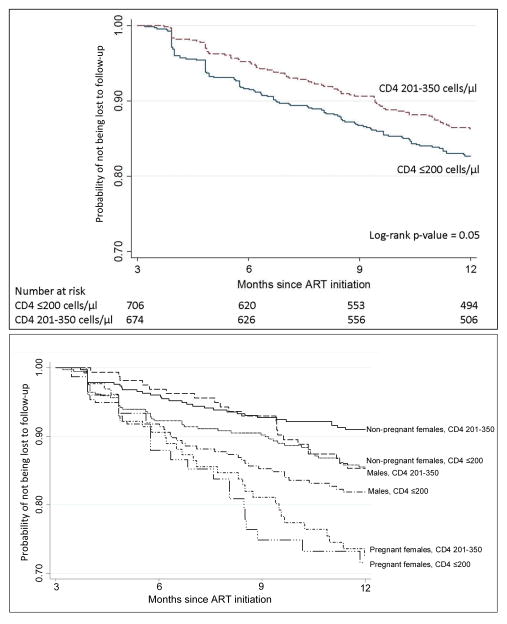
Within one year of ART initiation, 15.5% (95%CI: 13.0–18.2%) versus 12.7% (95%CI: 10.3–15.3%) were lost among those in the lower versus the higher CD4 group, respectively (Figure 1). Among non-pregnant females, we found a 42% reduction in loss to follow-up among women initiating ART at higher versus lower CD4 cell counts (aHR 0.58; 95%CI: 0.37–0.91) (Table 1). Males who initiated ART at higher CD4 counts were also at reduced risk of loss to follow-up compared to males who started ART at CD4 count ≤200 cells/μl (aHR 0.74; 95%CI: 0.44–1.23). However among pregnant women there was no association between CD4 category and loss to follow-up (aHR 0.95; 95%CI: 0.55–1.67). Additionally, younger adults (age 18–29 years) were 38% more likely to become lost to follow-up compared to adults age 30–39 years (aHR 1.38, 95%CI: 1.00–1.92), and unemployed patients were 51% (aHR 1.51, 95%CI: 1.34–2.00) more likely to become lost to follow-up. Estimates differed little when accounting for death as a competing risk. Figure 1 shows the gender variation in loss to follow-up: males were more likely to be lost than non-pregnant females and women pregnant at ART initiation were most likely to be lost.
Figure 1. Kaplan-Meier estimates of time to loss to follow-up by baseline CD4 value (top) and by baseline CD4 by gender/pregnancy at ART initiation status (bottom).
Note that the y-axes range from 0.7 to 1.0. Loss to follow-up is defined as not returning ≥3 months after the last scheduled visit; thus, person-time begins accumulating at 3 months.
Table 1.
Multivariate analysis of factors associated with patient loss to follow-up after ART initiation at an HIV treatment program in South Africa (N=1430).
| n (%) | Crude HR (95% CI) | Adjusted HR (95% CI) | Competing risk sHR* (95% CI) | |
|---|---|---|---|---|
| Non-pregnant female, CD4 ≤200 cells/μl | 50/369 (13.6) | 1 | 1 | 1 |
| Non-pregnant female, CD4 201–350 cells/μl | 33/380 (8.7) | 0.61 (0.39, 0.94) | 0.58 (0.37, 0.91) | 0.59 (0.38, 0.91) |
| Male, CD4 ≤200 cells/μl | 45/261 (17.2) | 1 | 1 | 1 |
| Male, CD4 201–350 cells/μl | 22/164 (13.4) | 0.74 (0.44, 1.23) | 0.74 (0.44, 1.23) | 0.74 (0.45, 1.24) |
| Pregnant at ART initiation, CD4 ≤200 cells/μl | 20/76 (26.3) | 1 | 1 | 1 |
| Pregnant at ART initiation, CD4 201–350 cells/μl | 32/130 (24.6) | 0.93 (0.53, 1.62) | 0.95 (0.55, 1.67) | 0.96 (0.56, 1.67) |
| Age at HIV testing | ||||
| 18–29 years | 74/383 (19.3) | 1.50 (1.10, 2.06) | 1.38 (1.00, 1.92) | 1.39 (1.00, 1.91) |
| 30–39 years | 83/605 (13.7) | 1 | 1 | 1 |
| 40 years and older | 45/392 (11.5) | 0.83 (0.58, 1.20) | 0.93 (0.64, 1.35) | 0.93 (0.64, 1.34) |
| Nationality | ||||
| Born in South Africa | 139/931 (14.9) | 1 | 1 | 1 |
| Born outside of South Africa | 63/449 (14.0) | 0.95 (0.70, 1.27) | 0.82 (0.61, 1.11) | 0.83 (0.61, 1.12) |
| Employment status | ||||
| Employed | 87/716 (12.2) | 1 | 1 | 1 |
| Unemployed | 115/664 (17.3) | 1.50 (1.14, 1.99) | 1.51 (1.34, 2.00) | 1.50 (1.13, 1.99) |
| TB at ART initiation | ||||
| Yes | 22/155 (14.2) | 0.97 (0.63, 1.52) | 1.01 (0.64, 1.59) | 1.00 (0.63, 1.59) |
| No | 180/1225 (14.7) | 1 | 1 | 1 |
HR, hazard ratio; CI, confidence interval; n (%) show proportion lost to follow-up in each category
Models are adjusted for all variables listed
Loss to follow-up is defined as not returning ≥3 months after the last scheduled visit; the HR models exclude 50 patients who died or transferred prior to 3 months follow-up (N=1380)
The competing risk subdistribution hazard ratio (sHR) model includes all 1430 patients and assesses patient loss to follow-up in the presence of the competing risk of death
During the first year of ART care, 29 patients (1.9%; 95%CI: 1.3–2.7%) died and 44 acquired TB (3.1%; 95%CI: 2.3–4.1%). Patients initiating ART at CD4 values 201–350 cells/μl were at much lower risk of death (aHR 0.34; 95%CI: 0.13–0.84) and incident TB (aHR 0.44; 95%CI: 0.23–0.85) than those with CD4 ≤200 cells/μl.
DISCUSSION
This is the first study from a routine clinical setting in South Africa to demonstrate patients can be initiated on ART at higher CD4 counts without increasing patient attrition. We found 42% (aHR 0.58; 95%CI: 0.37–0.91) reduced risk of loss to follow-up among non-pregnant females and 26% (aHR 0.74; 95%CI: 0.44–1.23) reduced risk among male patients who initiated ART at CD4 counts 201–350 cells/μl compared to those ≤200 cells/μl. This result is in concert with a 39% reduced loss among those initiating at CD4 cell counts >200 cells/μl versus ≤200 in Lesotho [11]. However our study highlights substantial variation by gender in reduced risk at initiating at higher CD4 cell counts. The association of initiating at higher CD4 counts was greatest among non-pregnant females and males, and null among pregnant women. Patient loss is a major challenge to providing effective HIV care and our results suggest that expanding ART eligibility to patients with higher baseline CD4 values can be done without increasing loss.
We found that loss to follow-up among males of both CD4 groups was substantially higher than non-pregnant females who initiate at CD4 201–350 cells/μl (Figure 1). Previous studies also have noted higher loss among men, [16–18] which suggests the need for specific retention interventions targeted to males. Loss to follow-up among women pregnant at ART initiation was the highest of any gender group, but interestingly, baseline CD4 did not substantially affect loss among pregnant women, possibly because loss was already so high. High loss to follow-up among pregnant women recently has been reported in other studies within South Africa [19–21], and is particularly concerning since HIV is the largest cause of maternal mortality in South Africa [22].
Additionally, our results confirm the considerable reductions in mortality and incident tuberculosis throughout the first year of ART seen among patients who present for care and initiate ART with a higher CD4 count versus those who present with advanced immunosuppression. These findings correspond with those of several studies in resource-limited settings that observed significantly reduced risk of death and opportunistic infections when initiating treatment at higher CD4 values [10–14] and support South Africa’s decision to revise its ART guidelines in 2011 to align with WHO recommendations.
Our study has several strengths. The unique study site allowed us to examine the impact of initiating at higher CD4 values within the context of routine, clinical care. Additionally, our analysis assessed the potential for the competing risk of death to bias our associations, but found little evidence of an important influence. We reduced the likelihood of death being misclassified as loss to follow-up by validating deaths using South Africa’s national death registry and conducting a sensitivity analysis of our primary outcome. However, the registry only includes patients with a valid South African ID number (47.9%) and included only deaths through data export in August 2011, thus it is possible that our estimate of mortality – particularly among patients lacking an ID number – may be underestimated.
Limitations to our analysis include use of data obtained from a single site and therefore findings may not be generalizable to all public-sector settings within South Africa or health care centers in other regions. As with most retention studies, we cannot know if a patient continued care at another facility due to unlinked data at facilities. Thus, loss to follow-up in this study must be understood as lost to the facility where ART was initiated. It is important not to interpret our results as evidence for when to start ART (i.e. a comparison of whether or not to initiate patients when their CD4 count first falls ≤350 or defer until CD4 counts reach <200). Our results only pertain to what happens to patients once they are diagnosed and initiate ART at their initial presentation CD4 value.
Our results show that adult, non-pregnant patients presenting for care and initiating ART at 201–350 cells/μl have reduced loss to follow-up than those presenting and initiating ART at ≤200 cells/μl, with significant variation by gender and pregnancy status. These are the first results to show South Africa’s 2011 expansion of ART treatment guidelines can be enacted without increasing attrition among patients initiating at higher CD4 values. As data become available from more sites that adopted the policy of initiating ART at higher CD4 cell counts, it will be important to perform similar evaluations at other sites in South Africa and other countries. Such information will be invaluable to national HIV/AIDS programs looking for guidance about the implications of earlier ART initiation, and may inform future expansions of the ART treatment criteria in South Africa.
Acknowledgments
Data collection occurred in collaboration with staff from Witkoppen Health and Welfare Centre and we are grateful for their cooperation and assistance.
This work was supported by USAID under the terms of agreement 674-A-00-08-00007-00 with Right to Care. Matthew Fox was supported by Award Number K01AI083097 from the National Institute of Allergy and Infectious Diseases (NIAID). The opinions expressed herein are those of the authors and do not necessarily reflect the views of NIH, NIAID, or Witkoppen Health and Welfare Centre.
Web Appendix Table 1. Characteristics of the study participants by baseline CD4 group
| Patient characteristic | Total (N=1430) | CD4 ≤200 cells/μl (n=743, 52.0%) | CD4 201–350 cells/μl (n=687, 48.0%) |
|---|---|---|---|
| Sex, n (%) | |||
| Female | 991 (69.3) | 471 (63.4) | 520 (75.7) |
| Male | 439 (30.7) | 272 (36.6) | 167 (24.3) |
| Age at HIV testing, median (IQR) | 34.3 (29.3, 41.2) | 35.2 (30.0, 42.2) | 33.3 (28.7, 39.9) |
| Age at HIV testing, n (%) | |||
| 18–29 years | 400 (28.0) | 182 (24.5) | 218 (31.7) |
| 30–39 years | 625 (43.7) | 327 (44.0) | 298 (43.4) |
| 40 years and older | 405 (28.3) | 234 (31.5) | 171 (24.9) |
| First CD4 value (cells/mm3), median (IQR) | 195 (103, 266) | 105 (55, 154) | 268 (239, 307) |
| First CD4 value, n (%) | |||
| <50 cells/μl | 169 (11.8) | 169 (22.8) | 0 (0.0) |
| 50–100 cells/μl | 182 (12.7) | 182 (24.5) | 0 (0.0) |
| 101–200 cells/μl | 392 (27.4) | 392 (52.8) | 0 (0.0) |
| 201–350 cells/μl | 687 (48.0) | 0 (0.0) | 687 (100.0) |
| Nationality, n (%) | |||
| Born in South Africa | 969 (67.8) | 504 (67.8) | 465 (67.7) |
| Born outside of South Africa | 461 (32.2) | 239 (32.2) | 222 (32.3) |
| Employment status, n (%) | |||
| Employed | 736 (51.5) | 391 (52.6) | 345 (50.2) |
| Not employed | 694 (48.5) | 352 (47.4) | 342 (49.8) |
| TB at ART initiation, n (%) | |||
| No | 1267 (88.6) | 619 (83.3) | 648 (94.3) |
| Yes | 163 (11.4) | 124 (16.7) | 39 (5.7) |
| Pregnant at ART initiation, n (%) | |||
| No | 1219 (85.2) | 664 (89.4) | 555 (80.8) |
| Yes | 211 (14.8) | 79 (10.6) | 132 (19.2) |
IQR, interquartile range
Footnotes
K.C. collected and analyzed the data, and wrote the first draft of the paper. A.P. supervised the study creation. M.F. supervised the data analysis and writing of the paper. All authors contributed to study design, revisions to the paper, and approved the final manuscript.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: 2010. [Accessed August 2012]. Available at http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. [Google Scholar]
- 2.Johnson LF. Access to antiretroviral treatment in South Africa, 2004–2011. South Afr J HIV Med. 2012;13(1):22–24. doi: 10.4102/sajhivmed.v18i1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Republic of South Africa Department of Health. [Accessed August 2012];National Antiretroviral Treatment Guidelines. (1). 2004 Available at http://apps.who.int/medicinedocs/en/m/abstract/Js17758en/
- 4.World Health Organization. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. Geneva: 2009. [Accessed August 2012]. Available at http://www.who.int/hiv/pub/arv/advice/en/index.html. [Google Scholar]
- 5.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. – 2010 rev. Geneva: 2010. [Accessed August 2012]. Available at http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed] [Google Scholar]
- 6.South African National AIDS Council. [Accessed August 2012];Statement on the Meeting of the South African National AIDS Council (SANAC) 2011 Available at http://www.sanac.org.za/files/uploaded/886_Plenary_Press_Statement_12Aug11.pdf.
- 7.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197(8):1133–44. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 8.Sterne JAC, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Severe P, Jean Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363(3):257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford N, Kranzer K, Hilderbrand K, et al. Early initiation of antiretroviral therapy and associated reduction in mortality, morbidity and defaulting in a nurse-managed, community cohort in Lesotho. AIDS. 2010;24(17):2645–50. doi: 10.1097/QAD.0b013e32833ec5b2. [DOI] [PubMed] [Google Scholar]
- 12.Fox MP, Sanne IM, Conradie F, et al. Initiating patients on antiretroviral therapy at CD4 cell counts above 200 cells/microl is associated with improved treatment outcomes in South Africa. AIDS. 2010;24(13):2041–50. doi: 10.1097/QAD.0b013e32833c703e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills EJ, Bakanda C, Birungi J, et al. Mortality by baseline CD4 cell count among HIV patients initiating antiretroviral therapy: evidence from a large cohort in Uganda. AIDS. 2011;25(6):851–5. doi: 10.1097/QAD.0b013e32834564e9. [DOI] [PubMed] [Google Scholar]
- 14.Moh R, Danel C, Messou E, et al. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS. 2007;21(18):2483–91. doi: 10.1097/QAD.0b013e3282f09876. [DOI] [PubMed] [Google Scholar]
- 15.Horne R, Cooper V, Gellaitry G, Date HL, Fisher M. Patients’ perceptions of highly active antiretroviral therapy in relation to treatment uptake and adherence: the utility of the necessity-concerns framework. J Acquir Immune Defic Syndr. 2007;45(3):334–41. doi: 10.1097/QAI.0b013e31806910e3. [DOI] [PubMed] [Google Scholar]
- 16.Toure S, Kouadio B, Seyler C, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Côte d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22(7):873–82. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15 (Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South African programme. PloS One. 2011;6(5):e19201. doi: 10.1371/journal.pone.0019201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B, Losina E, Stark R, et al. Loss to follow-up in a community clinic in South Africa--roles of gender, pregnancy and CD4 count. S Afr Med J. 2011;101(4):253–7. doi: 10.7196/samj.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyles TH, Wilkinson LS, Leisegang R, Maartens G. Factors influencing retention in care after starting antiretroviral therapy in a rural South african programme. PloS One. 2011;6(5):e19201. doi: 10.1371/journal.pone.0019201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Myer L, Cornell M, Fox MP, et al. Loss to follow-up and mortality among pregnant and non-pregnant women initiating ART: South Africa. 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. Paper 22. [Google Scholar]
- 22.National Committee on Confidential Enquiries into Maternal Deaths. [Accessed August 2012];Saving Mothers 2005–2007: Fourth Report on Confidential Enquiries into Maternal Deaths in South Africa. 2008 Available at www.doh.gov.za/docs/reports/2007/savingmothers.pdf.