Abstract
Purpose
Although established in the postresection setting, the prognostic value of carbohydrate antigen 19-9 (CA19-9) in unresectable locally advanced pancreatic cancer (LAPC) is less clear. We examined the prognostic utility of CA19-9 in patients with unresectable LAPC treated on a prospective trial of intensity modulated radiation therapy (IMRT) dose escalation with concurrent gemcitabine.
Methods and Materials
Forty-six patients with unresectable LAPC were treated at the University of Michigan on a phase 1/2 trial of IMRT dose escalation with concurrent gemcitabine. CA19-9 was obtained at baseline and during routine follow-up. Cox models were used to assess the effect of baseline factors on freedom from local progression (FFLP), distant progression (FFDP), progression-free survival (PFS), and overall survival (OS). Stepwise forward regression was used to build multivariate predictive models for each endpoint.
Results
Thirty-eight patients were eligible for the present analysis. On univariate analysis, baseline CA19-9 and age predicted OS, CA19-9 at baseline and 3 months predicted PFS, gross tumor volume (GTV) and black race predicted FFLP, and CA19-9 at 3 months predicted FFDP. On stepwise multivariate regression modeling, baseline CA19-9, age, and female sex predicted OS; baseline CA19-9 and female sex predicted both PFS and FFDP; and GTV predicted FFLP. Patients with baseline CA19-9 ≤90 U/mL had improved OS (median 23.0 vs 11.1 months, HR 2.88, P<.01) and PFS (14.4 vs 7.0 months, HR 3.61, P = .001). CA19-9 progression over 90 U/mL was prognostic for both OS (HR 3.65, P = .001) and PFS (HR 3.04, P = .001), and it was a stronger predictor of death than either local progression (HR 1.46, P = .42) or distant progression (HR 3.31, P = .004).
Conclusions
In patients with unresectable LAPC undergoing definitive chemoradiation therapy, baseline CA19-9 was independently prognostic even after established prognostic factors were controlled for, whereas CA19-9 progression strongly predicted disease progression and death. Future trials should stratify by baseline CA19-9 and incorporate CA19-9 progression as a criterion for progressive disease.
Introduction
Carbohydrate antigen 19-9 (CA19-9) is a tumor marker that has well established prognostic utility in patients with adenocarcinoma of the pancreas (1). The prognostic impact of baseline CA19-9 has been demonstrated in patients with all stages of disease treated with varying modalities, including surgery, chemotherapy alone, radiation therapy (RT) alone, and concurrent chemoradiation therapy (CRT) (1–6). For patients with localized disease, baseline CA19-9 has been shown be predictive of tumor resectability and early metastases, whereas postoperative CA19-9 has been demonstrated as prognostic for overall survival (OS) (2, 7–9). Furthermore, CA19-9 progression has been demonstrated to be closely correlated with time to radiographic progression, and to often precede radiographic progression in patients with both resectable and metastatic disease (10–12).
For patients with unresectable locally advanced pancreatic cancer (LAPC) treated with CRT, retrospective studies have suggested that CA19-9 response is predictive of treatment response and survival, with various criteria of assessing response suggested by different authors (3, 13, 14). The Radiation Therapy Oncology Group (RTOG) has recently prospectively validated postresection CA19-9 levels of 90 and 180 U/mL as prognostic for overall survival for patients receiving adjuvant CRT (8). To our knowledge, however, no studies have prospectively evaluated the prognostic value of baseline or posttherapy CA19-9 levels for patients undergoing definitive CRT for unresectable disease. The clinical utility of CA19-9 in such patients, therefore, has yet to be rigorously evaluated, and an optimal CA19-9 level for clinical application has yet to be identified or validated.
We have previously reported the results of a multi-institutional single-arm prospective phase 1/2 trial of intensity modulated radiation therapy (IMRT) with concurrent fixed-dose-rate gemcitabine in patients with unresectable LAPC (15). This study demonstrated a promising median survival of 14.8 months, and 12 of 50 patients initially deemed unresectable ultimately underwent resection, with R0 resections achieved in 10 patients and pathologic complete responses demonstrated in 2 patients. CA19-9 data were collected for all protocol patients both at enrollment and throughout follow-up after the completion of CRT. In the present analysis, we investigated the hypothesis that baseline and posttherapy CA19-9 predict for clinical outcome in patients receiving dose-escalated IMRT with concurrent gemcitabine for unresectable LAPC.
Methods and Materials
Patient eligibility and trial design
Forty-six patients at the University of Michigan were accrued on this prospective phase 1/2 trial approved by the University of Michigan and Rush University Medical Center Institutional Review Boards. The eligibility criteria, treatment regimen, and study design have previously been described in detail (15). In brief, patients were required to have a pathologic diagnosis of adenocarcinoma of the pancreas deemed unresectable because of locally advanced disease, established by radiographic criteria (>180° involvement of the superior mesenteric artery or celiac trunk or unreconstructable superior mesenteric vein/portal vein impingement) and without distant metastases. Resectability was determined by a multidisciplinary panel of surgeons, radiologists, and medical and radiation oncologists. Borderline resectable tumors were not allowed on this study. Zubrod performance status ≥2 was required.
All patients received gemcitabine, 1000 mg/m2 over 100 minutes, intravenously on days 1 and 8 of a 21-day cycle. One cycle of run-in chemotherapy was given before IMRT, followed by 2 cycles of gemcitabine given concurrently with IMRT (days 1, 8, 22, and 29). After IMRT, 4 additional cycles of gemcitabine were recommended.
The IMRT was delivered in 25 fractions over 5 weeks, with the radiation dose escalated from 50 to 60 Gy. The gross tumor volume (GTV) was defined on a pancreas protocol CT. The planning target volume (PTV) was GTV plus a 1-cm expansion. Active breathing control was used to minimize breathing motion, except in 4 patients in whom 4-dimensional CT was used to generate an internal target volume. The IMRT dose levels were assigned according to the Time-to-Event Continual Reassessment Method algorithm based on a simple model relating the probability of dose-limiting toxicity to dose, as previously described (15, 16). At the conclusion of the study, the maximum tolerated RT dose was 55 Gy in 25 fractions.
Patients were seen for follow-up 3, 8, and 13 weeks after completion of RT and then every 2 to 3 months. CA19-9 levels were determined at each follow-up visit. CT scans of the chest, abdomen (pancreas protocol), and pelvis were obtained on weeks 8 and 18 and every 2 to 3 months thereafter. All scans were centrally reviewed by 1 radiologist (I.R.F.). Progressive disease (PD) was defined by radiographic or biopsy evidence of progression, not by rising CA19-9 or initiation of palliative chemotherapy alone.
Baseline CA19-9 was obtained within 2 weeks of study registration, after biliary decompression and before the initiation of therapy. Eight patients with baseline CA19-9 <10 U/mL were considered nonsecretors and were excluded from this analysis. None of these patients subsequently experienced a rise in CA19-9 even at the time of disease progression, which confirmed their physiologic inability to express CA19-9, likely because of a lack of the Lewis antigen glycosyl-transferase expression that is required for CA19-9 synthesis. Confirmatory red blood cell phenotyping for Lewis A and Lewis B antigens was not performed.
Statistical methods
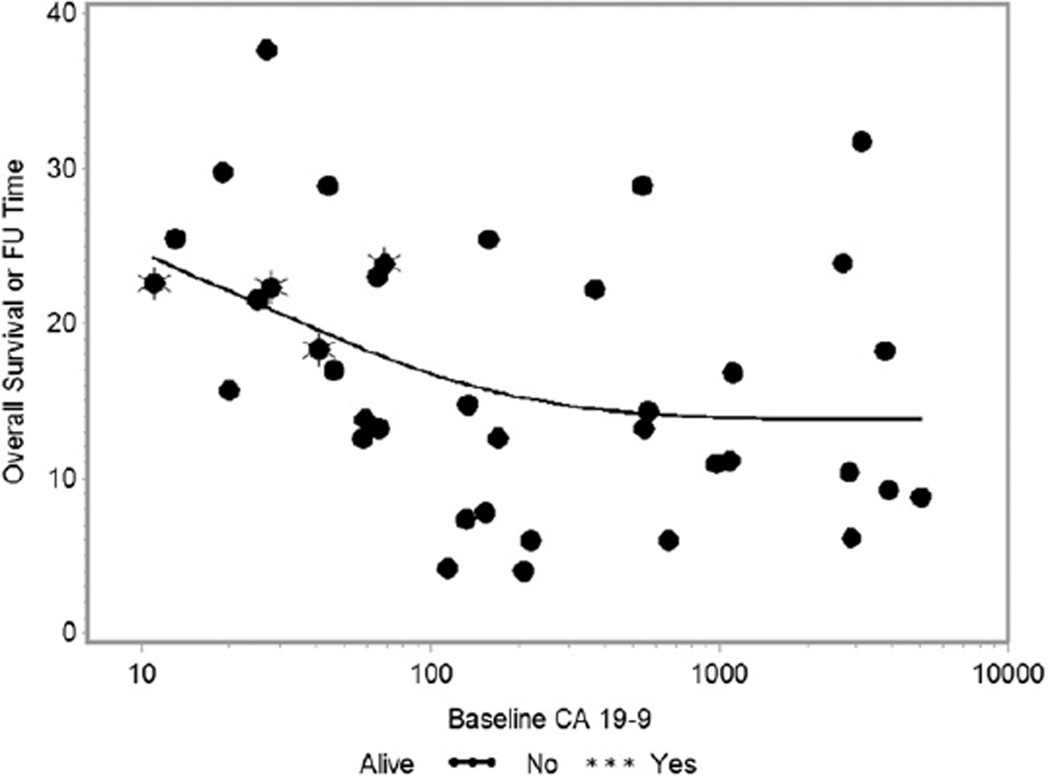
The clinical endpoints for statistical analysis included freedom from local progression (FFLP), freedom from distant progression (FFDP), progression-free survival (PFS), and overall survival (OS). Patients still alive without progression at the time of analysis were censored at the last date of radiographic assessment. The Kaplan-Meier method was used to summarize the time-to-event endpoints, and the log-rank test was used to compare survival curves between groups. Cox regression models were used to assess the effect of the predictors on each time-to-event outcome. Clinical variables incorporated into the Cox model included age, sex, race, performance status, clinical T stage, clinical N stage, GTV (cm3), RT dose, RT duration, hospitalization during treatment, and baseline CA19-9 at baseline and 3 months. CA19-9 values were log transformed before inclusion in regression models because of the approximately linear relationship between OS and CA19-9 on the log scale (Fig. 1). Stepwise forward regression modeling was used to build multivariate models. In addition to values of CA19-9 at baseline, absolute value at 3 months and rate of change during 0 to 3 months were considered. A time-dependent covariate, called CA19-9 progression, was defined as the first rise of CA19-9 over 90 U/mL after completion of CRT. All analyses were performed using SAS/STAT for Windows, version 9.3 (SAS Institute Inc 2008, Cary, NC).
Fig. 1.
Overall survival as a function of baseline carbohydrate antigen 19-9 (CA19-9) (log-scale). FU = follow-up.
Results
Thirty-eight patients with adequate CA19-9 data for analysis were included in the present analysis. The baseline characteristics of the cohort are shown in Table 1. The median follow-up time for the 4 remaining living patients was 22.4 months.
Table 1.
Baseline characteristics
| Age (median ±SD) | 62.0 ± 7.7 |
| Sex | |
| M | 23 (60.5%) |
| F | 15 (39.5%) |
| Race | |
| White | 34 (89.5%) |
| Black | 4 (10.5%) |
| Zubrod performance status | |
| 0 | 9 (23.7%) |
| 1 | 25 (65.8%) |
| 2 | 4 (10.5%) |
| T stage | |
| T3 | 8 (21.1%) |
| T4 | 30 (78.9%) |
| N stage | |
| N0 | 14 (36.8%) |
| N1 | 24 (63.2%) |
| AJCC stage | |
| II | 7 (18.4%) |
| III | 31 (81.6%) |
| GTV (cm3) | |
| Median ±S.D. | 47.9± 44.0 |
| Range | 17.1–169.6 |
| CA19-9 (U/mL) | |
| Median | 156 |
| Mean | 839 |
| SD | 1335 |
| Range | 11–5028 |
| Interquartile range (25%/75%) | 46–996 |
| ≤90 | 15 (39%) |
| >90 | 23 (61%) |
Abbreviations: AJCC = American Joint Committee on Cancer; CA19-9 = carbohydrate antigen 19-9; GTV = gross tumor volume; SD = standard deviation.
For the 38 evaluable patients, the median survival was 15.2 months (95% CI, 12.6–22.2), and 2-year OS was 26.6% (95% CI, 13.2–42.0). Median PFS was 8.6 months (95% CI, 6.5–10.6), with documented disease progression occurring in 30 patients (78.9%). Local progression occurred in 11 patients (29.0%) at a median of 15.4 months (range, 4.9–22.2 months). Distant progression occurred in 25 patients (65.8%) at a median of 8.1 months (range, 2.5–21.6 months). Six patients (16%) experienced both local and distant progression, with local progression preceding or occurring synchronously with distant progression in 4 of them. For the entire cohort, the median FFLP was 21.6 months (95% CI, 17.2enot estimable), and the median FFDP was 10.6 months (95% CI, 7.7–16.0).
Univariate predictors (Table 2) of OS were baseline CA 19-9 (hazard ratio [HR] 1.20, P = .038) and age (HR 1.05, P = .024). For PFS, baseline CA19-9 (HR 1.33, P = .005) and CA19-9 at 3 months (HR 1.44, P = .001) were significantly predictive. For FFLP, GTV (HR 1.02, P = .003) and black race (HR 4.74, P = .027) were predictive. For FFDP, CA19-9 at 3 months (HR 1.38, P = .013) was significantly predictive, whereas baseline CA19-9 (HR 1.23, P = .065) and female sex (HR 0.42, P = .053) were of borderline significance. Tumor stage, nodal stage, American Joint Committee on Cancer stage, RT dose level, RT duration, and hospitalization during treatment were all highly nonsignificant (P>.20) for all clinical endpoints tested (results not shown).
Table 2.
Cox univariate analysis of predictors of overall survival, progression-free survival, freedom from local progression, and freedom from distant progression
| Overall survival |
Progression-free survival |
Freedom from local progression |
Freedom from distant progression |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | P value | HR | P value | HR | Pvalue | HR | P value |
| Baseline CA19-9 | 1.20 | .038 | 1.33 | .005 | 1.18 | .303 | 1.23 | .065 |
| CA19-9 at 3 months | 1.10 | .32 | 1.44 | .001 | 1.08 | .667 | 1.38 | .013 |
| Age | 1.05 | .024 | 1.03 | .205 | 0.990 | .791 | 1.01 | .634 |
| Sex (F vs M) | 0.67 | .28 | 0.63 | .177 | 1.01 | .984 | 0.42 | .053 |
| Zubrod performance status | 1.76 | .12 | 1.51 | .212 | 1.71 | .395 | 1.22 | .605 |
| Race (black vs other) | 1.11 | .844 | 1.34 | .589 | 4.74 | .027 | 0.57 | .447 |
| Gross tumor volume (cm3) | 1.005 | .22 | 1.00 | .251 | 1.02 | .003 | 1.00 | .558 |
Abbreviations CA19-9 = carbohydrate antigen 19-9; HR = hazard ratio.
All variables were analyzed as continuous variables unless otherwise noted.
Stepwise multivariate regression modeling (Table 3) identified baseline CA19-9 (HR 1.37, P = .004), age (HR 1.05, P = .030), and female sex (HR 0.40, P = .034) as independent predictors of OS. For PFS and FFDP, baseline CA19-9 (HR 1.38, P = .001 for PFS; HR 1.40, P = .006 for FFDP) and female sex (HR 0.50, P = .050 for PFS; HR 0.265, P = .007 for FFDP) were both independently predictive. For FFLP, only GTV remained predictive, and no other predictors were significant when added to the model containing GTV.
Table 3.
Stepwise multivariate regression models for overall survival, progression-free survival, and freedom from distant progression
| Outcome | Hazard ratio | P value |
|---|---|---|
| Overall survival | ||
| Baseline CA19-9 | 1.37 | .004 |
| Age | 1.05 | .030 |
| Sex (F vs M) | 0.40 | .034 |
| Progression-free survival | ||
| Baseline CA19-9 | 1.38 | .001 |
| Sex (F vs M) | 0.50 | .050 |
| Freedom from distant progression | ||
| Baseline CA19-9 | 1.40 | .006 |
| Sex | 0.265 | .007 |
Abbreviation: CA19-9 = carbohydrate antigen 19-9.
All variables were considered as continuous variables unless otherwise noted. No models containing multiple variables for freedom from local progression were identified.
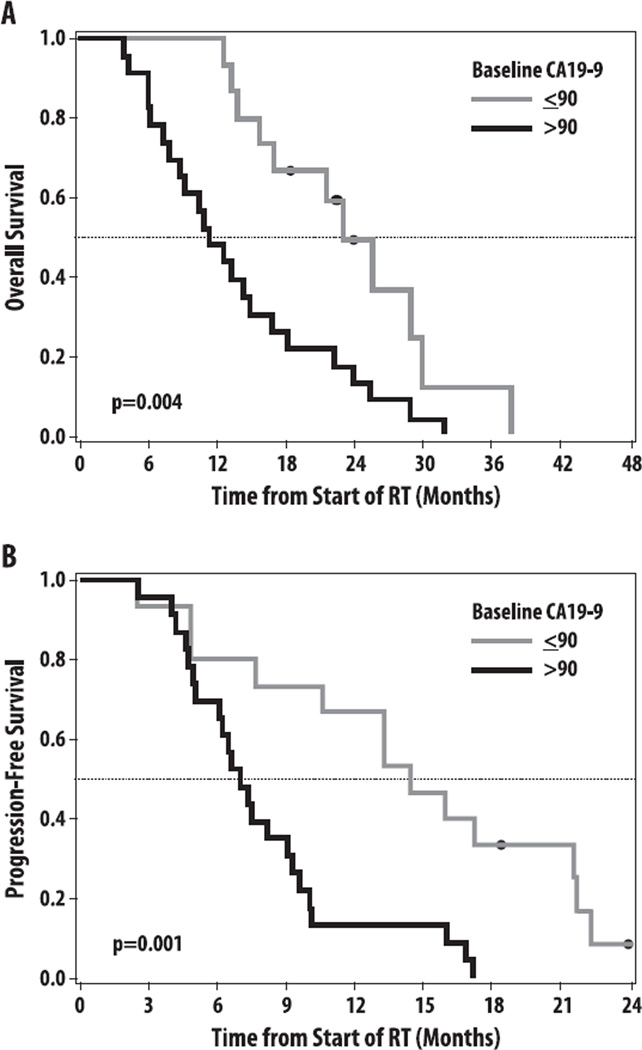
The OS and PFS were compared between subjects with baseline CA19-9 below/above a threshold level of 90 U/mL, based on the prognostic value of this level in the adjuvant setting (8).Twenty-three patients (61%) had baseline CA19-9 >90 U/mL and 15 patients (39%) had baseline CA19-9 ≤90 U/mL. The median OS was 11.1 versus 23.0 months (HR 2.88, P = .004), and the median PFS was 7.0 versus 14.4 months (HR 3.61, P = .001) for patients withCA19-9 >90 U/mL and ≤90 U/mL, respectively (Fig. 2). In sensitivity analyses, inclusion of the 8 nonsecretor patients who had baseline CA 19-9 <10 U/mL did not change the results qualitatively or in terms of statistical significance (data not shown). The relationship between baseline CA19-9 U/mL and OS is shown in Figure 1.
Fig. 2.
(A) Overall survival and (B) progression-free survival stratified by baseline carbohydrate antigen 19-9 (CA19-9) >90 U/mL versus ≤90 U/mL. RT = radiation therapy.
We also assessed the ability of CA19-9 to act as a disease-monitoring biomarker by including the longitudinally measured CA19-9 values as a time-dependent covariate in a Cox regression model. Thirty-seven patients had post-CRT CA19-9 levels available for analysis, of whom 34 (89.5%) had a decrease in CA19-9 levels compared with baseline. Among the 34 patients with a CA19-9 response, the best radiographic response was partial response in 10, stable disease in 23, and not evaluable in 1. Among the 23 patients with baseline CA19-9 >90 U/mL, 14 (60.9%) had a CA19-9 decrease below 90 U/mL after the completion of CRT, although OS (HR 0.77, P = .53) or PFS (HR 1.55, P = .27) did not differ between these 14 patients and the 9 patients in whom CA19-9 did not respond to less than 90 U/mL. We defined a time-dependent binary covariate as the presence of a rising CA19-9 level over 90 U/ mL to define CA19-9 progression. According to this definition, 27 patients (73.0%) had CA19-9 progression at a median of 7.3 months (range, 2.5–21.6 months). For the entire cohort, the median time to CA19-9 progression was 11.6 months (95% CI, 5.8–15.9), and the median time from progression to death was 8.0 months. CA19-9 progression preceded radiographic disease progression by 1 month or more in 11 patients (29.7%), and 2 patients died with progression by CA19-9 criteria alone (ie, without documented local or distant PD). CA19-9 progression over 90 U/mL was highly predictive of both OS (HR 3.65, P = .001) and PFS (HR 3.04, P = .001), and it was a stronger predictor of death than either local progression (HR 1.46, P = .42) or distant progression (HR 3.31, P = .004) (Table 4).
Table 4.
Predictive value of CA19-9 progression after completion of chemoradiation therapy
| Outcome | Hazard ratio | P value |
|---|---|---|
| Overall survival | 3.65 | .001 |
| Progression-free survival | 3.04 | .001 |
| Freedom from local progression | 2.44 | .158 |
| Freedom from distant progression | 1.75 | .206 |
Abbreviation: CA19-9 = carbohydrate antigen 19-9.
CA19-9 progression was defined as time of first rise of serum CA 19-9 over 90 U/mL.
Nine patients underwent resection at a median of 5.6 months after the completion of CRT, 8 of whom achieved an R0 resection. Patients undergoing resection had significantly longer OS than did those who did not undergo resection (median 25.5 vs 13.2 months, HR 0.39, P = .011). On multivariate analysis, only younger age (OR 0.91; 95% CI, 0.80–1.00; P = .037) was significantly associated with resection status, although T3 versus T4 tumor stage (OR 4.14; 95% CI, 0.93-19.25; P = .062), CA19-9 at 3 months (HR 0.72; 95% CI, 0.45–1.06; P = .098), female sex (OR 3.41; 95% CI, 0.82–15.8; P = .093), GTV (OR 0.99; 95% CI, 0.96–1.005; P = .16), and baseline CA19-9 (OR 0.81; 95% CI, 0.58–1.09; P = .162) trended toward significance. Patients with baseline CA19-9 <90 U/mL were more likely to undergo resection (7/16 patients, 46.7%) than were those with baseline CA19-9 <90 U/mL (2/22 patients, 9.1%) (OR 9.12, P = .014).
Discussion
Several retrospective reports have previously demonstrated that serum CA19-9 is prognostic for survival in patients undergoing CRT for unresectable LAPC (3–6). Postresection CA19-9 has also been prospectively validated in RTOG 9704 as a prognostic marker for OS in operable pancreatic cancer (8). To our knowledge, however, ours is the first study to confirm the prognostic value of CA19-9 in a prospective study of patients with unresectable pancreatic adenocarcinoma treated with definitive CRT. The prospective nature of our study is its primary strength, ensuring homogeneity of the patient population by prespecified standardization of all staging, treatment, follow-up, and data collection. This rigorous method serves to eliminate potential selection and reporting biases that were inherent to prior retrospective reports, thereby improving the broad generalizability of our results to other patients with unresectable LAPC.
In our study, baseline CA19-9 retained independent prognostic significance in patients receiving dose-escalated IMRT and concurrent gemcitabine for unresectable LAPC, even after established prognostic factors including age, sex, stage, and performance status were controlled for. This effect was particularly dramatic when patients were stratified by a cutoff value of 90 U/mL (median OS 11.1 vs 23.0 months; HR 2.88) for patients with CA19-9 >90 U/mL versus ≤90 U/mL, respectively. Our decision to dichotomize CA19-9 at 90 U/mL was motivated by the results of RTOG 9704, which showed a strikingly similar survival difference (median OS 10.4 vs 23 months, HR 3.4) in patients with postresection CA19-9 >90 U/mL versus ≤90 U/mL, as in our study, and led to the incorporation of patient stratification by postresection CA19-9 in RTOG 0848 (8). In our study, additional analysis of HR as a function of baseline CA 19-9 showed that dichotomization by baseline CA19-9 between 70 and 113 U/mL yielded the maximal HR for both OS and PFS (data not shown), further supporting our selection of 90 U/mL as an optimal cutpoint for baseline CA19-9. Although validation in an independent cohort remains necessary to establish whether 90 U/mL is the optimal baseline CA19-9 value for prognostic stratification of patients with unresectable LAPC, the overall prognostic impact of baseline CA19-9 in our study and other studies strongly supports its inclusion as a stratification factor for future randomized trials in unresectable LAPC, which to date have not incorporated CA19-9 for patient stratification (17).
Progression of CA19-9 over 90 U/mL after the completion of CRT was the strongest negative prognostic factor in our study, with a hazard ratio for OS exceeding that of either local or distant progression. This is consistent with data demonstrating that CA19-9 response can predict survival independently of CT imaging criteria response to chemotherapy in the setting of locally advanced and metastatic disease, and it is closely correlated with time to disease progression and may even precede radiographic progression in both the postresection and metastatic settings (10–12, 18). The observation that 2 patients in our study died after experiencing CA19-9 progression, yet without demonstrated local or distant PD, highlights the limitations of radiographic examination in detecting particularly local progression in patients with unresectable LAPC. Similar difficulties in radiographic diagnosis of local progression in this setting may well have contributed to the seemingly paradoxical results of the recently published Eastern Cooperative Oncology Group 4201 study, in which the addition of concurrent RT to gemcitabine improved OS without improving PFS (17). The finding that 11 patients experienced progression by CA19-9 criteria over 1 month before the clinical detection of PD further supports a role for routine CA19-9 surveillance after CRT, as endorsed by others (18). Given the superior prognostic utility of CA19-9 progression in our study and its potential benefits toward earlier detection of PD in patients who express CA19-9, we suggest that future studies of unresectable LAPC consider the inclusion of CA19-9 progression as a criterion for PD.
In our study, patients who were able to undergo surgical resection had significantly improved survival, with a median OS of 25.5 months for the 9 resected patients (23.6%) compared with 13.2 months for those who could not undergo resection (HR 0.39, P = .011). The significant proportion of patients who were converted to resectability by CRT was surprising, inasmuch as all patients treated on this study had unresectable disease at diagnosis. We were unable to identify any factors independently associated with resection other than younger age on multivariate analysis, although lower CA19-9 at 3 months, lower T stage, female sex, and smaller GTV size all trended toward significance. Inasmuch as the patients selected for surgery were those who had a favorable tumor response to CRT and no evidence of metastatic disease, it is possible that the improved survival reflects favorable tumor biology rather than the impact of surgical intervention, although our study was unable to address this question, given its nonrandomized single-arm design. An analysis of DPC4 status in this patient cohort is currently ongoing to determine whether intact DPC4 status may be predictive of response to intensified local therapy and conversion to surgical resectability, as has been proposed by others (19).
Even with aggressive multimodality therapy, unresectable LAPC continues to have a dismal prognosis. Given that approximately one third of patients with pancreatic cancer ultimately die of locally destructive disease, future improvements in local therapy will be essential to ultimately improving survival in this disease (19). In our study, only GTV was independently predictive for local progression on multivariate analysis, suggesting that larger tumors may most require further intensification of local therapy, although the radiation tolerance of surrounding normal tissue structures continues to pose significant barriers to further dose escalation efforts in pancreatic cancer (15, 20). Distant progression was associated with only baseline CA19-9 level on multivariate regression modeling, suggesting that patients with higher baseline CA19-9 levels should be potentially considered for intensification of systemic therapy, irrespective of radiographic or CA19-9 response to CRT.
In summary, our study shows that in patients with unresectable LAPC, baseline CA19-9 level >90 U/mL identifies those patients at highest risk for PD and death, and should be validated and used for patient stratification in future randomized trials of CRT. The strong negative prognostic value of CA19-9 progression over 90 U/mL after the completion of CRT suggests that this threshold for CA19-9 progression should be used as a criterion for PD and the initiation of salvage therapy in the management of unresectable LAPC.
Summary.
We investigated the prognostic utility of carbohydrate antigen 19-9 (CA19-9) in patients with unresectable pancreatic cancer treated on a prospective dose-escalation trial of intensity modulated radiation therapy with concurrent gemcitabine. Baseline CA19-9 was found to be highly prognostic for overall survival and progression-free survival, whereas CA19-9 progression over 90 U/mL after chemoradiation therapy was the strongest predictor of death. Future trials of chemoradiation therapy for unresectable pancreas cancer should incorporate baseline CA19-9 for patient stratification and CA19-9 progression as a criterion for disease progression.
Acknowledgments
Supported in part by R01 CA78554 (T.S.L.).
Footnotes
Presented at the 54th Annual Meeting of the American Society for Radiation Oncology (ASTRO), October 28–31, 2012, Boston, MA.
Conflict of interest: none.
References
- 1.Ritts RE, Pitt HA. CA 19-9 in pancreatic cancer. Surg Oncol Clin North Am. 1998;7:93–101. [PubMed] [Google Scholar]
- 2.Gattani AM, Mandeli J, Bruckner HW. Tumor markers in patients with pancreatic carcinoma. Cancer. 1996;78:57–62. doi: 10.1002/(SICI)1097-0142(19960701)78:1<57::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Koom WS, Seong J, Kim YB, et al. CA 19-9 as a predictor for response and survival in advanced pancreatic cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:1148–1154. doi: 10.1016/j.ijrobp.2008.06.1483. [DOI] [PubMed] [Google Scholar]
- 4.Micke O, Bruns F, Kurowski R, et al. Predictive value of carbohydrate antigen 19-9 in pancreatic cancer treated with radiochemotherapy. Int J Radiat Oncol Biol Phys. 2003;57:90–97. doi: 10.1016/s0360-3016(03)00524-8. [DOI] [PubMed] [Google Scholar]
- 5.Katz A, Hanlon A, Lanciano R, et al. Prognostic value of CA 19-9 levels in patients with carcinoma of the pancreas treated with radio-therapy. Int J Radiat Oncol Biol Phys. 1998;41:393–396. doi: 10.1016/s0360-3016(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 6.Hess V, Glimelius B, Grawe P, et al. CA 19-9 tumour-marker response to chemotherapy in patients with advanced pancreatic cancer enrolled in a randomised controlled trial. Lancet Oncol. 2008;9:132–138. doi: 10.1016/S1470-2045(08)70001-9. [DOI] [PubMed] [Google Scholar]
- 7.Safi F, Schlosser W, Falkenreck S, et al. Prognostic value of CA 19-9 serum course in pancreatic cancer. Hepatogastroenterology. 1998;45:253–259. [PubMed] [Google Scholar]
- 8.Berger AC, Garcia M Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol. 2008;26:5918–5922. doi: 10.1200/JCO.2008.18.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA 19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–2902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocha Lima CM, Savarese D, Bruckner H, et al. Irinotecan plus gemcitabine induces both radiographic and CA 19-9 tumor marker responses in patients with previously untreated advanced pancreatic cancer. J Clin Oncol. 2002;20:1182–1191. doi: 10.1200/JCO.2002.20.5.1182. [DOI] [PubMed] [Google Scholar]
- 11.Tian F, Appert HE, Myles J, et al. Prognostic value of serum CA 19-9 levels in pancreatic adenocarcinoma. Ann Surg. 1992;215:350–355. doi: 10.1097/00000658-199204000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong D, Ko AH, Hwang J, et al. Serum CA 19-9 decline compared to radiographic response as a surrogate for clinical outcomes in patients with metastatic pancreatic cancer receiving chemotherapy. Pancreas. 2008;37:269–274. doi: 10.1097/MPA.0b013e31816d8185. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan S, Rana V, Janjan NA, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer. 2006;107:2589–2596. doi: 10.1002/cncr.22328. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi H, Ohigashi H, Ishikawa O, et al. Serum CA 19-9 alterations during preoperative gemcitabine-based chemoradiation therapy for resectable invasive ductal carcinoma of the pancreas as an indicator for therapeutic selection and survival. Ann Surg. 2010;251:461–469. doi: 10.1097/SLA.0b013e3181cc90a3. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Josef E, Schipper M, Francis IR, et al. A Phase I/II Trial of Intensity Modulated Radiation (IMRT) Dose Escalation With Concurrent Fixed-dose Rate Gemcitabine (FDR-G) in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheung YK, Chappell R. Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics. 2000;56:1177–1182. doi: 10.1111/j.0006-341x.2000.01177.x. [DOI] [PubMed] [Google Scholar]
- 17.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez JM, Cowgill SM, Al-Saadi S, et al. CA 19-9 velocity predicts disease-free survival and overall survival after pancreatectomy of curative intent. J Gastrointest Surg. 2009;13:349–353. doi: 10.1007/s11605-008-0696-3. [DOI] [PubMed] [Google Scholar]
- 19.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy JD, Christman-Skieller C, Kim J, et al. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]